Q fever is a global zoonotic disease caused by the bacterium Coxiella burnetii. Human infections are mostly related to infected ruminants, e.g. sheep, goats and cows. In particular birth products of C. burnetii-infected animals are known to be highly contagious [Reference Aitken1, Reference Maurin and Raoult2], and airborne transmission is most common [Reference Hawker3]. C. burnetii is extremely infectious and weather resistant and may survive for several weeks in the environment [Reference Aitken1]. The incubation period is normally 2–3 weeks [Reference Maurin and Raoult2]. In humans, Q fever may cause influenza-like illness which can lead to severe respiratory symptoms; asymptomatic infections also occur. Both symptomatic and asymptomatic infections can lead to chronic infections with complications especially in patients with heart valve diseases or in pregnant women [Reference Maurin and Raoult2, Reference Raoult, Fenollar and Stein4]. In Germany, 100–500 symptomatic human infections with C. burnetii are reported annually, the trend is increasing, mostly as cases related to outbreaks. The largest previously described outbreak occurred in 2003, associated with a farmers' market in Soest [Reference Porten5]. Infected sheep have been implicated as the source of infection in 24 out of 40 documented outbreaks reported in Germany between 1947 and 1999 [Reference Hellenbrand, Breuer and Petersen6]. Epidemiological investigation of outbreaks has important implications for identifying means of prevention.
The outbreak described in the present paper was identified in June 2005, when general practitioners in Winzerla (11 500 inhabitants), a suburb of the city Jena in East Germany, reported 20 patients suffering from respiratory infections with fever, chills and headaches to the local health authorities. Because of sporadic Q fever infections in previous years, C. burnetti was quickly suspected as the cause of the described symptoms. Serological tests of some patients confirmed infection by C. burnetii. The suburb borders a meadow where 500 ewes grazed between 2 and 18 June 2005 and about 35 lambings occurred. The veterinary service investigated the flock of sheep using serological tests. Polymerase chain reaction tests of vaginal swabs from a ewe that had lambed on the meadow were also conducted. Both tests confirmed Q fever infection in the flock. Consequently, the sheep were removed to a remote meadow.
We carried out an investigation to describe the outbreak, to identify risk factors for infection, and to detect asymptomatic infections, in order to establish new or to strengthen existing recommendations for control.
We found cases through notifications to the local public health department, by contacting clinicians and laboratories and by offering a serological test to the local population. Participants of this test were recruited through newspapers and flyers distributed in the residential area. The main aim of the test was to detect asymptomatic infections, especially among risk groups.
We defined confirmed cases as residents or visitors of Jena-Winzerla in June 2005 with serological confirmation of acute C. burnetii-infection [using either complement-binding reaction (CBR) phase-2 titre ⩾1:80 or positive phase-2 IgM antibody in an ELISA test] between June and October of 2005. Asymptomatic cases were those serologically confirmed as having acute C. burnetii infection who did not have fever, headache or muscle pain for more than 1 day between 13 June and 28 July. Probable cases were defined as residents or visitors of Jena-Winzerla in June 2005 who reported fever, headache or muscle pain for at least 2 days between 13 June and 28 July 2005 but did not undergo serological testing in 2005.
CBR were performed in various private and hospital laboratories using different commercially available test kits. Confirmation was performed in the Q-fever Consulting Laboratory in Stuttgart with ELISA tests from Virion/Serion [Serion Elisa classic, Coxiella burnetii phase-1 (IgG, IgA), Coxiella burnetii phase-2 (IgG, IgM)].
We interviewed cases using a standardized questionnaire that included demographic and symptom details. We classified the cases in eight 50-m-wide bands, starting from the meadow, according to the distance between their place of residence and the edge of the meadow, sex and age group. For the calculation of attack rates (AR) and relative risks (RR) by sex, age and distance from residence to meadow, we defined the population living <400 m from the meadow as a cohort. For this exposed population, we used data from the registry office in Jena.
The University of Applied Science in Jena provided meteorological data (temperature, rainfall, wind speed and direction).
We identified 331 cases of Q fever, of which 160 were confirmed (including five asymptomatic cases) and 171 were probable cases. Symptom onset of the symptomatic cases occurred from 13 June to 24 July 2005. The median age was 43 years (range 14–99 years). Fifty-seven per cent of cases were men (n=188). Of all cases, 248 (75%) were living within 400 m from the sheep meadow. Twenty-six cases (8%) were hospitalized, mainly due to pneumonia.
The investigation of the cohort showed an overall AR in the area within 400 m of the meadow of 4·3%. The AR was 11·8% within 50 m and decreased with growing distance to 1·3% in the area 350–400 m away, leading to a risk 8·7 times higher when comparing these two distances [95% confidence interval (CI) 4·5–17·1]. The AR was higher in men than in women (RR 1·4, 95% CI 1·1–1·8) (Table 1). The AR was 2·3 times higher in the 25–64 years age group than in the other age groups (95% CI 1·7–3·0) (Table 2).
Table 1. The relative risk (RR) by distance from residence to meadow compared with area >350–400 m, Q fever outbreak, Jena 2005
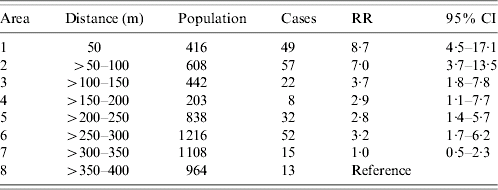
CI, Confidence interval.
Table 2. Attack rates (AR) by age group and sex (for population of Jena-Winzerla), Q fever outbreak, Jena 2005
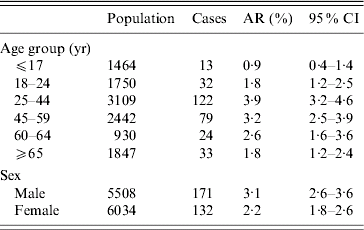
CI, Confidence interval.
Source: Population numbers for City of Jena, at 31 December 2004.
In total, 147 people chose to have the serological test offered to the inhabitants of the affected suburb in order to detect asymptomatic infections. However, only 50 of them had been free of symptoms (fever, headache or muscle pain) in the relevant period. The median age of the asymptomatic participants was 46 years, 66% were female. Sixty-six percent lived within 400 m of the meadow. An acute C. burnetii infection was detected in 10% of the 50 asymptomatic participants.
During June 2005 it was warm (mean temperature 17·3°C) and dry (rainfall 42·2 mm/month) in Jena. The wind was slight (mean speed 1·7 m/s) and came from variable directions. A heavy rainfall occurred on 30 June.
In the cohort study, the AR by distance from residence to meadow show a clear association between human Q fever infections and the proximity of residential areas to a sheep meadow. Cases occurred over a 6-week period. New cases continued to occur even 3 weeks (the maximum incubation period of Q fever [Reference Maurin and Raoult2]) after the removal of the sheep from the meadow on 18 June 2005. This is not surprising as it is known that C. burnetii survives well especially in dry weather conditions [Reference Aitken1]. No further cases were reported 3 weeks after the heavy rainfall on 30 June 2005, suggesting that the water removed most of the remaining infectious agents. The slight wind in June from several directions had no apparent influence on the transmission pattern of the disease in the affected suburb.
The higher AR in men is consistent with other studies [Reference Maurin and Raoult2, Reference Hawker3]. Possible explanations are protective oestrogen in women [Reference Leone7] or more severe courses leading to easier detection of infection in men [Reference Hawker3]. The difference might also be explained by different leisure activities undertaken by men and women; however, information about the leisure activities of all cases was not available.
This outbreak investigation provides AR by age groups as demanded in a recent study [Reference Parker, Barralet and Bell8]. The AR in the 25–64 years age group was significantly higher than the AR in other groups. This age group may be more active outdoors than others, and therefore more likely to be exposed to C. burnetii.
In other outbreaks the proportion of hospitalizations was about 2% [Reference Maurin and Raoult2, Reference Dupuis9]. The prolonged continuous exposure in this outbreak may have caused more severe illnesses which may explain the higher proportion of hospitalizations in Jena.
The serological test offered to detect asymptomatic infections was undertaken by many people who had been symptomatic but who had not seen their physician. The asymptomatic participants were older, more often female and tended to live further from the meadow than the symptomatic cases. The fact that the participants were not selected using probabilistic sampling could explain the very low proportion of asymptomatic cases in Jena compared to the 40–60% found in other studies [Reference Maurin and Raoult2, Reference Dupuis9].
The proximity between the sheep meadow and a nearby residential area has led to one of the largest documented Q fever outbreaks worldwide. The ongoing construction of suburban areas brings increasing numbers of people into closer proximity with animals not only in Germany. Q fever infections in these animals could lead to increased numbers of humans being infected by C. burnetii. The results of this investigation highlight the importance of following existing recommendations: that sheep should not be kept within 500 m of residential areas, and lambings should not occur outdoors [10]. This outbreak has led to improved communication between physicians, public health authorities, veterinarians and sheep farmers in the affected area. However, further improvement in cooperation of the involved parties, also in other regions and countries, is required to enforce the strategies to prevent future human Q fever infections.
ACKNOWLEDGEMENTS
We thank our colleagues from the local public health and veterinary offices in Jena, for their contribution in the outbreak investigation, as well as the clinicians and microbiologists who helped diagnose and treat patients. We also thank Naomi Boxall, Lisa King and Suzi Lyons for editing this paper.
DECLARATION OF INTEREST
None.




