The grass carp (Ctenopharyngodon idella) production in China reached 5·6 million tons in 2020, which was the highest freshwater fish production in China, and it is widely accepted by consumers due to its richness in protein and PUFA(Reference Han, Huang and Mahunu1). Nutrients deposition and muscle fibre characteristics can reflect the quality of fish. It has been reported that the unbalanced diet or improper aquaculture mode markedly reduces the quality of fish, such as a decrease in nutritional value and change in texture parameters(Reference Lv, Ma and Hu2).
In recent years, the high-fat diet (HFD) has been widely applied in aquaculture industry to save protein ingredients, which are of high price. As a result, the production cost of aquafeed is reduced to a certain extent, and the excretion of nitrogen during the process of aquaculture is also reduced(Reference Jia, Cao and Du3). However, a study has shown that more than 6 % of lipids in the diet was harmful to grass carp, including growth performance and nutritional composition(Reference Du, Liu and Tian4). Also, the HFD led to excessive liver fat deposition and oxidative stress, which seriously affected the health and quality of grass carp (C. idella) and tilapia (Oreochromis niloticus)(Reference Jia, Cao and Du3,Reference Tang, Hu and Peng5) . A large amount of vegetable oil in HFD does not contribute much to the accumulation of highly unsaturated fatty acids in fish. More seriously, the HFD led to the decline of processing quality and eating taste of fish, such as looseness of fish meat, lower pH value, and reduced water holding capacity (WHC)(Reference Lv, Ma and Hu2). In addition, lipid is the main energy source for fish to overwinter, so lipid accumulation before overwintering has naturally occurred in fish(Reference Morgan6,Reference Sun, Wu and Ji7) . At this time, the HFD will lead to a further decline in the flesh quality of cultured fish(Reference Zhang, Limbu and Zhao8). Therefore, research is required to explore approaches for improving flesh quality in farmed fish fed with HFD, especially before overwintering.
Nutritional regulation is increasingly applied to improve the edible quality of cultured fish. For example, feeding with faba bean for 90 to 120 d before grass carp was put on the market, the hardness and elasticity of muscle increased obviously(Reference Yu, Fu and Wang9). The optimal dietary riboflavin improved fish quality of grass carp by reducing lipid peroxidation and protein oxidation(Reference Jiang, Chen and Liu10). And dietary glutamine enhanced the nutrition and healthcare substances, mouthfeel parameters, and the synthesis of type I collagen in grass carp(Reference Ma, Feng and Wu11). However, most studies focusing on fish quality regulation are carried out in a fast-growing season of fish with optimum water temperature(Reference Jiang, Chen and Liu10,Reference Ma, Feng and Wu11) . Few studies have been conducted before overwintering in a relatively cold environment.
Se, as a micronutrient, is necessary for humans and animals. As a constituent of selenoproteins, Se has structural and enzymic roles, in the latter context being best known as an antioxidant and catalyst for the production of active thyroid hormone(Reference Rayman12). According to previous research, the dietary Se requirements for young grass carp were estimated to be 0·546–0·604 mg/kg diet(Reference Zheng, Jiang and Feng13). According to Liu et al. (Reference Liu, Yu and Wang14), dietary 0·3 or 0·6 mg/kg nano-Se was the health-giving concentration. Se deficiency leads to a decrease in fish feed intake and immunity and an increase in mortality. Dietary nano-Se alleviated intestinal inflammation and hepatopancreas oxidative stress of grass carp induced by HFD(Reference Liu, Yu and Wang14,Reference Liu, Yu and Li15) . Studies also showed that dietary yeast-Se promoted the synthesis of protein in trout muscle and stimulated the hypertrophy and growth of white muscle in trout by upregulating the expression of selenoprotein(Reference Wang, Wang and Zhang16). However, studies on the impact of dietary Se on nutrients deposition and muscle fibre formation in fish are still rare. It has been confirmed that dietary nano-Se decreased the lipid accumulation of grass carp fed HFD by regulating the expression level of genes taken part in the lipid metabolism(Reference Liu, Yu and Wang14). In addition, the research shows that compared with organic Se and inorganic Se, nano-Se has a greater impact on the growth performance and antioxidant defense system of carp(Reference Saffari, Keyvanshokooh and Zakeri17). Nano-Se has been developed to supplement Se due to its good water solubility and low toxicity(Reference Yang, Li and Wang18).
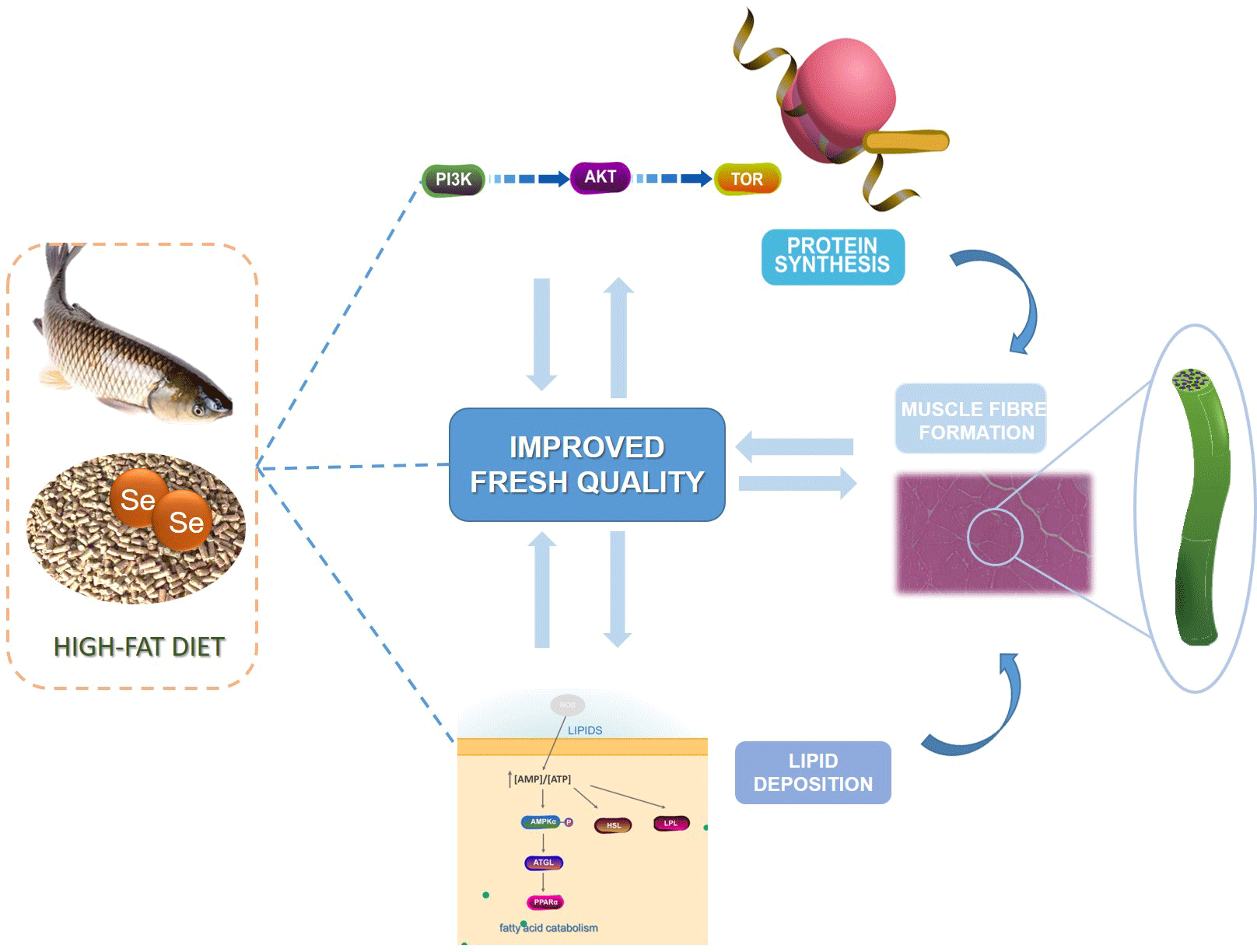
Thus, the current study was the first to probe into the improving effect of dietary nano-Se on nutrients deposition and muscle fibre formation of grass carp fed with HFD before overwintering. The lipid deposition, protein synthesis and muscle fibre formation of grass carp fed the regular diet (RD), HFD and HFD added with nano-Se (0·3 or 0·6 mg/kg) were tested. And the signaling pathways involved in lipid deposition, protein synthesis and muscle fibre formation were investigated. The current study will help to reveal the possible regulation mechanism of dietary nano-Se on nutrients deposition and muscle fibre formation in grass carp fed with HFD before overwintering.
Material and methods
Experimental diets and fish management
The experiment performed in accordance with animal welfare and ethics, and the procedures were approved by the Animal Care and Use Committee of Northwest A&F University (DKLS2021017).
Four experimental diets were designed, namely RD, high-fat diet (HFD) or the HFD supplemented with 0·3 or 0·6 mg/kg of nano-Se (HSe0·3 or HSe0·6). The lipid levels of RD and HFD are 48 and 88 g/kg(Reference Liu, Yu and Wang14). According to Liu et al. (Reference Liu, Yu and Li15) and Zheng et al., the nano-Se levels are 0·3 and 0·6 mg/kg in HSe0·3 and HSe0·6, respectively. Supplementary Table 1 (online Supplementary Table S1) shows the detailed information of ingredients composition and nutrient content of different diets. Grass carp was obtained from a fishery company in Xianyang (Shaanxi Province, China) and adapted to experimental conditions for 14 d.
Subsequently, 180 healthy grass carp (161·3 g ± 0·43 g) were selected and randomly divided into twelve net cages (2 m × 1 m × 1 m) in outdoor cement pools. And three cages were allocated for each kind of experimental diet (n 3). The feeding trial was conducted from 17 September to 15 November 2021. During the feeding experiment, fish were fed three times (08.00, 12.30 and 17.00) a day to reach apparent saturation. The cement pool was supplied with micro-flowing water to maintain the proper water quality. Water temperature decreased from 24°C to 18°C following the decrease in air temperature. The Dissolved oxygen > 5·0 mg/l; pH was 7·0 ± 0·5; ammonia content was 0·10 ± 0·03 mg/l.
Sample collection
After 60 d of feeding trial, fish were fasted for 24 h, anaesthetised with 90 mg/l MS-222 and then weighed. The white muscles at the junction of the dorsal fin and lateral scales of grass carp were rapidly separated. A small piece of left side muscle was frozen in liquid nitrogen to analyse the expression of genes and proteins. Meanwhile, about 1 cm × 0·5 cm × 0·5 cm of muscle was fixed in 4 % paraformaldehyde for histological analysis, and most of the rest sample was used for muscle texture detection. The muscle on the right side was cut into three equal parts. It is used to analyse nutrient content, WHC and the relevant indicators after refrigeration.
Nutritional composition analysis
The nutritional components of muscle, including moisture, crude lipid and crude protein, were determined according to AOAC method(Reference Horwitz and Latimer19). The freeze-drying method was adopted for muscle moisture, Soxhlet extraction method was adopted for crude lipid and Johan Kjeldahl method was adopted for crude protein(Reference Wang, Liu and Feng20).
The determination process of Se content in muscle was similar to the description of Wang et al. (Reference Wang, Li and Lu21). Briefly, about 0·2 g of freeze-dried tissue sample was put into a poly tetra fluoroethylene tube and pre-digested the sample with 5 ml of HNO3 at 120°C for 30 min. Added 1 ml HNO3 and 1 ml H2O2 into the pre-digested samples and carried out microwave digestion (Multiwave PRO, Anton Paar Gmbh). The procedure of microwave digestion was as follows: heating 6 min, reaching 120°C, constant temperature 1 min; heating 3 min, reaching 150°C, constant temperature 5 min and heating 5 min, reaching 180°C, constant temperature 10 min. Remove acid: 160°C, 40 min in an acid driving instrument. Added 5 ml 6 M HCl for reduction at 100°C for 30 min, and then the Se contents were measured using LC-AFS-8530 (Haiguang Instrument). Se standard solution comes from China National Standard Material Research Center (GSB 04-1751-2004).
The fatty acid in muscle was extracted by the chloroform-methanol method and modified from the method of Folch et al. (Reference Folch, Lees and Stanley22). Briefly, taken about 0·2 g of ground freeze-dried muscle sample and added 5 ml of prepared methanol: chloroform (v/v = 1:2) and fatty acid internal standard (C11, 0·83 mg/ml). The sample was shaken at a speed of 180 rpm for 1–2 h, filtered with quantitative filter paper, then added 4 ml ddH2O and and centrifuged at 3000 g for 5 min. The supernatant was dried for about 40 min with a nitrogen blower (Jin Tan, dcy, China). Added 1 ml 0·4 M KOH methanol solution and allowed to stand for 30 min. Then added 1 ml of pure chromatographic n-hexane, shook for 1 min. Added 2 ml ddH2O, 3000 g for 5 min and the fatty acid content in the sample was performed using a Gas Chromatography (Nexis GC-2030; Shimadzu Corporation) with Nexis SH-Rt-2560 column [100 m (l) 0·25 mm (ID) 0·20 μm film thickness; Shimadzu Corporation]. And the GC analysis protocols were carried out with reference to Ye et al. (Reference Ye, Li and Cao23).
Flesh quality determination
The instrument used in this experiment was the Texture Analyzer (TMS-pilot 753303, FTC). Two fish pieces (1 cm ×1 cm × 1 cm) of one fish were used for muscle texture analysis. One of the pieces used to determine the hardness (g), adhesiveness (g), cohesiveness (ratio), springiness (mm), gumminess (g) and chewiness (mj). Another piece is used to detect muscle shear force (N).
WHC of fish muscle was evaluated by dripping loss and cooking loss. For dripping loss, the sample was weighed (5 g ± 0·5) and then hung inside a sealed plastic bottle with a rope. After storing at 4°C for 24 h, weighed the sample again(Reference Ali, Rajput and Li24). For cooking loss, the sample was removed from subcutaneous fat and connective tissue on the surface and weighed (5 g ± 0·5), then put in sealed plastic bags and water bath at 80°C for 30 min. After air drying, the juice on the surface of the sample was absorbed with absorbent paper and then weighed the sample(Reference Jiang, Wen and Liu25). The dripping loss and cooking loss were calculated as follows:
Dripping loss (%) = (initial sample weight−final sample weight)/initial sample weight × 100.
Cooking loss (%) = (raw fillet weight−cooked fillet weight)/raw fillet weight × 100.
Use a pH-meter (PHS-3C, Leici) to measure the pH value of fish meat in fish/water (1:10, mg/ml) homogenate after being stored at 4°C for 24 h (pH24 h). The malondialdehyde content of fish meat after being stored at 4°C for 48 h (MDA48 h) was measured using the kit No. A003-1-2 from Nanjing Jiancheng Bioengineering Institute, Nanjing, China.
Histological evaluation
Muscle fixed with 4 % paraformaldehyde was embedded in paraffin and about 5 μm-thick stained with haematoxylin and eosin. An optical microscope (Nikon Ni-U) was used to observe and take photos. As described by Ma et al. (Reference Ma, Feng and Wu11), fibre density was calculated as the number of fibres per mm2 of muscle cross-sectional area. Assuming that muscle fibres were roughly cylindrical, calculated the average diameter of muscle fibres in the visual field according to S = πr2 (S and r represent area and radius, respectively). More than 1000 muscle fibres were measured for each group.
Real-Time PCR analysis
The steps were as follows: the total RNA in the muscle sample was extracted with AG RNAex Pro Reagent from Accurate Biotechnology (Hunan) Co., Ltd. Reverse transcription reagent: HiScript II Q Select RT SuperMix (Vazyme). qPCR reagent: ChamQ SYBR qPCR Master Mix (Vazyme). The reaction volume was 10 μl, including 1 μl of template cDNA, 5 μl of SYBR Mix, the forward and reverse primers (10 p mol/l) (0·2 μl) and 3·6 μl of ddH2O. The reaction condition was 95°C for 2 min, 40 cycles (95°C for 5 s) and 60°C for 30 s, in the end, a melting curve analysis in the range of 60–95°C to verify that a single PCR product was produced. The primer sequences are listed in Supplementary Table S2. The β-actin was selected as the reference gene in this research. And the results were analysed using the 2–ΔΔCt method(Reference Livak and Schmittgen26). The primers amplified efficiency reached 97–102 %.
Western blot
The western blot was carried out in accordance with Liu et al. (Reference Liu, Yu and Li15), and antibodies used in this study include glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000, #GB11002, Servicebio), PI3 Kinase p110 (1:1000, #4255, Cell Signaling Technology), Serine/threonine kinase (AKT) (1:1000, #9272, Cell Signaling Technology), mTOR (1:1000, #2983, Cell Signaling Technology), p70 S6 Kinase alpha (1:1000, #WL03839, Wanlei Bio.), MyoD (1:1000, #WL04662, Wanlei Bio.), adipose triglyceride lipase (ATGL) (1:1000, #ab99532, Abcam), amp-activated protein kinase (AMPK) (1:1000, #5831, Cell Signaling Technology) and HSL (1:1000, #WL02643, Wanlei Bio.). The gray level of the image was analysed using Image J software. The reference protein (GAPDH) was used to calculate the relative expression of a target protein.
Statistical analyses
All data in this study were expressed as mean ± sd. SPSS 25·0 was used for one-way ANOVA. Levene’s test was used for the homogeneity test of data variance, and multiple comparisons were used for significance analysis. P values less than 0·05 were considered statistically significant. In addition, the histological pictures were measured and processed by Image J.
Results
Growth parameters of grass carp
The growth parameters of grass carp are shown in Supplementary Table S3. The specific growth rate of grass carp had no significant change between RD and HFD groups (P > 0·05). However, the specific growth rate of grass carp in HSe0·3 group and HSe0·6 group showed an increasing trend compared with the HFD group, although no significant difference was observed (P > 0·05).
The nutritional components in grass carp muscle
Figure 1(a), (b) and (c) show the Se contents in muscle, hepatopancreas and intestine. Compared with the RD, HFD significantly reduced the content of Se in hepatopancreas (P < 0·05). No significant change of Se in muscle and intestine was observed between RD and HFD groups. However, HFD supplied with nano-Se markedly increased the content of Se in muscle, hepatopancreas and intestine compared with the group HFD, except for the HSe0·3 group in muscle.

Fig. 1. Effects of dietary nano-Se on the nutritional components in muscle of grass carp. Se levels in the (a) muscle, (b) hepatopancreas and (c) intestine. The (d) moisture, (e) crude protein and (f) crude lipid content in muscle. (g) PCA of fatty acid content in muscle of grass carp fed with different diets (n 4). (h) EPA and (I) DHA content. Values were presented as mean ± sds (n 3). Significant differences were indicated by different letters (P < 0·05).
Additionally, the changes of nutritional components including moisture, crude protein and crude lipid in muscle are shown in Fig. 1(d), (e) and (f), respectively. Compared with the RD, HFD significantly increased the lipid level and reduced the protein content in muscle (P < 0·05). In contrast, 0·3 or 0·6 mg/kg of nano-Se supplement in HFD significantly reduced the lipid level and increased the protein content in muscle (P < 0·05). There was no significant difference in the moisture level of muscle among the four groups.
Fatty acid profiles in the muscle of grass carp are shown in Supplementary Table S4. PCA is applied to analyse the fatty acid profiles in muscle (Fig. 1(g)). The result of PCA clearly shows that the samples of the RD group and HFD group were well distinguished in the distribution map. However, the samples of HSe0·3 and HSe0·6 groups have overlapping parts with those of the RD and HFD groups. Especially, EPA (C20:5n3) and DHA (C22:6n3) contents in the muscle of HFD group significantly decreased compared with that in the RD group (P < 0·05). Interestingly, compared with HFD group, the DHA content in the muscle of the HSe0·6 groups significantly increased (Fig. 1(h) and (i)) (P < 0·05).
Water holding capacity in grass carp muscle
The dripping loss and cooking loss of grass carp muscle are shown in Fig. 2(a) and (b). HFD markedly increased the dripping loss of grass carp muscle compared with the RD (P < 0·05). But compared with HFD, the nano-Se in HFD significantly decreased the dripping loss in grass carp muscle (P < 0·05). Cooking loss of grass carp muscle also showed an increasing trend in HFD group compared with the RD group. And the cooking loss of grass carp muscle showed decreasing trend in the HSe0·3 group and the HSe0·6 group in comparison with the HFD group. However, there was no significant difference in cooking loss between different groups (P > 0·05).

Fig. 2. The water holding capacity (WHC) included the (a) dripping loss and (b) cooking loss. (c) pH24 h and (d) MDA48 h of grass carp in muscle. The pH24 h represents the pH value after being stored at 4°C for 24 h; MDA48 h represents the malondialdehyde value after being stored at 4°C for 48 h. The heatmap (e) represents texture quality changes of skeletal muscle of grass carp (red, high index; blue, low index). Values were presented as mean ± sds (n 3). Significant differences were indicated by different letters (P < 0·05).
Changes of pH24 h and MDA48 h in grass carp muscle
As presented in Fig. 2(c), there was no significant difference between RD group and HFD group in the pH value of grass carp flesh muscle at 24 h. However, dietary nano-Se at 0·3 or 0·6 mg/kg significantly increased the pH24 h value of grass carp muscle in the RD and the HFD groups (P < 0·05).
Besides, as presented in Fig. 2(d), the HFD caused a significant increase of MDA48 h level in grass carp muscle in comparison with that of the RD (P < 0·05). And the level of MDA48 h in grass carp muscle showed decreasing trends in HSe0·3 and HSe0·6, but no significant difference was observed (P > 0·05).
Textural quality of grass carp muscle
The texture characteristics of grass carp muscle are shown in Fig. 2(e). Here, the HFD markedly increased the cohesiveness of grass carp muscle compared with the RD (P < 0·05). The hardness of grass carp muscle decreased from 10·38 (RD group) to 8·79 (HFD group), but no significant difference was observed (P > 0·05).
The adhesiveness, springiness and chewiness showed an increasing trend in the HSe0·6 group in comparison with the HFD group, but no significant difference was observed. The springiness and chewiness significantly increased in the group HSe0·6 in comparison with RD the group (P < 0·05).
Changes in muscle histology
Muscle fibres were observed under the microscope (Fig. 3(a)). Obviously, muscle fibre density significantly decreased and muscle fiber diameter significantly increased in the group HFD compared with the group RD (P < 0·05) (Fig. 3(b) and (c)). Besides, dietary nano-Se at 0·3 or 0·6 mg/kg markedly increased the muscle fibre density and decreased the muscle fibre diameter compared with the group HFD (P < 0·05).

Fig. 3. Effects of dietary nano-Se on muscle morphology of grass carp fed with high-fat diet (HFD). (a) Microstructure observation (H.E., scale bar, 100 μm); (b) muscle fibre density and (c) diameter. More than 1000 muscle fibres were measured for each group. Significant differences were indicated by different letters (P < 0·05).
The mRNA expression related to lipid deposition, protein synthesis and muscle fibre formation
The expression levels of lipolysis-related genes are shown in Fig. 4(a)–(d). Compared with the RD, the mRNA levels of PPARα, ATGL, LPL and HSL were downregulated in HFD group compared with the RD group, although the significant differences were only observed in LPL and HSL. However, dietary nano-Se at 0·3 or 0·6 mg/kg markedly induced the expression of those four genes in comparison with HFD (P < 0·05).

Fig. 4. Effects of dietary nano-Se on the gene mRNA expression levels of (a) PPARα, (b) ATGL, (c) LSL and (d) HSL and (e) the proteins expression levels of p-AMPK, ATGL and HSL related to lipid deposition. Values were presented as mean ± sds (n 3). Significant differences were indicated by different letters (P < 0·05).
In Fig. 5(a) and (b), HFD significantly downregulated the expression levels of PI3K and AKT compared with RD (P < 0·05), and the supplement of nano-Se significantly increased the expression levels PI3K and AKT compared with HFD (P < 0·05). Then, in Fig. 5(c) and (d), HFD also decreased the mRNA levels of TOR and S6K1 compared with RD, although no significant difference was observed. And compared with HFD, the supplement of nano-Se markedly enhanced the expression of TOR gene (P < 0·05). Besides, dietary nano-Se at 0·3 mg/kg in HFD markedly enhanced S6K1 gene expression (P < 0·05) compared with the HFD. Interestingly, the gene expression levels of 4E-BP1 and GLDH were not significantly different among the four groups (Fig. 5(e) and (f)).

Fig. 5. Effects of dietary nano-Se on the gene mRNA expression levels of (a) PI3K, (b) AKT, (c) TOR, (d) S6K1, (e) 4E-BP1 and (f) GLDH and (g) the proteins expression levels of PI3K, AKT, TOR and S6K related to protein synthesis. Values were presented as mean ± sds (n 3). Significant differences were indicated by different letters (P < 0·05).
The mRNA expression level of MyoG was markedly downregulated in HFD group compared with that in the RD group (P < 0·05) (Fig. 6(a)). And then, the mRNA expression levels of MyoG and MyoD were both significantly upregulated by 0·3 or 0·6 mg/kg of dietary nano-Se compared with the HFD (P < 0·05).

Fig. 6. Effects of dietary nano-Se on the gene mRNA expression levels of (a) MyoG and MyoD and (b) the protein expression levels of MyoD related to muscle fibre formation. Values were presented as mean ± sds (n 3). Significant differences were indicated by different letters (P < 0·05).
The expression of proteins related to lipid deposition, protein synthesis and muscle fibre formation
In Fig. 4(e), HFD depressed the expression level of p-AMPKα significantly in muscle compared to RD, but HFD supplemented with 0·3 or 0·6 mg/kg nano-Se markedly enhanced the level of p-AMPKα compared with HFD (P < 0·05). Besides, HFD decreased the HSL protein expression level in muscle, but the nano-Se in HFD markedly increased the ATGL and HSL protein expression levels in comparison with HFD (P < 0·05).
Similar to the gene expression results, in Fig. 5(g), HFD markedly decreased the protein expression levels of PI3K, TOR and S6K in muscle. However, HFD supplemented with 0·3 or 0·6 mg/kg nano-Se markedly upregulated the protein expression of PI3K, AKT, TOR and S6K compared with HFD (P < 0·05).
Besides, as shown in Fig. 6(b), there was no significant difference in protein expression levels of MyoD between the RD and the HFD groups. But nano-Se at 0·3 or 0·6 mg/kg in HFD markedly increased the MyoD protein expression levels compared with the HFD (P < 0·05).
Discussion
Nano-Se in high-fat diet regulated the lipid deposition in the muscle of grass carp
High-fat content in fish muscle is considered to be an important factor leading to the softening of fish texture, and it will also shorten the storage time of fish(Reference Bjørnevik, Espe and Beattie27). Malondialdehyde, as a product of lipid peroxidation, can be used as an early indicator of the freshness of fish with high-fat content(Reference Zhao, Chong and Tang28). It is widely reported that HFD leads to lipid accumulation in the muscle of cultured fish, such as Nile tilapia (O. niloticus)(Reference Lv, Ma and Hu2) and grass carp (C. idella)(Reference Yuan, Liang and Liu29), and leads to an increase in lipid peroxidation in fish(Reference Lv, Ma and Hu2,Reference Liu, Yu and Wang14) . In this study, the HFD significantly promoted the deposition of lipid in the muscle of grass carp. As such, obviously decreased hardness in HFD group was observed in comparison with RD group. Moreover, the HFD significantly increased MDA48 h level in muscle of grass carp compared with the RD, which means that the acceleration of lipid peroxidation and the reduction in the freshness of grass carp induced by HFD.
It has been confirmed that dietary Se reduced the crude lipid content in the whole body of fish(Reference Liu, Yu and Wang14). In this study, the addition of 0·3 or 0·6 mg/kg nano-Se notably reduced the lipid deposition in muscle which was promoted by the HFD. Moreover, compared with the RD, the addition of 0·6 mg/kg nano-Se in HFD significantly increased the springiness and chewiness of muscle in grass carp, which may enhance the firmness of grass carp muscle. In addition, the lipid peroxidation promoted by HFD decreased to some extent in the muscle of grass carp fed with HFD added with nano-Se. And these positive effect of dietary nano-Se is helpful to improve the taste and post mortem storage time of grass carp.
To further reveal the molecular mechanism of the decrease of lipid deposition by nano-Se, we further determined the expression level of genes and proteins related to lipid metabolism in the muscle of grass carp. AMPK is switched on by an increase in the AMP/ATP ratio and is considered the key regulator of lipid metabolism, which can switch on the catabolic pathway and ATP-consuming processes(Reference He, Guo and Tan30). HFD markedly decreased the p-AMPK expression level, indicating the inhibition of the AMPK pathway in grass carp caused by HFD in this research. As a result, the mRNA level and expression of HSL, an important lipolytic enzyme responsible for the hydrolysis of triacylglycerols(Reference Sun, Yang and Xiao31) significantly decreased. Meanwhile, the mRNA expression level of lipoprotein lipase, which releases fatty acids from lipoprotein-associated triacylglycerols(Reference Liu, Yu and Wang14,Reference Kaneko, Shirakami and Yamada32) was also significantly suppressed. Hence, these results may consequently contribute to the lipid accumulation in the muscle of grass carp fed with HFD. Our previous study has been reported that the addition of nano-Se decreased the levels of crude lipid in the hepatopancreas and muscle of grass carp(Reference Zheng, Jiang and Feng13). Similarly, in this study, the supplement of nano-Se decreased the deposition of lipid in the muscle through activating the AMPK pathway. One possible mechanism of this effect is that Se induced phosphorylation of AMPK, thus mediating the process of lipolysis. In response, the downstream proteins of AMPK, such as hormone-sensitive lipase (HSL) and ATGL, which is an enzyme that starts the hydrolysis of triacylglycerol were both significantly upregulated. Moreover, the expression of downstream genes of AMPK, including ATGL, LPL, HSL and peroxisome proliferators-activated receptors (PPARα), which is a transcription factor that promotes β-oxidation of fatty acids,(Reference Lee, Kim and Park33) was all markedly enhanced by the supplement of nano-Se. Above all, the evidence indicates that nano-Se can reduce the deposition of lipid in muscle by regulating the AMPK activity.
Surprisingly, nano-Se in HFD promoted the deposition of beneficial fatty acids of grass carp fed with HFD in this study. Fish has attracted much attention because it is rich in highly unsaturated fatty acids. Unfortunately, in this study, HFD significantly reduced EPA (C20:5n3) and DHA (C22:6n3) contents in the muscle of grass carp, which means declined nutritional quality of grass carp. However, in muscle, the DHA content markedly increased in HSe0·6 group in comparison with that in HFD group. And the EPA level also showed an increasing trend in HSe0·3 group and HSe0·6 group compared with that in HFD group, although no significant difference was observed. Its mechanism still needs to be explored in future study.
Nano-Se in high-fat diet promoted the protein synthesis in the muscle of grass carp
Protein plays a key role in human health and nutrition(Reference Han, Huang and Mahunu1). Fish is considered to be one of the best sources of protein. Therefore, protein content is an important index to evaluate fish quality. It has been reported that HFD decreased protein deposition in the muscle of Nile tilapia(Reference Lv, Ma and Hu2). In this study, HFD also markedly decreased crude protein deposition in the muscle of grass carp, which could be the output of lowered protein synthesis and increased protein breakdown(Reference Lv, Ma and Hu2). Interestingly, dietary nano-Se at 0·3 or 0·6 mg/kg markedly increased protein deposition in muscle of grass carp. This positive effect of dietary Se also can be confirmed by the study of Wang et al. (Reference Wang, Wang and Zhang16), in which Se-yeast increased protein deposition in rainbow trout muscle by promoting the synthesis of protein. Hence, the dietary nano-Se may also promote protein synthesis in muscle and contribute to the deposition of protein in grass carp.
Based on the above assumption, to confirm the change of synthesis of protein in the muscle of grass carp regulated by dietary Se, the expression of genes and proteins involved in the synthesis of protein were further determined in the muscle of grass carp. The mammalian target of rapamycin (mTOR) signalling pathway is very critical for protein synthesis of muscle, which is considered to play a central role in skeletal muscle nutrition and energy-sensing(Reference Wang, Liu and Feng20). And it has been reported that dietary Se can promote the synthesis of protein in rainbow trout through a persistently high activity of the TORC1 pathway(Reference Wang, Wang and Zhang16). And Zhao et al. (Reference Zhao, Barcus and Kim34) demonstrated that a high-Se diet leads to a 10–14 % increase in the concentration of protein in pig muscle, which is related to the activation signal of protein synthesis of mTOR and P70 S6K. In this research, the mRNA level and protein expression of TOR and S6K in muscle both decreased caused by HFD. However, dietary nano-Se markedly upregulated the mRNA level and proteins expression of TOR and S6K in muscle, which reveals that dietary nano-Se can activate the TOR pathway. As such, the increased protein deposition in the muscle of grass carp was observed.
The PI3K/AKT/TOR pathway plays a pivotal role in physiological processes, including cell proliferation, adhesion, migration, invasion, energy metabolism, protein synthesis and myoblast proliferation(Reference Li, Fu and Pang35). In the current study, the PI3K/AKT/TOR pathway in muscle of grass carp was obviously suppressed by the HFD. It may also be related to the lower Se content in the HFD. However, the expression levels of mRNA and protein of PI3K and AKT both significantly increased in HFD added with nano-Se, which indicates that nano-Se may promote the muscle protein synthesis by activating PI3K/AKT/TOR pathway, thus increasing the protein deposition in grass carp. In addition, the high retention rate of muscle protein obtained by dietary nano-Se may also be owing to the increase of lipid catabolism which can be saving protein.
Nano-Se in high-fat diet promoted the muscle fibre formation of grass carp
Fibre characteristic is a key factor affecting flesh quality(Reference Wang, Jiang and Alhamoud36). Many dietary factors affect the muscle fibre characteristic in fish, such as dietary lipid sources affected the number of white muscle fibres in rainbow trout(Reference Fauconneau, Andre and Chmaitilly37), and fish species with thin muscle fibres were firmer than species with thick muscle fibres(Reference Hurling, Rodell and Hunt38). In the present study, the HFD significantly reduced the density of muscle fibres and increased the diameter of muscle fibres. However, dietary nano-Se markedly reversed this trend. Further, studies have confirmed that fibre density has positive correlations with the scores of ‘chewiness’ and ‘firmness’(Reference Liang, Hu and Dong39). As such, chewiness and springiness of grass carp flesh were both increased in HSe0·6 group following the increase of muscle fibre density in this study.
To further reveal the mechanism of the muscle fibre formation promoted by dietary Se, the expression of genes and protein involved in muscle fibre formation were further determined in the muscle of grass carp. The development and growth of muscle is a complex dynamic process, including the recruitment of new muscle fibres (proliferation) and the growth of existing muscle fibres (hypertrophy)(Reference Fauconneau, Andre and Chmaitilly37). Myogenic determination, differentiation and muscle growth remain under the control of specific gene products belonging to myogenic factors of the MyoD family(Reference Wirth-Dzicioowska, Zimowska and Gajewska40). Mainly, myogenic determining factors (MyoD) and myogenin (MyoG) are critical transcription factors in the MyoD family which are essential in skeletal muscle development. MyoD is required for the specification and proliferation of myoblasts, and MyoG is required for the differentiation of myoblasts into multinucleated myotube which is an important structure of muscle fibres. HFD significantly depressed the expression of MyoG of grass carp in this research, suggesting the inhibition of muscle fibre formation by HFD. As such, the muscle fibre density of grass carp was accordingly reduced. However, the nano-Se markedly upregulated the mRNA level of MyoG and MyoD and expression of MyoD in the muscle of grass carp, indicating the increase of proliferation of myoblasts and differentiation of myoblasts into multinucleated myotubes.
In addition, the increase of muscle fibre density and the decrease of fish muscle fibre diameter means the increase of connective tissue in the muscle, which improves the WHC of muscle(Reference Rant, Radzik-Rant and Swiatek41). WHC is an important factor affecting the texture of fish and can be evaluated by cooking loss and dripping loss. HFD has been confirmed to cause a significant decrease in WHC in the muscle of Nile tilapia (O. niloticus)(Reference Lv, Ma and Hu2). Similarly, the HFD significantly increased the dripping loss of muscle in grass carp in this research. But the increase of muscle fibre density and the decrease of fish muscle fibre diameter owing to the addition of 0·3 or 0·6 mg/kg nano-Se in HFD may be the important reason for the markedly reduced dripping loss of muscle in grass carp. In addition, the decreased pH value of muscle post mortem may affect both muscle proteins and endogenous proteolytic enzymes, thus reducing the WHC of muscle(Reference Hultmann, Phu and Tobiassen42). In this study, the post-mortem muscle pH24 h of the grass carp in the HSe0·3 group and the HSe0·6 group improved considerably compared with the RD and the HFD group. Hence, the increased muscle pH by dietary nano-Se may be another reason for the improved WHC of grass carp muscle.
Conclusion
The results of the present study demonstrated that dietary nano-Se at 0·3 or 0·6 mg/kg significantly reduced lipid content, dripping loss and fibre diameter, but increased protein content, post-mortem pH24 h and muscle fibre density of muscle in grass carp fed with HFD. Notably, dietary nano-Se decreased lipid deposition in muscle by activating the AMPK pathway and increased protein synthesis and fibre formation in muscle via TOR and MyoD pathways. In summary, dietary nano-Se can regulate the nutrients deposition and muscle fibre formation in grass carp fed with HFD, which exhibit potential benefit for improving flesh quality of grass carp fed with HFD. However, the improved effects of dietary nano-Se on flesh quality of grass carp still need to be further confirmed using market size grass carp.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114523000892
Acknowledgements
This research was funded by the National Key Research and Development Program of China (Project No. 2019YFD0901002), Northwest A & F University Young Talent Training Program (2452018030), Postdoctoral Science Foundation of China (2016M600821) and Key Research and Development Project of Shaanxi Province (2018NY-008).
S. L.: Methodology, investigation, data curation, writing—original draft, formal analysis; H. Y.: conceptualisation, methodology, resources, supervision, project administration, funding acquisition, writing—review and editing; L. Z.: methodology, investigation, data curation; X. Z.: methodology, investigation, data curation; P. L.: investigation; C. W.: investigation G. L.: investigation; P. H.: investigation; C. Z.: investigation; H. J.: resources.
There are no conflicts of interest to declare.