There is a substantial body of research demonstrating both increased morbidity(Reference Barrington1, Reference Barry, Sinclair and Kelly2) and premature mortality(Reference Balanda and Wilde3) among Irish adults of low socio-economic status (SES), in comparison with their more advantaged peers. International research has suggested that such health inequalities may be at least partially attributable to differences in food and nutrient intakes across the socio-economic spectrum(Reference James, Nelson and Ralph4). However, while literature describing dietary and nutritional inequalities among Irish adults has been published(Reference Friel and Conlon5–Reference Layte, Harrington and Sexton7), it has been a challenge for the larger national nutrition surveys conducted in recent years(Reference Harrington, Robson and Kiely8–Reference Walton11) to capture the food and nutrient intake patterns of the very poorest members of Irish society. The methodological constraints that limit the recruitment of such subjects in national nutrition surveys have highlighted the need for focused, dedicated research in this area. For example, the Low Income Diet and Nutrition Survey (LIDNS) in the UK(Reference Nelson, Erens and Bates12) was commissioned specifically to examine food and nutrient intake patterns among a carefully selected population of low-SES consumers in that country.
Research in many countries has highlighted profound differences in food group and nutrient intakes across the socio-economic spectrum. For example, lower consumption of fruit and vegetables has been consistently demonstrated among low-SES groups in the UK(Reference Shohaimi, Welch and Bingham13), Europe(Reference Boylan, Lallukka and Lahelma14), Norway(Reference Wandel15), the Netherlands(Reference Kamphuis, van Lenthe and Giskes16), Denmark(Reference Groth, Fagt and Brondsted17), Australia(Reference Giskes, Turrell and Patterson18), New Zealand(Reference Metcalf, Scragg and Davis19) and the USA(Reference Dubowitz, Heron and Bird20). Similarly, lower consumption of ready-to-eat breakfast cereals has been reported among low-SES groups in the UK(Reference Lang, Thane and Bolton-Smith21), France(Reference Touvier, Méjean and Kesse-Guyot22), Spain(Reference Aranceta, Perez-Rodrigo and Ribas23), the USA(Reference Siega-Riz, Popkin and Carson24) and Australia(Reference Mishra, Ball and Arbuckle25), while lower wholemeal bread intakes have been observed among low-SES men and women in the UK(Reference Gray and Leyland26). Lower fish intakes have also been observed among low-SES respondents in Switzerland(Reference Galobardes, Morabia and Bernstein27), Italy(Reference Vannoni, Spadea and Frasca28) and Spain(Reference Aranceta, Perez-Rodrigo and Ribas23), while higher intakes of processed red meats(29–Reference Cosgrove, Flynn and Kiely31), chips and fried potatoes(Reference Barker, Lawrence and Crozier32, Reference Deshmukh-Taskar, O'Neil and Nicklas33), potato-based snacks(Reference Barker, Lawrence and Crozier32, Reference Vinkeles Melchers, Gomez and Colagiuri34), white bread(Reference Barker, Lawrence and Crozier32, Reference Darmon and Drewnowski35), and sugar-sweetened foods and drinks(Reference Gray and Leyland26, Reference Bhargava and Amialchuk36) have also been widely reported among these socially disadvantaged groups.
These dietary differences have been shown to lead to unfavourable macronutrient(Reference James, Nelson and Ralph4, Reference Darmon and Drewnowski35) and micronutrient(Reference Giskes, Turrell and Patterson18) intakes among low-SES groups. The present study aimed to determine whether similar socio-economic differences in diet occur among young Irish women and, if so, whether they are associated with poorer nutritional intakes among these low-SES women.
Methods
Data collection
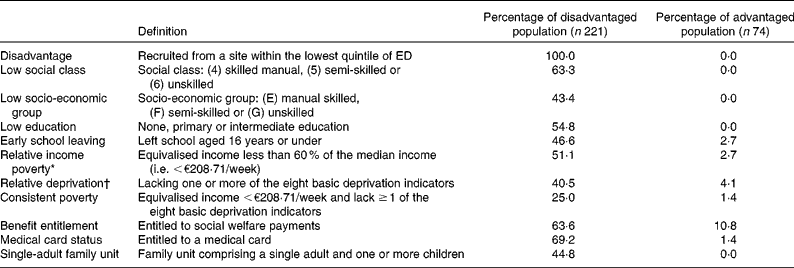
A total of 295 women aged 18–35 years were recruited from areas around Dublin using a de novo socio-economic sampling frame that ranked electoral districts (ED) based on a composite score for six socio-economic indicators. In order to construct this sampling frame, small-area population statistics were derived from national census data, enabling prevalence estimates for unemployment, low occupational social class, low socio-economic group, low formal education, single-parent household structure and local authority accommodation to be calculated for each of the 353 ED in Greater Dublin. Each of the ED was then ranked from 1 to 353 for each of these socio-economic parameters. For each ED, the ranking scores for each of these six socio-economic parameters were multiplied together. The product of the ranking scores for each ED was finally used to score each ED from 1 to 353, with larger numbers indicating a greater level of overall disadvantage. Using this system, the most disadvantaged quintile of ED (i.e. the seventy-one ED with the largest scores) was identified. From these seventy-one ED, areas were identified in North, South, West and City Centre Dublin. Within these areas (four in North Dublin, three in South Dublin, four in West Dublin and four in the City Centre), fifteen recruitment sites (e.g. local community development schemes, training centres, crèches) were selected. Ultimately, 221 disadvantaged women were recruited from these sites. A reference population of seventy-four women was recruited from areas in the top four quintiles to establish a non-disadvantaged comparison group that would be broadly representative of the wider ‘non-poor’ female population in this age group.
Several indices were subsequently used to assess the SES of each respondent. These included educational attainment and occupational social class, in addition to material indices of disadvantage including ‘at risk of poverty’ status, relative deprivation and consistent poverty. At risk of poverty (relative income poverty) was calculated by comparing equivalised household income against the 60 % median income threshold(37). Relative deprivation was assessed by determining whether the respondents had experienced the enforced absence (due to financial constraint) of one or more basic necessities from a list of eight(37), while consistent poverty was identified if a respondent reported being ‘at risk of poverty’ in addition to experiencing enforced absence of one or more of the eight basic markers of deprivation(37). The derivation of relative deprivation is now defined as the enforced absence of two or more basic necessities from a revised list of eleven(38); however, this itinerary had not been finalised or implemented during the design or early data collection phases of the present study.
Habitual dietary intake was assessed by an interviewer-assisted questionnaire in a group setting, using a semi-structured weekly diet history according to a previously described protocol(Reference Black, Welch and Bingham39). A facilitator outlined each mealtime and inter-meal period to the group in turn, with each respondent recording each component of her typical weekly menu at the appropriate location on her diet history questionnaire. Thereafter, three fieldworkers (one dietitian and two final-year nutrition and dietetics students) conducted face-to-face interviews with each respondent in the group to elicit the types of foods consumed at these meal- and snack times and the frequency with which these foods were consumed. Portion sizes for each food were quantified using verbal descriptions from the respondents expressed in terms of typical household measures and were further explicated using an atlas of food portion sizes(40) and an itinerary of average portion sizes and typical weights of common foods as required(Reference Mills and Patel41). Information regarding the type, brand, frequency and dosage of any dietary supplements taken was also sought. A new food code was created for each of these supplements in a nutrient analysis package (Weighed Intake analysis Software Package (WISP) version 3.0; © Tinuviel Software Limited, 2005) using nutritional composition data derived from the manufacturer or taken directly from the nutrition label on the product. Where the brand was unknown to the respondent, the food code for the most commonly cited brand of that supplement type after all dietary records had been entered was used as the default.
Quantitative demographic, ecological, socio-economic, health behavioural and attitudinal data were simultaneously collected by a questionnaire at this time. Body weight was also measured to the nearest 0·2 kg using a Seca Compact Digital Floor Scale III, model 888 (Seca Limited), while height was measured to the nearest 0·5 cm using a collapsible ‘Leicester Height Measure’ stadiometer (CMS Weighing Equipment). Waist circumference was measured on the left hand side around the umbilicus, at the mid-point between the lower rib margin and the supra-iliac crest on the mid-axillary line. These measurements were taken to the nearest 0·5 cm with a Seca Circumference Measuring Tape model 200 (Seca Limited), held snugly against the skin as described in the North/South Ireland Food Consumption Survey (NSIFCS)(Reference McCarthy, Harrington and Kiely42). Overall, anthropometric indices (weight, height and waist circumference) were measured for 292 of the 295 respondents.
Data management
For each respondent, the total weekly intake for each food in the diet history (i.e. the g per portion multiplied by the number of servings per week) and each dietary supplement was entered into a MS Excel® spreadsheet. Using the formula function, the weekly intake data were then divided by 7 to yield an average daily intake (i.e. amount per d) for all of the foods and supplements recorded in the respondent's diet history. These average daily intakes for each food and supplement were subsequently entered into WISP version 3.0, which generated food group and nutrient intake estimates based on McCance and Widdowson's The Composition of Foods 6th Edition(43) and Supplements.
WISP version 3.0 generated seventeen default food groups, from which ten broad food groups were initially created. In order to assess differences in the intakes of foods within these groups, six of the ten food groups (milk and dairy foods, starchy carbohydrates, meat and meat products, beverages, potatoes and fish) were disaggregated manually. This was done by examining all of the 295 diet records from WISP, highlighting the foods in each of these six broad food groups, and then separating the foods in each group into their constituent subgroups. This created twenty-three new subgroups from the six original larger food groups, including categories such as low-fat milk and full-fat milk; white, brown and wholemeal breads; and sugar-sweetened and non-sugar-sweetened beverages, the latter of which included all teas, coffees, squashes and waters. These disaggregated food group data were added to the original food and nutrient intake data from WISP, and these were then merged with the demographic, ecological, socio-economic, health behavioural and attitudinal data, creating a final relational database that covered all of these parameters for all the 295 respondents. The full database was exported to a statistical software package (SPSS version 16.0; SPSS, Inc., 2007) for subsequent analyses.
To address the issue of dietary misreporting, the 292 respondents for whom body weight data were available were stratified into one of four relative physical activity categories, based on habitual vigorous activity levels and estimated daily sitting times. In order to do this, the respondents were asked questions adapted from the short version of the International Physical Activity Questionnaire (IPAQ)(Reference Booth44) about how long they were seated for on a typical weekday and a typical weekend day, and about their duration of vigorous physical activity on a typical weekday and a typical weekend day. Weighted average daily durations spent at each of these activity levels were calculated from these data, permitting the respondents to be classified into one of three tertiles for sitting time (low, moderate or high) and as ‘exercisers’ or ‘non-exercisers’ based on their participation or non-participation in vigorous activity. Those in the highest tertile for sitting time (i.e. the least active) were attributed a ‘sedentarism’ score of 1, with those in the moderate category receiving a score of 2 and those in the lowest category (i.e. the most active) receiving a score of 3. Non-exercisers who did not participate in vigorous activity were similarly given a score of 1, while the ‘exercisers’ were allocated a score of 2. The respondents then had their sedentarism (sitting) scores and their vigorous activity scores multiplied together, generating overall relative physical activity scores from 1 (least active) to 6 (most active). These overall scores were finally used to classify the 292 respondents into four relative activity categories: low (n 64); low to moderate (n 96); moderate to high (n 66) and high (n 66).
Based on published estimates of typical physical activity levels among women(45–Reference Black, Coward and Cole47) and considering the demonstrably low overall levels of vigorous activity in our study population (two-thirds of participants performed no vigorous activity at all), this cohort was deemed to have habitual levels that lay at the lower reaches of the documented physical activity spectrum for young women. Accordingly, the four relative physical activity categories were ‘mapped’ to a series of physical activity level estimates (1·40, 1·48, 1·56 and 1·64) according to previously described protocols(Reference Black48). Lower physical activity level ‘cut-off’ thresholds for the respondents in each physical activity level category were then calculated(Reference Black48). Those whose energy intake (EI) divided by their calculated BMR (EI/BMR) fell below the calculated cut-off threshold for their category were classified as dietary ‘under-reporters’ (n 53), while in all categories, those with an EI/BMR greater than 2·5 were classified as dietary ‘over-reporters’ (n 23). These under-reporters and over-reporters were excluded from further dietary and nutrient analyses.
Statistical analysis
Compliance with dietary fibre, macronutrient, vitamin and mineral intake guidelines was compared between the disadvantaged and non-disadvantaged groups, using cross-tabulation with χ2 analyses and reporting Yates' continuity correction for all 2 × 2 analyses. For the macronutrients, this first necessitated that their percentage contribution to food EI (i.e. to EI after the contribution from alcohol had been excluded) be derived. Notwithstanding the fact that these are population guidelines, the respondents were categorised as ‘compliers’ if their intake exceeded the population guideline (in the case of carbohydrate and protein) or fell below the population guideline (in the case of total fat, saturated fat and non-milk extrinsic sugars (NMES)). ‘Non-compliers’ for the various macronutrients were those whose percentage of food energy fell below the population guideline in the case of carbohydrate and protein and those whose percentage of food energy fell above the population guideline in the case of total fat, saturated fat and NMES. In addition to macronutrient compliance, dichotomous categorical variables were also created to compare compliance with dietary fibre, NSP, alcohol, vitamin and mineral intake guidelines between the groups, again by means of cross-tabulation with χ2 analyses. Compliance thresholds for dietary fibre and NSP were defined according to the WHO/Food and Agriculture Organization(49) and UK Department of Health(50) guidelines, respectively. Compliance thresholds for macronutrient intakes were defined according to the UK percentage food energy guidelines(50) and Irish alcohol unit intake guidelines(51). For each vitamin and mineral, the compliance threshold was defined as the Irish estimated average requirement (EAR)(52), with the exception of Na, where in the absence of an EAR, the compliance threshold was defined at the population maximum recommended intake(53).
All of the food group intakes were non-normally distributed, and intake comparisons between the disadvantaged and non-disadvantaged populations were made by non-parametric Mann–Whitney U tests. The percentage of consumers for each food group was also compared between the disadvantaged and non-disadvantaged populations. This was done by first classifying each respondent as a consumer (intake >0 g/d) or a non-consumer (intake = 0 g/d) for each of the different food groups of interest. These dichotomous categorical variables were then individually cross-tabulated against disadvantaged/non-disadvantaged status, with χ2 analyses and Yates' continuity correction being applied in each case to determine the statistical significance of their associations.
Differences in energy, dietary fibre and macronutrient intakes between the disadvantaged and non-disadvantaged populations were analysed using parametric independent samples t tests for those whose intakes were normally distributed (energy, carbohydrate, total fat, saturated fat, monounsaturated fat, polyunsaturated fat and protein) and by non-parametric Mann–Whitney U tests for those whose intakes were non-normally distributed (dietary fibre, NSP, NMES, cholesterol and alcohol). Macronutrient intakes were compared between the disadvantaged and non-disadvantaged populations with the contribution of alcohol excluded (i.e. comparison of percentages of energy from food only). This was done to ensure that high alcohol intakes did not artifactually reduce the calculated percentage of energy derived from fat, saturated fat or NMES, leading to erroneous conclusions about the overall quality of such diets.
Differences in vitamin and mineral intakes and dietary micronutrient density between the disadvantaged and non-disadvantaged groups were similarly assessed by parametric independent samples t tests for normally distributed parameters (Na, Zn and P) and by non-parametric Mann–Whitney U tests for non-normally distributed parameters (vitamins A, B1, B2, B5, B6, B12, C, D and E, niacin, folate, carotene, K, Fe, Ca, Mg and Cu).
Vitamin and mineral intake and compliance analyses were performed with dietary supplements included to compare total intakes and adequacy of these nutrients according to SES. Vitamin and mineral density analyses, however, were performed with supplements excluded to assess whether there were differences in the dietary concentration of these micronutrients between the two groups.
The two-sided significance of all results was assessed at the P< 0·05 level.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Dublin Institute of Technology Ethics Committee, 2005. Written informed consent was obtained from all subjects.
Results
Sample population
The disadvantaged population differed significantly from the non-disadvantaged population in terms of both the social (occupational social class, education and single-adult family structure) and material (income, deprivation and consistent poverty) indices of disadvantage used by the Irish Central Statistics Office(37) (Table 1). The disadvantaged women in the present study also had rates of relative income poverty or ‘at risk of poverty’ (51·1 %), relative deprivation (40·5 %) and consistent poverty (25 %) that were substantially greater than those observed in the national population (16·5, 24·4 and 5·1 %, respectively). The disadvantaged respondents' poverty rates were also considerably higher than those of identifiably vulnerable population groups in Irish national surveys, such as those in lone-parent households (37·6 % relative income poverty rate) and the unemployed (17·5 % consistent poverty rate)(54). While all the respondents were aged 18–35 years, the ‘disadvantaged’ sample was younger than the reference ‘non-disadvantaged’ peer group (25·1 (sd 5·7) v. 26·9 (sd 3·9) years, P= 0·011). Overall, 90·7 % of the overall population was Caucasian Irish, with 3·6 % from other EU member states, 3·4 % of Black African ethnicity, 1·7 % classified as travellers and 0·6 % from Asia. This compared to the most recent National Census data at the time (2006), which classified 87·4 % of the population as Irish, 0·5 % as Irish Travellers, 6·9 % as ‘Any other White background’, 1·0 % as African, 0·1 % as ‘Any other Black background’, 0·4 % as Chinese, 0·9 % as ‘Any other Asian background’ and 1·1 % as ‘Other including Mixed background’(55).
Table 1 Socio-economic characteristics of the full study population (n 295)

ED, electoral district.
* Equivalised income calculated on 1·0 (first adult), 0·5 (second and subsequent adults) and 0·3 (children under 14 years) scales used by the Central Statistics Office, Ireland(37).
† The eight ‘basic necessities’ selected by the Central Statistics Office(37) to describe relative deprivation in Ireland are not having new, but second-hand clothes, a meal with meat, chicken or fish every second day, a warm, water-proof coat, two pairs of strong shoes, and a roast or its equivalent once per week or having debt problems arising from normal living expenses (or availing of charity), a day in the last 2 weeks without a substantial meal and needing to go without eating during the last year through lack of money.
Food group intakes
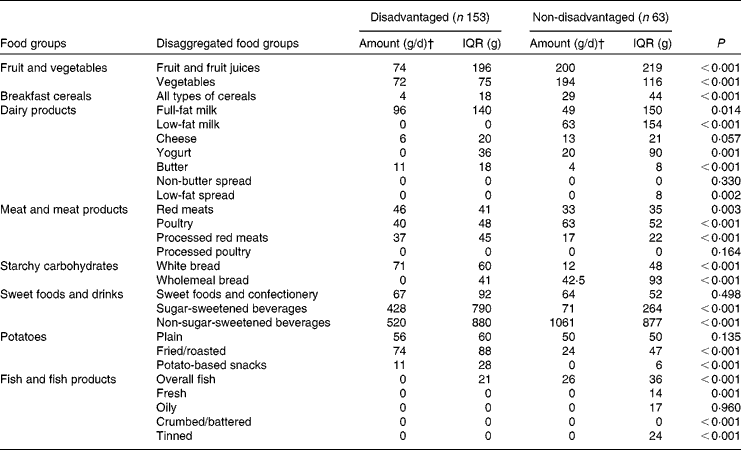
Median intakes of fruit and fruit juices, vegetables, breakfast cereals, low-fat milk, yogurt, low-fat spread, poultry, wholemeal bread, non-sugar-sweetened beverages, fresh fish and tinned fish were all significantly lower among the disadvantaged women than among their more affluent peers. Conversely, median intakes of full-fat milk, butter, red meats, processed red meats, white bread, sugar-sweetened beverages, fried and roasted potatoes and potato-based snacks were all significantly higher among the disadvantaged women than among the non-disadvantaged women (Table 2).
Table 2 Differences in food group consumption between the disadvantaged and non-disadvantaged respondents* (n 216)

IQR, interquartile range.
* All food group intakes were non-normally distributed, with intake comparisons between the disadvantaged and non-disadvantaged populations being performed using non-parametric Mann–Whitney U tests.
† Median values used for comparison.
For fruit, vegetables, breakfast cereals, low-fat milk, butter, processed red meats, wholemeal bread, sugar-sweetened beverages, fried and roasted potatoes, potato-based snack foods and fish, two- to threefold disparities in intake (and considerably more in some cases) were observed, indicating profound dietary differences between the two groups (Table 2).
Most of these discrepancies in food group intake were mediated by differences in both ‘intake level’ (i.e. portion size and frequency of consumption) and ‘prevalence of consumption’ (i.e. the percentage of consumers within the respective populations). For example, there were significantly fewer consumers of fruit juices (69 v. 94 %), breakfast cereals (58 v. 86 %), low-fat milk (24 v. 64 %), oily fish (4 v. 36 %) and tinned fish (15 v. 47 %) (all P< 0·001), yogurt (36 v. 61 %, P= 0·001), low-fat spread (20 v. 39 %, P= 0·007), poultry (87 v. 98 %, P= 0·018), wholemeal bread (49 v. 73 %, P= 0·002), non-sugar-sweetened beverages (82 v. 95 %, P= 0·020) and fresh fish (12 v. 31 %, P= 0·001) in the disadvantaged group than in the non-disadvantaged group. A higher percentage of disadvantaged than non-disadvantaged women consumed full-fat milk (80 v. 58 %, P= 0·001), butter (80 v. 59 %, P= 0·002), white bread (88 v. 64 %, P< 0·001), sugar-sweetened beverages (82 v. 61 %, P= 0·002), fried and roasted potatoes (88 v. 73 %, P= 0·020) and potato-based snacks (71 v. 38 %, P< 0·001).
However, when non-consumers were removed, considerable differences in intake level between the disadvantaged and non-disadvantaged consumers persisted for several food groups. Disadvantaged consumers had higher daily intakes of butter (14 v. 7 g, P= 0·002), red meats (47 v. 39 g, P= 0·002), processed red meats (40 v. 20 g, P< 0·001), white bread (78 v. 31 g, P< 0·001), sugar-sweetened beverages (601 v. 200 g, P< 0·001), fried and roasted potatoes (89 v. 30 g, P< 0·001) and potato-based snacks (17 v. 9 g, P< 0·001); and lower daily intakes of fruit and fruit juices (145 v. 214 g, P= 0·006), breakfast cereals (17 v. 30 g, P< 0·001), poultry (43 v. 66 g, P= 0·004), wholemeal bread (41 v. 71 g, P= 0·012) and non-sugar-sweetened beverages (671 v. 1074 g, P= 0·001) than the non-disadvantaged women who consumed these food groups.
Nutrient compliance
Energy, fibre and macronutrients
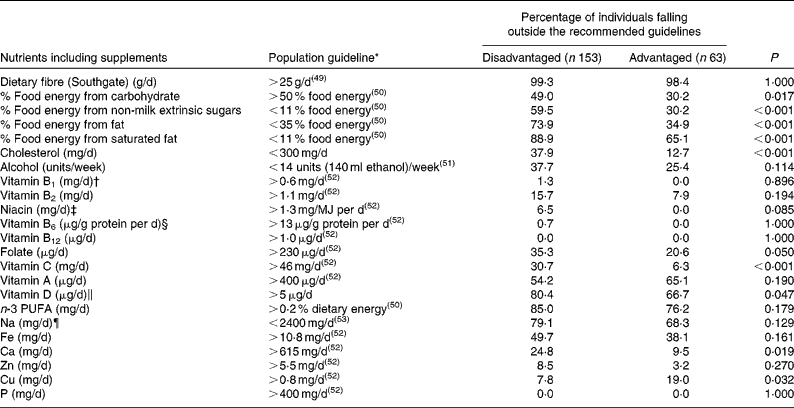
Non-compliance with macronutrient intake guidelines occurred more often among the disadvantaged women, who were significantly more likely than their non-disadvantaged peers to fall short of the recommended carbohydrate intake and to exceed the recommended intake guidelines for fat, saturated fat and NMES. Additionally, three times more disadvantaged than non-disadvantaged respondents exceeded the recommended 300 mg of dietary cholesterol per d, while a considerable majority of both groups failed to meet dietary fibre and n-3 fatty acid guidelines (Table 3).
Table 3 Differences in achievement of the recommended dietary fibre, macronutrient, cholesterol, alcohol, vitamin and mineral intakes between the disadvantaged and non-disadvantaged respondents (n 216)

* Population intake guidelines defined in terms of the WHO/Food and Agriculture Organization guidelines on dietary fibre(49), UK Department of Health guidelines on macronutrient intake(50), Irish alcohol intake guidelines(51) and current Irish estimated average requirements (EAR)(52).
† EAR for vitamin B1 set at 72 μg/MJ per d and assumed at 0·6 mg/d for a daily energy intake of approximately 8·4 MJ.
‡ EAR for niacin set at 1·3 mg/MJ per d and assumed at 11 mg/d for a daily energy intake of approximately 8·4 MJ.
§ EAR for vitamin B6 set at 13 μg/g protein per d and assumed at 1·1 mg/d for a daily protein intake of approximately 85 g.
∥ EAR for vitamin D assumed at 5 μg/d (i.e. half of the maximum of the current RDA).
¶ Target maximum recommended intake set at 2400 mg/d by the Food Safety Authority of Ireland(53).
Micronutrients
While differences in vitamin and mineral compliance were less pronounced between the two groups, the disadvantaged women were significantly more likely to fall short of the EAR for folate, vitamin C, vitamin D and Ca (Table 3). In the case of folate, while 35·3 % of the disadvantaged women (and 20·6 % of the non-disadvantaged women) failed to achieve the EAR of 230 μg/d(52), just 0·5 % of the disadvantaged women and only 5·4 % of the non-disadvantaged women took a 400 μg/d supplement of folic acid as recommended for all women of child-bearing potential(52). A significant percentage of both populations also failed to achieve the requisite intake of vitamin C, vitamin A, Fe, Ca and especially vitamin D, while a majority of both groups exceeded the recommended intake of Na.
Nutrient intakes
Energy, dietary fibre and macronutrients
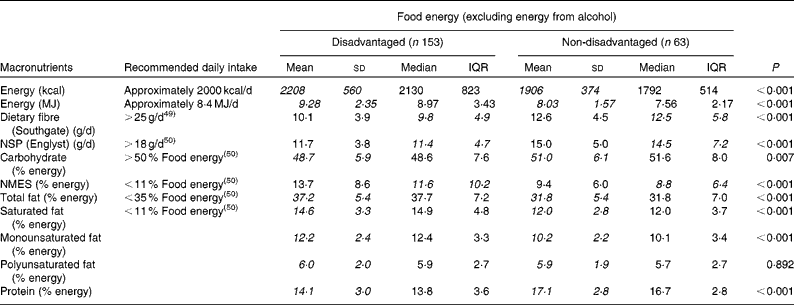
Mean and median energy consumption and dietary fibre and macronutrient intakes differed considerably between the two groups, when the contribution from alcohol was excluded (Table 4). Mean daily EI was 1·25 MJ greater in the disadvantaged group than in the non-disadvantaged group (1·46 MJ with alcohol included). Dietary fibre, NSP, carbohydrate and protein intakes were lower, and fat, saturated fat, NMES and cholesterol intakes were higher, among the disadvantaged respondents in comparison with their more affluent peers.
Table 4 Differences in energy, dietary fibre and macronutrient intakes (excluding alcohol) between the disadvantaged and non-disadvantaged respondents* (Mean values and standard deviations; medians and interquartile ranges (IQR), n 216)

NMES, non-milk extrinsic sugars.
* Energy, carbohydrate, total fat, saturated fat, monounsaturated fat, polyunsaturated fat and protein intakes are normally distributed and socio-economic differences in mean intakes (italicised values) between the disadvantaged and non-disadvantaged groups are assessed by parametric methods (independent samples t tests). Dietary fibre, NSP and NMES intakes are non-normally distributed and socio-economic differences in median intakes (italicised values) between the disadvantaged and advantaged groups are assessed by non-parametric methods (Mann–Whitney U tests).
Micronutrients
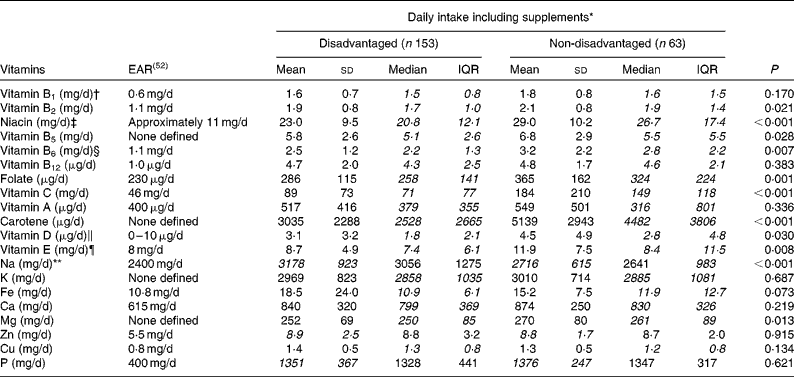
Mean and median vitamin and mineral intakes differed substantially between the disadvantaged and non-disadvantaged women, with the contribution from supplements both included and excluded. With supplements included, median intakes of vitamin B2, niacin, vitamin B5, vitamin B6, folate, vitamin C, carotene, vitamin D and vitamin E were all lower among the disadvantaged women in comparison with the non-disadvantaged women (Table 5). Regarding mineral consumption, median intakes of Mg were lower, while mean Na intakes were higher among the disadvantaged women.
Table 5 Differences in vitamin and mineral intakes (including supplements) between the disadvantaged and non-disadvantaged respondents (Mean values and standard deviations; medians and interquartile ranges (IQR), n 216)

EAR, estimated average requirement.
* Where mean intakes for the disadvantaged and non-disadvantaged groups are in italics (Na, Zn and P), intakes of that nutrient are normally distributed and comparison between the two groups is by parametric independent samples t tests. Where median intakes for the disadvantaged and non-disadvantaged groups are in italics (vitamins A, B1, B2, B5, B6, B12, C, D and E, niacin, folate, carotene, K, Fe, Ca, Mg and Cu), intakes of that nutrient are non-normally distributed and comparison is by means of non-parametric Mann–Whitney U tests.
† EAR for vitamin B1 set at 72 μg/MJ per d and assumed at 0·6 mg/d for a daily energy intake of approximately 8·4 MJ.
‡ EAR for niacin set at 1·3 mg/MJ per d and assumed at 11 mg/d for a daily energy intake of approximately 8·4 MJ.
§ EAR for vitamin B6 set at 13 μg/g protein per d and assumed at 1·1 mg/d for a daily protein intake of approximately 85 g.
∥ EAR for vitamin D assumed at 5 μg/d (i.e. half of the maximum of the current RDA).
¶ RDA for vitamin E previously set at 8 mg/d for women aged 18–64 years (Irish RDA, 1983); no current Irish EAR.
** Target maximum recommended intake set at 2400 mg/d by the Food Safety Authority of Ireland(53).
The use of dietary supplements was more prevalent among the non-disadvantaged women than among the disadvantaged women (48·4 v. 30·5 %, P= 0·011), with multivitamins and multiminerals, cod-liver oil, vitamin C, fish oils and Fe the most commonly used preparations. Even with the contribution from these supplements excluded, however, several micronutrient differences persisted between the two groups. For example, mean niacin intakes (20·3 v. 23·9 mg/d, P= 0·001) and median vitamin C (59 v. 112 mg/d, P< 0·001) and carotene (2528 v. 4482 μg/d, P< 0·001) intakes remained significantly lower among the disadvantaged women, while there was also a tendency towards lower folate intakes (252 v. 273 μg/d, P= 0·060) in this group. Notwithstanding the fact that both group means fell below the 400 μg/d EAR, vitamin A intakes excluding the contribution from supplements were significantly higher in the disadvantaged group. For mineral intakes, mean Fe (10·2 v. 11·4 mg/d, P= 0·011) and median Mg (250 v. 259 mg/d, P= 0·035) intakes were significantly lower among the disadvantaged women than among their non-disadvantaged peers, while mean Na intakes (3178 v. 2716 mg/d, P< 0·001) remained significantly higher among the poorer women.
Nutrient density
Micronutrients
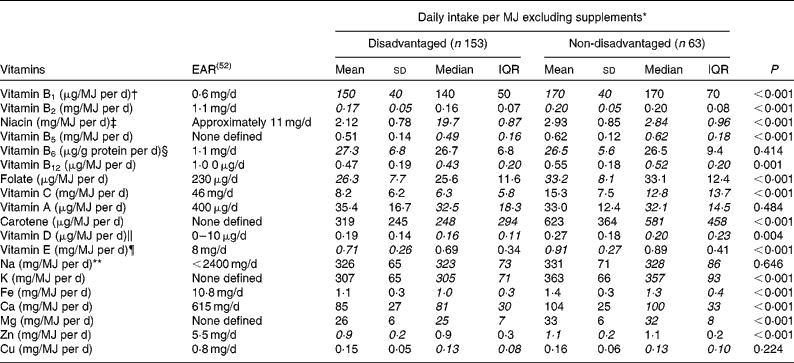
Further analyses examined the vitamin and mineral densities of the diet per MJ of energy consumed to elucidate differences in the micronutrient quality of the diet between the disadvantaged and non-disadvantaged cohorts. Here, with the contribution of supplements excluded, nutrient densities for vitamin B1, vitamin B2, niacin, vitamin B5, vitamin B12, folate, vitamin C, carotene, vitamin D and vitamin E were all significantly lower among the disadvantaged women (Table 6). For the minerals investigated, dietary K, Fe, Ca, Mg and Zn densities per MJ of energy consumed were all significantly lower among the disadvantaged women than among the non-disadvantaged women (Table 6).
Table 6 Differences in vitamin and mineral densities per MJ of energy consumed (excluding supplements) between the disadvantaged and non-disadvantaged respondents (Mean values and standard deviations; medians and interquartile ranges (IQR), n 216)

EAR, estimated average requirement.
* Where the mean intakes per MJ for the disadvantaged and non-disadvantaged groups are in italics (vitamins B1, B2, B6, E, folate and Zn), intakes of that nutrient per MJ are normally distributed and comparison between the two groups is by means of parametric independent samples t tests. Where the median intakes per MJ for the disadvantaged and non-disadvantaged groups are in italics (vitamins A, B5, B12, C and D, carotene, Na, K, Fe, Ca, Mg and Cu), intakes of that nutrient per MJ are non-normally distributed and comparison between the two groups is by means of non-parametric Mann–Whitney U tests.
† EAR for vitamin B1 set at 72 μg/MJ per d and assumed at 0·6 mg/d for a daily energy intake of approximately 8·4 MJ.
‡ EAR for niacin set at 1·3 mg/MJ per d and assumed at 11 mg/d for a daily energy intake of approximately 8·4 MJ.
§ EAR for vitamin B6 set at 13 μg/g protein per d and assumed at 1·1 mg/d for a daily protein intake of approximately 85 g.
∥ EAR for vitamin D assumed at 5 μg/d (i.e. half of the maximum of the current RDA).
¶ RDA for vitamin E previously set at 8 mg/d for women aged 18–64 years (Irish RDA, 1983); no current Irish EAR.
** Target maximum recommended intake set at 2400 mg/d by the Food Safety Authority of Ireland(53).
Discussion
Methodology
The present study aimed to establish how the food group and nutrient intake patterns of young, low-SES Irish women differed from those of their more affluent peers. The sampling frame for the recruitment of our disadvantaged women was developed de novo using small-area population statistics data from the most recent national census, reflecting similar approaches used previously for socio-economic health research in Ireland(Reference Kelly56). The post hoc profile of our disadvantaged women confirms their low SES and supports the use of such multi-component sampling tools. Notwithstanding this fact, however, the data presented do raise the prospect that the dietary and nutritional deficits identified here may be still more pronounced in areas (and among groups) where these markers of material disadvantage are even more preponderant.
While the exclusive recruitment of our study population from the Greater Dublin area is a limitation, there is no reason to believe that the profound nutritional deficits identified among these young women are not characteristic of other young, urban women of similar SES across Ireland. Although non-probability, purposive sampling was selected for our study design, efforts were made to ensure that the respondents were recruited from a geographically disperse number of areas in North, South, West and City Centre Dublin, with a roughly equal number of low-SES recruitment sites being selected in each region. However, it must be recognised that in the absence of a comprehensive, multi-tiered and explicit sampling frame and the application of a robust probabilistic algorithm to select participants within this frame, our purposive sampling method lacks the rigour of a full probability-based sampling protocol. The propensity for sampling bias is increased where ‘judgement’ or ‘assumption’ rather than randomisation distribution is used for participant selection in this way. While use of an area-level sampling frame to identify ED of low SES, along with the equal selection of low-SES ED from the four geographic regions of Greater Dublin, was undertaken in an effort to mitigate such bias and enhance the representativeness of our low-SES sample, the inherent limitations of this sampling protocol must be acknowledged.
The small sample size of our study population also needs to be addressed. By convention, a power of 80 % and a significance level of 5 % were selected to limit the chance of type 1 error (i.e. false positive findings) to less than 5 %. The minimum number of disadvantaged and non-disadvantaged respondents required for the comparison of mean macronutrient (fat, saturated fat, carbohydrate and protein) intakes between these two independent samples was calculated using standard errors from a similarly sized population of young women in the NSIFCS (n 269), according to previously described protocols(Reference Daly and Bourke57). This yielded a minimum sample size of sixty-three in each group. However, this does not automatically infer that this sample size will suffice for comparative analyses of other nutrient intakes between the low-SES women and their reference group, and this limitation needs to be acknowledged.
Because dietary misreporting constitutes a significant problem in nutritional surveys(Reference Livingstone and Black58), unreliable dietary records were identified and removed from our food group and nutrient analyses(Reference Samaras, Kelly and Campbell59). This process, however, only excludes unreliable dietary records on the basis of implausible EI v. an individual's calculated energy requirements. It does not eradicate other sources of potential error such as inaccuracy and imprecision of dietary reporting(Reference Nelson, Beresford, Kearney, Gibney, Margetts, Kearney and Arab60) and possible selective, preferential misreporting of certain food groups(Reference Scagliusi, Polacow and Artioli61).
It could also be argued that the diet history method facilitates the omission of consumed foods, by failing to provide ‘cues’ to assist dietary recall in the way that a FFQ might. Additionally, some studies have suggested a lower internal consistency and a greater divergence of population EI with the diet history method compared with weighed intake records(Reference Black, Welch and Bingham39), while interviewer bias and social desirability bias may be further concerns with this methodology. Nonetheless, as a single-pass, non-presumptive and readily comprehensible way of estimating habitual dietary intakes in an inaccessible population whose intakes are thought to deviate from those of the wider population, this diet history protocol was deemed a more suitable instrument than either a 24 h recall or a FFQ.
Finally, the inherent limitations of nutrient conversion from dietary records must be stated, as the UK Food Composition database upon which WISP version 3.0 is based, itself has several deficits. For example, the dietary fibre content of most foods as measured by the Southgate(49) and Englyst(50) methods is available on the database. However, the Association of Organic and Analytic Chemists method(Reference Prosky, Asp and Schweizer62), which measures not just NSP, but also resistant starches, lignins and fructans and is arguably an analytically superior estimate of dietary fibre in food, remains unavailable for many foods in the UK food composition database. Similarly, the food composition databases upon which WISP version 3.0 is based are significantly incomplete for Se, iodine and trans-fatty acids, meaning that output results for these nutrients could not be reliably reported.
Dietary intakes
There is significant epidemiological evidence from Ireland(Reference Balanda and Wilde3, Reference Layte, Nolan and Nolan63) and elsewhere(Reference Marmot, Allen and Goldblatt64–Reference Singh and Siahpush66) that those in the lower socio-economic strata experience poorer health outcomes and have significantly greater premature mortality than their more affluent peers. There is also a wealth of data describing the consumption of lower-quality diets among low-SES groups(Reference Friel, Kelleher and Nolan6, Reference Darmon and Drewnowski35, Reference Mullie, Clarys and Hulens67). Several researchers have linked these two phenomena, highlighting the role of poor diet in socio-economic health inequalities(Reference James, Nelson and Ralph4, Reference Anderson68).
The present study clearly demonstrates the existence of less favourable dietary habits among a group of young, socially disadvantaged women from Dublin, in comparison with those of their more affluent peers. Additionally, while 48·4 % of the non-disadvantaged women reported taking dietary supplements, this estimate fell to 30·5 % among the disadvantaged women. This prevalence of usage among the disadvantaged women is comparable to that reported for all women aged 18–64 years in the National Adult Nutrition Survey (NANS)(Reference Walton11). However, the discordant estimates between the two groups also highlight the fact that those who have potentially most to gain from using these products are relatively less likely to use them than their better nourished, wealthier counterparts.
While the socio-economic differences in dietary intakes highlighted by the present study are profound, the reasons for these differences are more elusive. Putative barriers to healthy diet among low-SES groups include poor nutritional knowledge(Reference McLeod, Campbell and Hesketh69), inadequate food preparation skills(Reference Larson, Perry and Story70), high cost of healthy food(Reference Monsivais and Drewnowski71, Reference Aggarwal, Monsivais and Cook72), poor local food environment(Reference Layte, Harrington and Sexton7, Reference Zenk, Lachance and Schulz73, Reference Inglis, Ball and Crawford74), low perceived control and self-efficacy(Reference Barker, Lawrence and Crozier32, Reference Lawrence, Skinner and Haslam75, Reference Lawrence and Barker76) and poorer health-related and dietary attitudes(Reference Lawrence, Skinner and Haslam75–Reference Hearty, McCarthy and Kearney77).
Nutritional consequences
Whatever the origin of these dietary inequalities, the present study highlights the adverse impact that poor dietary habits have on the nutritional intake of low-SES Irish women. The lower dietary fibre and carbohydrate intakes and the higher fat, saturated fat, NMES and Na intakes observed among the low-SES women are wholly consistent with low-cost diets, which are low in fruit, vegetables, wholemeal bread, breakfast cereals and fish and high in processed meats, butter, full-fat (rather than low-fat) milk, sugar-sweetened beverages, fried potatoes and potato-based snacks(Reference James, Nelson and Ralph4, Reference Andrieu, Darmon and Drewnowski78–Reference van Lee, Geelen and van Huysduynen81). Similarly, the lower absolute and energy-adjusted intakes of vitamin C, folate, carotene, vitamin D, vitamin E, K, Fe, Ca and Mg observed among the low-SES women, along with the high prevalence of micronutrient intake inadequacy in this group, are reflective of lower fruit, vegetable, breakfast cereal, unprocessed meat, fish and overall milk intakes, foods that constitute the richest dietary sources of these nutrients.
Health implications
The health consequences of these aberrant food group and nutrient intake patterns are well established. Diets high in red and processed meats, fat and saturated fat have been associated with increased risk of overweight and obesity(Reference Bray, Paeratakul and Popkin82), elevated LDL-cholesterol levels(Reference Jakobsen, O'Reilly and Heitmann83), increased risk of colorectal cancer(84) and greater mortality(Reference Pan, Sun and Bernstein85). Similarly, diets high in refined, extrinsic sugars have been associated with overweight and obesity(Reference Fitch and Keim86, Reference Livesey, Taylor and Hulshof87) and the metabolic syndrome(Reference Livesey, Taylor and Hulshof87), with high intakes of sugar-sweetened beverages, and in particular fructose-containing drinks, being strongly linked to multiple metabolic risk factors(Reference Stanhope, Schwarz and Keim88, Reference Hu and Malik89). In the present study, sugary drinks contributed 6 % of the total energy among the disadvantaged respondents v. 2 % among the non-disadvantaged women. High alcohol intakes have also been associated with increased cardiometabolic risk(Reference Foerster, Marques-Vidal and Gmel90), increased likelihood of colorectal cancer(Reference Giovannucci91), lower bone mineral density and increased fracture risk(Reference Berg, Kunins and Jackson92) and higher overall mortality among socially disadvantaged women(Reference Herttua, Makela and Martikainen93).
The micronutrient deficits observed among the disadvantaged women also have significant health consequences. For example, low folate intake and status have been associated with increased serum homocysteine levels and cardiovascular risk(Reference Wald, Wald and Morris94), as well as elevated cancer risk(Reference Giovannucci91, Reference Figueiredo, Levine and Grau95). Low intakes of several antioxidants including vitamin C and vitamin E have been inconsistently associated with increased cardiovascular(Reference Hamilton96) and cancer risks(84), while high Na, low Ca and low K intakes have been implicated in hypertension(Reference McCarron and Reusser97, Reference He and MacGregor98) and in poorer skeletal health(Reference Teucher, Dainty and Spinks99). Several micronutrient deficits observed among the low-SES women including low Fe(Reference Lee, Kim and Kim100), Ca(Reference Bergel and Barros101) and folate(102, Reference Sinclair, Allegrucci and Singh103) intakes may also exert deleterious effects on the health of their offspring.
Interventions
The depth and breadth of the nutritional deficits elicited by their poor dietary patterns commend these low-SES women as a primary target for diet-related public health interventions. Fortunately, the candidate food groups for such interventions have been largely established. For example, the significant vitamin and mineral intakes achievable from fruit and vegetables(Reference Vincent104, Reference Darmon, Darmon and Maillot105), breakfast cereals(Reference Galvin, Kiely and Flynn106, Reference Deshmukh-Taskar, Radcliffe and Liu107), wholegrain cereals(Reference Maillot, Darmon and Darmon108), milk and dairy products(Reference Vincent104, Reference Nicklas, O'Neil and Fulgoni109) and fish(Reference Maillot, Darmon and Darmon108, Reference Gebauer, Psota and Harris110, Reference Bonham, Duffy and Robson111) are well known. However, apart from their own valuable micronutrient contributions, there is also considerable evidence that increasing the intake of these foods would displace the intake of other more energy-dense, nutrient-deplete foods from the diet. For instance, a higher intake of breakfast cereals has been consistently associated with lower overall fat intakes(Reference Galvin, Kiely and Flynn106, Reference Deshmukh-Taskar, Radcliffe and Liu107). Conversely, high sugar-sweetened beverage intake has been associated with reduced milk intake(Reference Cavadini, Siega-Riz and Popkin112) and higher processed meat consumption with lower fish and poultry intakes(Reference Guenther, Jensen and Batres-Marquez30, Reference Cosgrove, Flynn and Kiely31). In the case of high-fat, high-sugar foods, there is clear evidence that their displacement effect on micronutrient-dense foods exerts a deleterious impact on overall nutrient intake and adequacy(Reference Bhargava and Amialchuk36). The interplay between these competing low-energy, micronutrient-rich foods and their high-energy, nutrient-dilute alternatives is, therefore, a critically important consideration in optimising food-based dietary guidelines for young women of low SES.
In the present study, a lower percentage of the disadvantaged women consumed fruit and fruit juices, breakfast cereals, fish, wholemeal bread, low-fat milk and low-fat spread, and a higher percentage of these disadvantaged women consumed sugar-sweetened drinks and potato-based snacks. Low-SES women should, therefore, be advised and facilitated to introduce these foods de novo into their diets. Indeed, there is a synergistic ‘displacement’ benefit to be gained by explicitly recommending that fruit replace potato-based snacks, that low-fat milk or fruit juices replace sugar-sweetened beverages, that wholemeal bread replace white bread, that fish replace processed red meats, that breakfast cereals replace other breakfast foods such as processed meats and that low-fat spread replace butter.
The fact that fruit and fruit juice, vegetable, breakfast cereal, poultry and wholemeal bread intakes remain lower and that red meat, processed red meat, white bread, sugar-sweetened beverage, fried potato and potato-based snack intakes remain higher in this low-SES group when non-consumers are excluded indicates that frequency of consumption is also a crucial component of these dietary inequalities. Therefore, additional guidance should be given to low-SES women who already consume these healthy foods to increase their frequency of consumption, with the displacement of less healthy alternatives again being a key objective.
Conclusion
The present study highlights the presence of endemic food group and nutrient intake deficits among young women of low SES in Ireland. Although a coincident biomarker analysis to assess the nutritional status of these women would have been illuminating, their food and nutrient intakes alone suggest that many may experience deficiency of one or more nutrients. While such nutritional inadequacies portend obvious deleterious effects for these women themselves, their public health impact is compounded by the critical importance of nutrients such as Fe, folate, vitamin A, vitamin D and Ca to the optimal growth of their offspring in utero (Reference Rao, Padmavathi and Raghunath113, Reference Barker, Lampl and Roseboom114). Our findings constitute an evidence base for diet-related interventions in such groups and have enabled us to suggest several explicit food-based dietary guidelines. However, psychosocial, sociocultural and ecological impediments to the adoption of such guidelines abound among low-SES women and remain critical barriers to be overcome in the success of any such interventions.
Acknowledgements
The present study was supported by the Food Safety Promotion Board (SafeFood) (grant no. 03CR/06). We gratefully acknowledge Dr Clare Corish for assistance in the preparation of the final manuscript. Finally, we acknowledge with thanks the generosity of the community development workers and young women who gave of their time to participate in our study, and without whom this work would not have been possible. The contributions of the authors are as follows: D. M. A. M., researcher, was responsible for the fieldwork and manuscript preparation; K. M. Y. was the project supervisor and manuscript editor; J. W. and M. O. were the fieldworkers; C. S. was responsible for the disaggregation of food groups and data analysis; J. M. K. was the principal investigator and grant recipient. None of the authors has any conflict of interests.