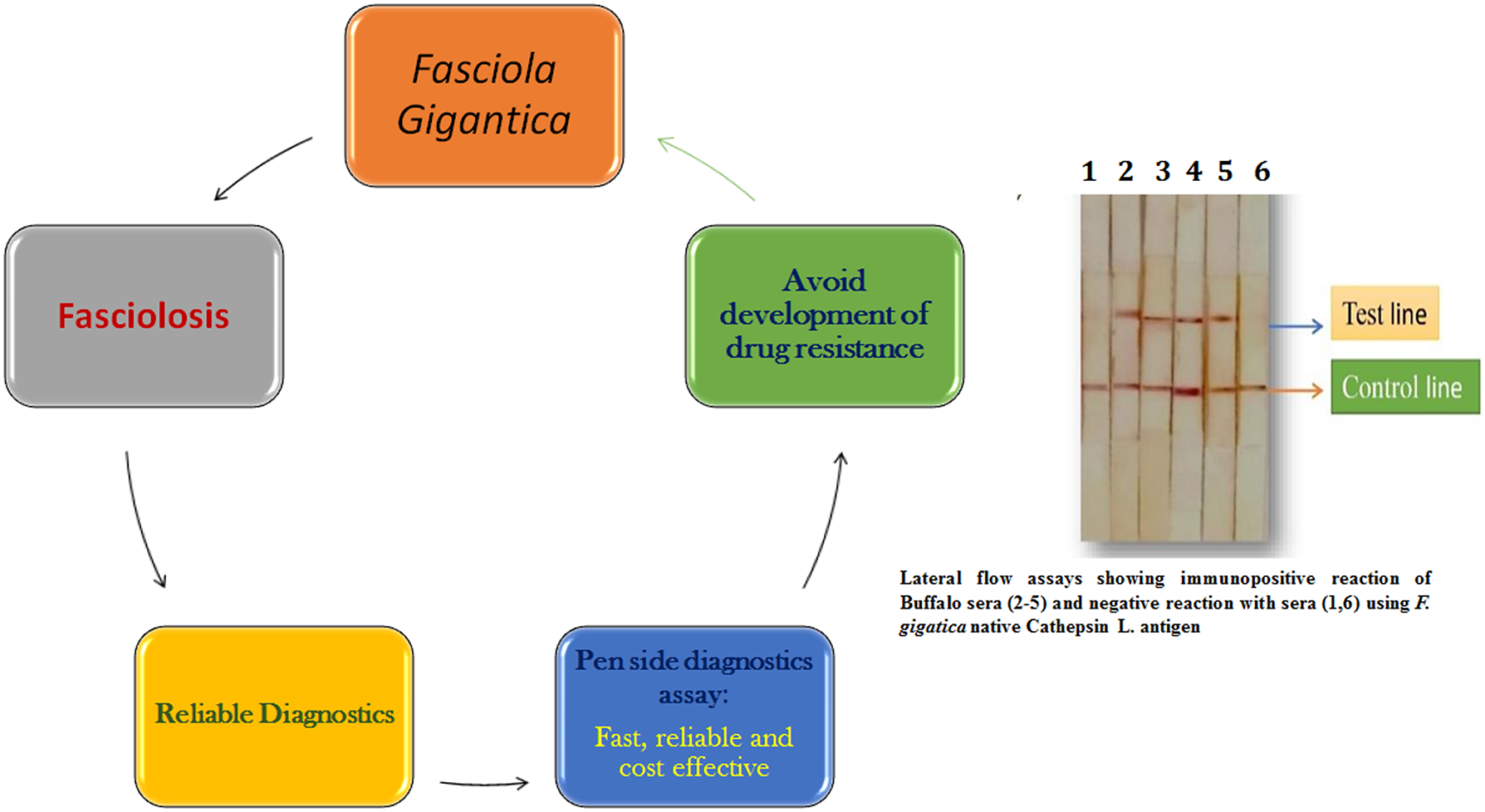
Introduction
Fasciolosis is a parasitic disease caused by two species of the liver fluke Fasciola hepatica and F. gigantica. The prominent definitive hosts are ruminants including sheep, goat, cattle, and buffaloes, with humans as accidental hosts. The life cycle of Fasciola involves an intermediate aquatic snail and a definitive host (Andrews Reference Andrews and Dalton1999). Regardless of the definitive host, which can either be an animal or a human, the life cycle of both species of Fasciola is the same (Figure 1). However, the intermediate snail host differs for both species. The metacercariae (infective larval stage) present on foliage and in water are ingested by grazing animals or by humans, initiating the course of infection in the host. Livestock is an important economic resource for rural families, but the economic productivity of this resource is reduced by fasciolosis, eventually curbing household income and posing a threat to health security (Carmona & Tort Reference Carmona and Tort2017; Webb & Cabada Reference Webb and Cabada2018). Production losses associated with infection and overt clinical disease results in significant costs to the global farming industry, estimated over US $3 billion per year (Spithill et al. Reference Spithill, Smooker, Sexton, Bozas, Morrison, Creaney, Parsons and Dalton1999). These costs are largely unquantified at a national or regional level, whilst at a farm level the fluke affects milk yield, carcass composition, and weight gain (Charlier et al. Reference Charlier, Vercruysse, Morgan, van Dijk and Williams2014).
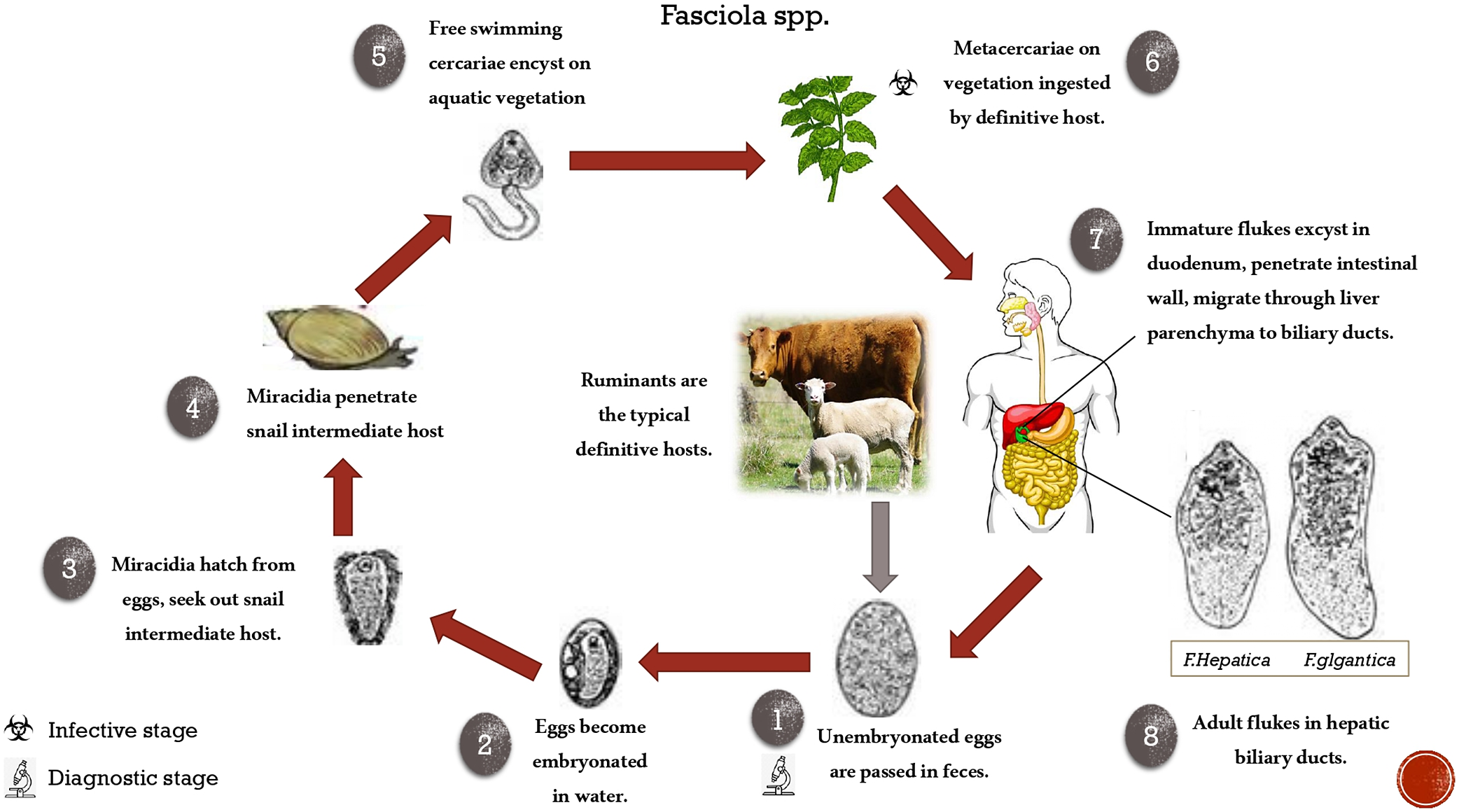
Figure 1. Complete life cycle of Fasciola.
Human cases of fasciolosis are increasing rapidly, with approximately 17 million people infected worldwide and about 180 million at risk of infection (Arifin et al. Reference Arifin, Höglund and Novobilský2016; Amiri et al. Reference Amiri, Shemshadi, Shirali, Kheirandish and Fallahi2021). However, the actual number of people infected must be much higher than the number of documented cases because many cases remain unreported in the developing countries of Asia and Africa (Mas-Coma Reference Mas-Coma2005; Mirzadeh Reference Mirzadeh, Jafarihaghighi, Kazemirad, Sabzevar, Tanipour and Ardjmand2021). The World Health Organization (WHO) has classified fasciolosis as a neglected tropical disease, and significant steps are to be taken to combat the disease at the global level (WHO 2018).
Control of fasciolosis involves preventing animals’ access to wet pastures or water bodies infected with Fasciola infected snails, proper drainage of water bodies to shrink snail habitat, proper management of grazing areas of livestock, and reliable diagnosis and treatment of the definitive host. A wide range of anthelmintics are available for the treatment of infection, but widespread and non-selective use of these anthelmintics has led to the development of resistance against them (Fairweather et al. Reference Fairweather, Brennan, Hanna, Robinson and Skuce2020; Beesley et al. Reference Beesley, Cwiklinski, Allen, Hoyle, Spithill, La Course, Williams, Paterson and Hodgkinson2023). Several benzimidazole drugs are being used in Fasciola treatment, but triclabendazole is the drug of choice as it works against all the fluke stages (Fairweather Reference Fairweather2005). However, resistance to this drug has been reported in both species (Kelley et al. Reference Kelley, Elliott, Beddoe, Anderson, Skuce and Spithill2016; Caravedo & Cabada Reference Caravedo and Cabada2020; Fairweather et al. Reference Fairweather, Brennan, Hanna, Robinson and Skuce2020). A possible substitute to chemotherapy could be a vaccine, but vaccine development has remained a challenge and no commercial vaccine is available yet (Caravedo & Cabada Reference Caravedo and Cabada2020). Therefore, in the absence of vaccines, control of Fasciola infection in animals relies on the judicious use of anthelminthics and on accurate diagnosis of the parasite infection. In view of the challenges posed by anthelmintic resistance and lack of vaccines, reliable and timely diagnosis plays a crucial role in the control of the disease in livestock. This review focuses on the advancements made in diagnostic approaches against Fasciola species in animals and in humans.
Diagnostic approaches for Fasciola infection in livestock
Traditionally, Fasciola infections have been diagnosed by detecting eggs in the faeces (Anderson et al. Reference Anderson, Luong, Vo, Bui, Smooker and Spithill1999). However, because the prepatent period of the parasite is a long duration of 10–12 weeks (depending on the host and the fluke species), egg counts are only useful in post-patent infection. Nonetheless, faecal detection of Fasciola eggs is considered a gold standard in the routine diagnosis of infection in individual animals. Serological diagnosis is preferred because anti-Fasciola antibodies can be detected at an early stage of infection and can thus facilitate early chemotherapeutic intervention. In recent decades, several immunodiagnostic assays with higher sensitivity and specificity have been developed (Table 1). Antibody detection enzyme-linked immunosorbent assays (ELISAs) have been developed and commercialized for routine use in the diagnosis of Fasciola infection in livestock. Their diagnostic sensitivity and specificity have been reviewed by several groups (Alvarez Rojas et al. Reference Alvarez Rojas, Jex, Gasser and Scheerlinck2014; Lalrinkima et al. Reference Lalrinkima, Lalchhandama, Jacob, Raina and Lallianchhunga2021). Most of these antibody-detection ELISAs are based on cysteine protease antigens of F. hepatica and F. gigantica. Cysteine proteases of Fasciola are the key molecules involved in several biological processes of the parasite (Berasain et al. Reference Berasain, Carmona, Frangione, Dalton and Goni1997; Dalton et al. Reference Dalton, Neill, Stack, Collins, Walshe, Sekiya, Doyle, Mulcahy, Hoyle, Khaznadji, Moiré, Brennan, Mousley, Kreshchenko, Maule and Donnelly2003). The major cysteine proteases are members of the families cathepsin (cat)-L and cat-B and are secreted in abundance by the immature and adult fluke. Cat-L proteases have been successfully expressed as recombinant antigens that have shown significant diagnostic potential for fasciolosis in animals and humans equally. F. gigantica cat-L protease-based ELISAs have been developed and tested for the prepatent diagnosis of F. gigantica infection in cattle, buffaloes, and sheep (Dixit et al. Reference Dixit, Yadav and Sharma2002, Reference Dixit, Yadav and Sharma2004, Reference Dixit, Dixit and Sharma2008; Raina et al. Reference Raina, Yadav, Sriveny and Gupta2006; Sriveny et al. Reference Sriveny, Raina, Yadav, Chandra, Jayraw, Singh, Velusamy and Singh2006; Varghese et al. Reference Varghese, Raina, Nagar, Garg, Banerjee, Maharana and Kollannur2012; Kumar et al. Reference Kumar, Varghese and Solanki2017). In addition to cat-L protease-based diagnostic assays, attempts are being made to develop cat-B based assays as well, particularly because these proteins are secreted at an early stage of the infection. Expression of several members of the cat-B gene family (cat B1–cat B7) in both species of Fasciola has been reported in the last decade, indicating that cat-B proteins are performing diverse functions in the parasite. Cat-B proteases are expressed by Fasciola at the host gut penetration stage, and their expression is continued until the flukes are immature (Law et al. Reference Law, Smooker, Irving, Piedrafita, Ponting, Kennedy, Whisstock, Pike and Spithill2003; Meemon et al. Reference Meemon, Grams, Vichasri-Grams, Hofmann, Korge, Viyanant, Upatham, Habe and Sobhon2004; Cancela et al. Reference Cancela, Acosta, Rinaldi, Silva, Durán, Roche, Zaha, Carmona and Tort2008; Sethadavit et al. Reference Sethadavit, Meemon, Jardim, Spithill and Sobhon2009; Siricoon et al. Reference Siricoon, Vichasri Grams, Lertwongvisarn, Abdullohfakeeyah, Smooker and Grams2015). However, expression of cat-L proteins is predominant, with a decline in the expression of cat-Bs as the parasite matures (Chantree et al. Reference Chantree, Wanichanon, Phatsara, Meemon and Sobhon2012). Fasciola cat-B proteins are also secreted like cat-Ls, which are immunogenic and easily exploited in diagnostic assays of Fasciola. In fact, both cat-L and cat-B proteins can be used in combination in an immunodiagnostic assay for enhancing the detection sensitivity of prepatent and patent infections (Aftab et al. Reference Aftab, Lall, Bisen, Anandanarayanan, Rialch, Chamuah, Yadav, Silamparasan and Raina2020). Recently, Jacob et al. (Reference Jacob, Sengupta, Pavithra, Chandu and Raina2023) reported a first effort to use recombinant cat B-5 antigen to develop ELISA that showed 95.3 % sensitivity and 92.4 % specificity (with a cut-off of 60% positive) in the serosurveillance of bovine tropical fasciolosis in India.
Table 1: Diagnostic methods used for detection of fasciolosis

Detection of antibodies secreted in milk is another approach to diagnosing Fasciola infection in animals. Several serum antibody tests have been adapted to use either in individual or pooled milk samples, providing rapid and less costly methods for monitoring infection status in dairy herds. Most tests show good diagnostic sensitivity and specificity, and several ELISAs are commercially available for detecting the infection in both milk and serum samples. However, anti-Fasciola antibodies secreted in milk can be detected in lactating animals only, and the test cannot be useful for screening non-lactating dairy herds.
Antibody-detection ELISAs are typically developed for a single host species where antibody conjugates are host specific. Incidentally, buffaloes constitute a sizable population of the livestock in the Indian sub-continent and in other Asian and African countries. These animals contribute significantly to the milk and meat industries of these countries. A high prevalence of F. gigantica infection has been reported in buffaloes in India (reviewed by Lalrinkima et al. Reference Lalrinkima, Lalchhandama, Jacob, Raina and Lallianchhunga2021). Typically, fasciolosis in buffaloes is detected using anti-bovine IgG immunoglobulin conjugates as anti-buffalo IgG conjugates are not commercially available. This compromises the sensitivity of the detection assay due to a lack of host-specific antibody conjugates. Therefore, anti-buffalo IgG-specific antibody conjugates must be made commercially available to enhance the sensitivity of these tests. Ideally, immunodiagnostic assays will be developed for detection of infection caused by specific parasites. However, assays in the multiplex format for rapid and high-throughput diagnosis are required to cover the spectrum of helminths co-infecting a host. Karanikola et al. (Reference Karanikola, Krücken, Ramünke, de Waal, Höglund, Charlier, Weber, Müller, Kowalczyk, Kaba, von Samson-Himmelstjerna and Demeler2015) developed a magnetic bead-based assay using fluorescence detection for simultaneous detection of antibodies against F. hepatica, Cooperia oncophora, and Dictyocaulus viviparus in cattle sera, which was shown to be highly sensitive and specific in comparison to serological and coprological diagnostic techniques. In the tropical environments where various trematode species like F. gigantica, amphistomes, schistosomes, and nematodes, particularly Haemonchus contortus, are co-infecting cattle, buffalo, sheep, and goats, immunodiagnostic assays in the multiplex format can save costs and time.
Antigen detection assays
Coprological detection of Fasciola antigens has been adopted as a diagnostic method with high sensitivity and specificity. It detects infection in its early prepatency to patent stage. Several diagnostic kits are commercially available based on the coprodetection of both F. hepatica and F. gigantica infection in livestock. However, coprodetection is not found to be reliable in some cases because in the acute phase of infection, false negative results are obtained (Mokhtarian et al. Reference Mokhtarian, Meamar, Khoshmirsafa, Razmjou, Masoori, Khanmohammadi, Akhlaghi and Falak2018). Monoclonal antibody-based sandwich ELISAs have been developed for the detection of circulating antigens in the sera or faeces of infected animals. Demerdash et al. (Reference Demerdash, Diab, Aly, Mohamed, Mahmoud, Zoheiry, Mansour, Attia and El-Bassiouny2011) reported sandwich ELISA with two monoclonal antibodies for diagnosis of fasciolosis where coproantigens present in stool samples gave better results, with sensitivity, specificity, and diagnostic efficacy of 96.0%, 98.2, and 97.1%, respectively. The MM3-COPRO test, based on the MM3 monoclonal antibody that binds to both Cat L-1 and Cat L-2 proteases (Mezo et al. Reference Mezo, González-Warleta, Carro and Ubeira2004), is commercially available through BIO X Diagnostics (La Jemelle, Belgium). This test has been shown to have high diagnostic specificity (100.0%), with no cross-reaction with soluble fractions of Paramphistomum cervi, Taenia hydatigena, coccidia, and gastrointestinal nematodes (Kajugu et al. Reference Kajugu, Hanna, Edgar, Forster, Malone, Brennan and Fairweather2012). However, ELISA sensitivity can sometimes be compromised by variability in the concentration of cathepsin proteinases in faecal samples and by differences in the between-batch performance of the test (Martinez-Sernandez et al. Reference Martínez-Sernández, Muiño, Perteguer, Gárate, Mezo, González-Warleta, Muro, Correia da Costa, Romarís and Ubeira2011). Brockwell et al. (Reference Brockwell, Elliott, Anderson, Stanton, Spithill and Sangster2013) and Palmer et al. (Reference Palmer, Lyon, Palmer and Forshaw2014) improved the sensitivity of MM3-COPRO ELISA using a customized cut-off for sheep and cattle whilst maintaining the specificity above 99.0%. A coproantigen test using a streptavidin-polymerized horseradish peroxidase conjugate was evaluated and was sufficiently sensitive to detect infection with a single fluke (Martınez-Sernandez et al. 2016). A wide range of antigens from F. hepatica including excretory/secretory products, tegumental components, crude extracts from adult worms, and recombinant proteins (cathepsins, a heat shock protein, and a saposin-like protein) have been incorporated into antigen detection assays (reviewed by Alvarez Rojas et al. Reference Alvarez Rojas, Jex, Gasser and Scheerlinck2014). Refinement of all these diagnostic assays will further enhance their reliability in terms of sensitivity and specificity. Antigen detection assays have an advantage over antibody detection assays as the latter suffer from cross-reactivity and cannot differentiate between current and past infection.
Nucleic acid detection-based diagnostic assays
Molecular diagnostic methods have been tested for furthering the sensitivity and the specificity of available diagnostic tests. Conventional polymerase chain reaction (PCR) and nested-PCR based on cytochrome C oxidase 1 gene and ITS-2 sequence have been tested for diagnosis of sheep, cattle, and buffalo fasciolosis. These tests detected parasite DNA by 2 weeks post-infection in faecal samples (Martinez-Perez et al. Reference Martínez-Pérez, Robles-Pérez, Rojo-Vázquez and Martínez-Valladares2012; Robles-Perez, et al. Reference Robles-Pérez, Martínez-Pérez, Rojo-Vázquez and Martínez-Valladares2013; Ayaz et al. Reference Ayaz, Ullah, AbdEl-Salam, Shams and Niaz2014). As both Fasciola species may overlap in distribution in some countries of Africa and Asia, a single-step duplex PCR was developed for their simultaneous detection using faecal samples (Le et al. Reference Le, Nguyen, Nguyen, Doan, Le, Hoang and De2012). However, there are issues at times with reproducibility of results of PCR protocols using faecal samples. A pen-side test based on loop-mediated isothermal amplification (LAMP) is an alternative to PCR to detect fluke DNA in the host faeces (Martinez-Valladares & Rojo-Vazquez Reference Martínez-Valladares and Antonio Rojo-Vázquez2016). Ghodsian et al. (Reference Ghodsian, Rouhani, Fallahi, Seyyedtabaei and Taghipour2019) have described a LAMP assay that could detect even a single egg in faecal samples. There was no cross-amplification of other helminths including Taenia saginata, Dicrocoelium dendriticum, and F. gigantica. Molecular methods like PCR, real-time PCR, and LAMP detect very low amounts of Fasciola DNA from biological samples such as sera and faeces (Carnevale et al. Reference Carnevale, Pantano, Kamenetzky, Malandrini, Soria and Velásquez2015; Tran & Phung Reference Tran and Phung2020; Shi et al. Reference Shi, Li, Huang, Yao, Chen, Du and Yang2020; Amiri et al. Reference Amiri, Shemshadi, Shirali, Kheirandish and Fallahi2021). Adoption of an integrated liver fluke management plan would benefit from a simple, rapid, field-deployable diagnostic assay for detection of F. hepatica and F. gigantica in the environment and the host. Therefore, a rapid DNA test using LAMP was developed and optimized for detection of F. hepatica DNA in host faeces and water samples (Tran et al. Reference Tran, Porcher, Pane and Ravaud2022). The Tran et al. assay is fast, with amplification in ≤20 min, and highly sensitive, with a detection limit of 5×10−4 ng /μL. The assay does not require a commercial kit for the extraction of DNA from faecal and water samples. The assay is pending field validation but highlights its potential to inform local treatment decisions in real-time for management of liver fluke infections.
Since F. hepatica and F. gigantica co-exist in the countries of Asia and Africa, there are possibilities of the existence of hybrid forms of the parasite in the regions of parasite sympatry. A growing number of reports of hybridization between F. hepatica and F. gigantica in Southeast Asia are available now, but there is no empirical evidence supporting introgression between these two parasites (Calvani et al. Reference Calvani and Šlapeta2021). Introgression between these two species, if it exists, may have other important human and animal health impacts, including increased infectivity, virulence, and pathophysiology. Therefore, new diagnostic tools for the characterization of hybridization and introgression events between Fasciola spp. in the areas of parasite sympatry are needed.
Deep amplicon sequencing or metabarcoding, using next generation sequencing platforms, has revolutionized the study of microbial communities in humans, animals, and the environment. Parasites often exist in complex communities within a host or in the external environment. The nemabiome refers to the community of nematodes that inhabit a single host animal or environmental niche (Francis & Slapeta Reference Francis and Šlapeta2022). Nemabiome sequencing provides a detailed picture of the species composition of the gastrointestinal parasite community in large sample sets and has vast potential for applications in diagnostics, surveillance, and research (Avramenko et al. Reference Avramenko, Redman, Lewis, Bichuette, Palmeira, Yazwinski and Gilleard2017; Costa-Junior et al. Reference Costa-Junior, Chaudhry, Silva, Sousa, Silva, Cutrim-Júnior, Brito and Sargison2021; Sargison et al. Reference Sargison, Chambers, Chaudhry, Costa Júnior, Doyle, Ehimiyein, Evans, Jennings, Kelly, Sargison, Sinclair and Zahid2022; De Seram et al. Reference De Seram, Redman, Wills, de Queiroz, Campbell, Waldner, Parker, Avramenko, Gilleard and Uehlinger2022). However, such an approach is yet to be applied to determine species composition of trematode parasites, including liver flukes, amphistomes, and schistosomes, infecting our livestock. This approach will further enhance future diagnostics of helminth infections in large sample sets.
Diagnostic approaches for human fasciolosis
The World Health Organization has recommended confirming Fasciola diagnosis in humans based on clinical signs by supplementing with either egg detection or immunological techniques (WHO 2018). Specific assays based on native or recombinant antigens have been developed to enhance the reliability of human immunoassays. These immunoassays mostly use Fasciola cysteine proteinases as antigens with high specificity (Rokni et al. Reference Rokni, Massoud and Hanilo2003; Robinson et al. Reference Robinson, Menon, Donnelly, Dalton and Ranganathan2009; Rojas-Caraballo et al. Reference Rojas-Caraballo, López-Abán, Pérez del Villar, Vizcaíno, Vicente, Fernández-Soto, del Olmo, Patarroyo and Muro2014). Recombinant proteins and synthetic peptides based on cysteine proteases are used in immunodiagnostic assays (Intapan et al. Reference Intapan, Tantrawatpan, Maleewong, Wongkham, Wongkham and Nakashima2005; Tantrawatpan et al. Reference Tantrawatpan, Maleewong, Wongkham, Wongkham, Intapan and Nakashima2005; Gonzales-Santana et al. Reference Gonzales Santana, Dalton, Vasquez Camargo, Parkinson and Ndao2013; Meshgi et al. Reference Meshgi, Jalousian, Fathi and Jahani2018). The cysteine protease-based ELISAs were further simplified by using cystatin-capture (CC)-ELISA to detect anti-cysteine protease antibodies without the need for a purified or recombinant cysteine protease; CC-ELISA using whole worm extract was reported suitable for practical diagnosis of fasciolosis (Ikeda Reference Ikeda1998; Tantrawatpan et al. Reference Tantrawatpan, Maleewong, Wongkham, Wongkham, Intapan and Nakashima2005; Tran et al. Reference Tran, Ton Nu, Intuyod, Dao, Pinlaor, Nawa, Choowongkomon, Geadkaew-Krenc, Kosa, Grams and Pinlaor2019). Pen-side diagnostic tests have been evaluated to make diagnosis easier, faster, and more cost-effective. Martınez-Sernandez et al. (2011) developed a lateral flow test (SeroFluke) for the serodiagnosis of human fasciolosis. In comparison with an ELISA test (MM3-SERO), the SeroFluke test showed excellent specificity and sensitivity and could be used with serum or whole blood samples. Lateral flow assays using Fasciola specific antigens will likely be future pen-side diagnostic tests for both human and animal fasciolosis.
Human fasciolosis in the Indian sub-continent
Although fasciolosis in humans is distributed worldwide, it is more prevalent in countries associated with extensive livestock rearing. In India, fasciolosis is reported in patient case reports, and most of the time it is a surprise diagnosis (Ramachandran et al. Reference Ramachandran, Ajjampur, Chandramohan and Varghese2012; Yatoo et al. 2021). There are no seroepidemiological studies being carried out for snail borne trematode infections in humans in India or its neighbouring countries. Therefore, there are no data available on the prevalence or endemicity of this zoonotic parasite in humans on the Indian sub-continent. In most human cases of Fasciola infection in India, the species was reported as F. hepatica, with only a few reports incriminating it as F. gigantica (reviewed by Lalrinkima et al. Reference Lalrinkima, Lalchhandama, Jacob, Raina and Lallianchhunga2021). No molecular markers were used for detection of the Fasciola species in these reports. Therefore, describing the parasite as F. hepatica in these human infections does not seem authentic, as F. hepatica has not yet been reported in the plains of India. Human fasciolosis cases in India and its adjoining countries like Pakistan, Nepal, Bangladesh, and Sri Lanka could be higher than reported in medical journals, as these more rural populations are at risk of infection due to the close association of humans with domestic livestock and their access to snail-infested water bodies. Also, lack of skilled healthcare workers available for the diagnosis and lack of public awareness of the risks of water-borne trematode diseases are other factors contributing to the spread of Fasciola infection in human populations in rural areas.
Future directions
Accurate and rapid diagnoses of Fasciola infection in animals and humans are due to advancements in the available diagnostic tests. Fasciola-specific antigens that are expressed in the early, prepatent stage of the parasite hold promise for reliable and prepatent diagnosis of Fasciola infection. Both the molecular and serodiagnostic tests developed thus far have definitely enhanced the reliability of Fasciola diagnosis in animals and humans. Future state-of-the-art technologies will provide more accurate, faster, and more cost-effective diagnoses of Fasciola infection that will allow targeted treatment and more accurate prevalence surveys. A pen-side diagnostic test based on a lateral flow assay or a DNA test like LAMP would be quick, simple, and cost-effective, enabling clinicians to treat animals in a targeted manner to avoid development of drug resistance to the limited available flukicides. Climatic changes are also a compelling force for strengthening diagnostic methodologies as the disease may emerge in newer areas that are currently Fasciola free. The commercial kits produced in developed countries are not affordable for routine screening in endemic areas in developing and resource-poor nations. Therefore, research on Fasciola diagnosis has to be strengthened locally by the nations where this infection is endemic.
Acknowledgments
The authors are thankful to the Director, ICAR-Indian Veterinary Research Institute-Izatnagar, for allowing Andleeb Aftab to use laboratory facilities at the Division of Parasitology, ICAR-Indian Veterinary Research Institute, Izatnagar.
Financial support
Not applicable
Competing interests
None
Ethical standards
Not applicable