In 1996, the Department of Health published a review of drug services in England (Department of Health, 1996) and stated that for patients on methadone maintenance, daily doses of over 50 mg were associated with lower rates of heroin use. International research supports this assertion, with results showing that patients on higher doses have better outcomes (Reference Farrell, Ware and MattickFarrell et al, 1994). Community pharmacy surveys of methadone prescribing in the UK show that over 50% of patients receive daily doses lower than 50 mg (Reference Strang, Sheridan and BarberStrang et al, 1996; Reference Strang and SheridanStrang & Sheridan, 1998; Reference Strang and SheridanStrang & Sheridan, 2001), with the mean dose being 49 mg. In the National Treatment Outcome Research Study, the mean methadone dose prescribed by specialist drug services in the UK was 48 mg (Reference Gossop, Marsden and StewartGossop et al, 1999). These findings have led to concerns that specialist services are under-treating opiate-dependent patients.
A limitation of the pharmacy surveys is that patients in receipt of two prescriptions for different formulations of methadone, such as ampoules and mixture, are counted twice. Furthermore, patients on detoxification programmes, slow reduction regimens and methadone maintenance are grouped together, thus skewing the results to the left. The aim of this study was to audit long-term methadone prescribing at an inner-city, specialist NHS drug service and to compare the results with national data.
The study
This study was performed over 2 weeks, from 15.7.02 to 26.7.02.
The service
The North Camden Drug Service covers the northern half of the London borough of Camden (population 101,700 and Jarman index=32.9). Although there are areas of affluence (e.g. Hampstead Village), it has pockets of deprivation (e.g. Gospel Oak, Camden Town and Kentish Town). Camden & Islington have the fourth-highest number of drug users notified to the Regional Drug Misuse Database in the whole Thames Region (Department of Health, 1998).
The following on-site treatment services are available: out-patient detoxification, long-term prescribing, keyworking, psychological therapies, psychiatric and medical interventions, an alcohol programme, a crack cocaine programme, needle exchange, hepatitis testing and vaccination, alternative therapies and benefits advice. There is an on-site pharmacy and prescriptions are also dispensed at community pharmacies. Only four local pharmacies have so far been approved to take part in the supervised methadone scheme. There were 620 episodes of care at our service between 3 April 2001 and 31 April 2002, including 304 new assessments.
Data extraction and analysis
Patients were excluded if they were on the waiting list, not receiving controlled drugs or on the structured detoxification programme. Data were extracted from the following three electronic databases: (i) the patient registration database, covering 19 items including basic demographic information, drug use, service allocated and date of allocation; (ii) the prescribing software (Advantage-Altrix), which produces patients' prescriptions; and (iii) a database of urine drug screens for the last 7 months (provided by the Department of Chemical Pathology at the Royal Free Hospital). Anonymised data were collated and entered onto the Statistical Package for the Social Sciences. Eleven variables were used: age, gender, prescribed opioid, dose, formulation, frequency of pick-up, supervised consumption, duration of current treatment episode, number of urine drug screens in the past 7 months, proportion of urine screens positive for non-prescribed opiates and treatment programme (slow reduction or maintenance). Slow reduction was empirically defined as any methadone regimen not forming part of the structured detoxification programme, in which there had been two or more successive reductions in dose since January 2000 (when the prescribing database began). All other non-reducing regimens were considered as maintenance.
The t-test or one-way analysis of variance were used to compare the means between groups when data followed a normal or near-normal distribution. Categorical data were analysed using the χ2 test. Parametric but highly-skewed data were analysed using the Mann—Whitney U or Kruskal—Wallis tests. Pearson's correlation was used to test the association between two variables. To compare the proportion of urines positive for non-prescribed opiates between maintenance patients on different methadone formulations, a paired analysis of matched cases was used with matching on gender, age, methadone dose and duration of treatment. The paired Wilcoxon signed ranks test was used because of the asymmetric distribution of this variable.
Findings
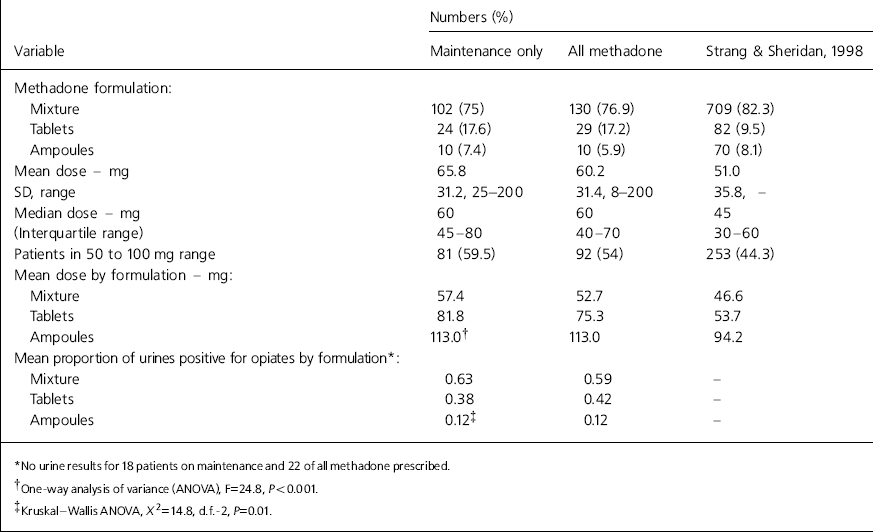
During the study period, 174 patients were receiving a prescription for opioids. The characteristics of these patients are shown in Table 1. Of the 169 patients on methadone, 33 were on a slow reduction regimen and the rest on maintenance. There were no statistically significant differences in age or gender distribution between patients on methadone maintenance and slow reduction, and they had been in treatment for similar lengths of time (means=59.5 and 53.5 months, respectively). The slow reduction group had a lower mean proportion of urines positive for non-prescribed opiates compared to the maintenance group (0.47 v. 0.54, respectively), despite being on lower doses of methadone (mean=36.9 mg, SD=19.5, range=8-70 mg). In Table 2, the prescribing characteristics of all patients on long-term methadone and those specifically on methadone maintenance are compared with data from the south east England pharmacy survey (Reference Strang and SheridanStrang & Sheridan, 1998). Because Strang and Sheridan's (Reference Strang and Sheridan1998) survey was unable to distinguish between maintenance and reduction prescriptions, the most appropriate comparison is with the ‘all methadone’ column.
Table 1. Characteristics of opioid prescribed patients from an inner London drug service (n=174)
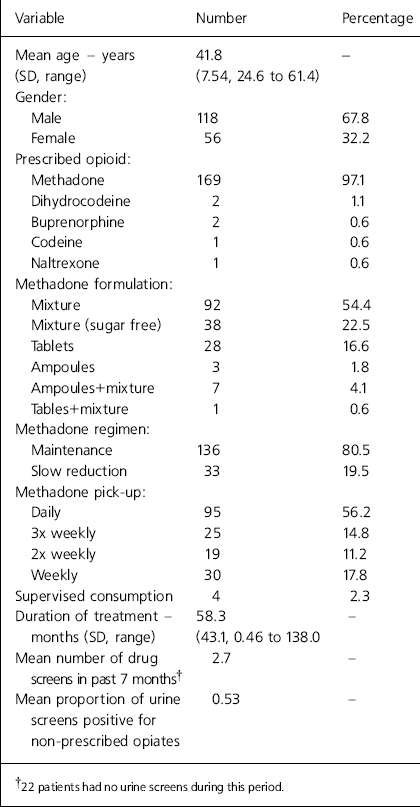
| Variable | Number | Percentage |
|---|---|---|
| Mean age — years (SD, range) | 41.8 (7.54, 24.6 to 61.4) | — |
| Gender: | ||
| Male | 118 | 67.8 |
| Female | 56 | 32.2 |
| Prescribed opioid: | ||
| Methadone | 169 | 97.1 |
| Dihydrocodeine | 2 | 1.1 |
| Buprenorphine | 2 | 0.6 |
| Codeine | 1 | 0.6 |
| Naltrexone | 1 | 0.6 |
| Methadone formulation: | ||
| Mixture | 92 | 54.4 |
| Mixture (sugar free) | 38 | 22.5 |
| Tablets | 28 | 16.6 |
| Ampoules | 3 | 1.8 |
| Ampoules+mixture | 7 | 4.1 |
| Tables+mixture | 1 | 0.6 |
| Methadone regimen: | ||
| Maintenance | 136 | 80.5 |
| Slow reduction | 33 | 19.5 |
| Methadone pick-up: | ||
| Daily | 95 | 56.2 |
| 3× weekly | 25 | 14.8 |
| 2× weekly | 19 | 11.2 |
| Weekly | 30 | 17.8 |
| Supervised consumption | 4 | 2.3 |
| Duration of treatment — months (SD, range) | 58.3 (43.1, 0.46 to 138.0 | — |
| Mean number of drug screens in past 7 months† | 2.7 | — |
| Mean proportion of urine screens positive for non-prescribed opiates | 0.53 | — |
Table 2. Prescribing characteristics of patients on methadone maintenance, all long-term methadone patients and Strang & Sheridan's (Reference Strang and Sheridan1998) pharmacy survey
| Numbers (%) | |||
|---|---|---|---|
| Variable | Maintenance only | All methadone | Reference Strang and SheridanStrang & Sheridan, 1998 |
| Methadone formulation: | |||
| Mixture | 102 (75) | 130 (76.9) | 709 (82.3) |
| Tablets | 24 (17.6) | 29 (17.2) | 82 (9.5) |
| Ampoules | 10 (7.4) | 10 (5.9) | 70 (8.1) |
| Mean dose — mg | 65.8 | 60.2 | 51.0 |
| SD, range | 31.2, 25-200 | 31.4, 8-200 | 35.8, — |
| Median dose — mg | 60 | 60 | 45 |
| (Interquartile range) | 45-80 | 40-70 | 30-60 |
| Patients in 50 to 100 mg range | 81 (59.5) | 92 (54) | 253 (44.3) |
| Mean dose by formulation — mg: | |||
| Mixture | 57.4 | 52.7 | 46.6 |
| Tablets | 81.8 | 75.3 | 53.7 |
| Ampoules | 113.0† | 113.0 | 94.2 |
| Mean proportion of urines positive for opiates by formulation*: | |||
| Mixture | 0.63 | 0.59 | — |
| Tablets | 0.38 | 0.42 | — |
| Ampoules | 0.12‡ | 0.12 | — |
The proportion of urines positive for non-prescribed opiates was inversely correlated with methadone dose (-0.26, P=0.004) and duration of current treatment episode (-0.31, P=0.001).
Patients receiving methadone ampoules had the lowest proportion of urines positive for illicit opiates, followed by those on tablets and then those on mixture. However, patients on tablets and ampoules were receiving higher doses of methadone. To investigate whether the lower level of illicit opiate use was best-explained by methadone dose or formulation, a paired analysis of patients on methadone tablets with those on mixture was performed with matching on gender, age, methadone dose and duration of treatment. As some patients had no urine results, there were only 19 pairs in this analysis. The proportion of urines positive for illicit opiates was slightly lower in the tablet (0.40) compared with the mixture group (0.54). However, this difference was not statistically significant (P=0.36). Patients on methadone ampoules were not included in this analysis due to insufficient numbers.
Discussion
Although other UK drug services must have surveyed their methadone prescribing, we have been unable to find any published studies on either Medline or EMBASE. In this study, we have been able to focus on patients in methadone maintenance and have shown that the mean dose is 65.8 mg, with 67.7% of patients taking 50 mg or more. These figures are encouraging, but still suggest that a minority of patients are on a sub-therapeutic dose. There may be good clinical reasons why some patients are on lower doses, such as a low level of dependency, and without more detailed information, generalisations about under-treatment may be premature. However, the inverse relationship between methadone dose and the proportion of urines positive for non-prescribed opiates, a measure of treatment compliance, gives some support to the under-treatment hypothesis. Other areas in the UK may not have the same level of service as that found in Camden and this too may influence the level of methadone prescribing.
The proportion of patients on methadone tablets (17.2%) was much higher than in Strang and Sheridan's (Reference Strang and Sheridan1998) survey (9.5%). The reasons for this are unclear. A previous prescribing policy in operation at this service seems to have allowed long-term compliant patients to move onto methadone tablets, if they were no longer injecting. A more restrictive policy was introduced in early 2000, bringing prescription into line with the Department of Health's Clinical Guidelines (Department of Health, 1999). Patients on combination therapy who are HIV-positive are often prescribed methadone tablets because they complain that mixture causes nausea. Since the change in policy, the number of patients receiving methadone tablets has fallen from 44 to 28. The percentage of patients receiving methadone ampoules is lower (5.9%) than Strang and Sheridan's (Reference Strang and Sheridan1998) figure (8.1%). Again, the stricter prescribing policy introduced in 2000 led to the number of patients on ampoules falling from 16 to 10.
Initial analysis suggested that patients on methadone ampoules or tablets were less likely to use illicit opiates than those taking mixture. However, when matched for gender, age, methadone dose and duration of treatment, drug formulation no longer had an independent effect on illicit drug use.
The inverse relationship between illicit heroin use and length of time in treatment is encouraging, suggesting that patients continue to improve and reduce their drug use over time. There was also an inverse relationship between methadone dose and the proportion of urines positive for illicit opiates, though the correlation was not strong. Two factors are likely to influence this relationship: (i) whether all the methadone prescribed is being taken and (ii) individual variation in the rate of methadone metabolism. Supervised methadone consumption has been advocated in an attempt to reduce the diversion of prescribed methadone onto the black market. In North Camden, we are still at an early stage of implementing supervised consumption in local pharmacies. We are also investigating the cost—benefit of having an on-site pharmacy, which would allow us to supervise more prescribed patients. The second factor in the equation is the rate at which methadone is metabolised. Studies suggest that there is considerable individual variation, due to different levels of activity of the cytochrome P450 enzymes (Reference Eap, Bertschy and BaumannEap et al, 1998). We argue that monitoring serum methadone levels is a better way of determining methadone dose than using the arbitrary maximum doses set by many clinics. This is not a routine test in the UK and could have considerable costs. However, if its use was restricted to patients who persistently use heroin in addition to their methadone, to investigate the possibility of a pharmacokinetic explanation, the cost would be less prohibitive.
Looking at methadone dose and urine drug screens in isolation gives limited information on the multifaceted nature of treatment outcome. Consequently, we plan to do a larger and more detailed survey of patients on methadone maitenance, in the whole of Camden and Islington, to gain a better perspective of our prescribing practices and the clinical effectiveness of this intervention. The present study has acted as a useful pilot exercise, to identify problems that might arise with a larger survey.
Declaration of interest
John Dunn has received travel expenses and an honorarium for attending a meeting organised by Napp Pharmaceuticals, who produce Subsitol (slow release morphine).
Acknowledgements
I would like to thank Doborah Zador for her helpful comments and both Brendon Gregory and Martin Fajimi for their assistance with the databases.





eLetters
No eLetters have been published for this article.