For nearly two decades it has been recognised that the immune system plays a role in depression. Reference Smith1 Systemic immune activation has been documented in major depression, with reports of increased levels of pro-inflammatory cytokines Reference Maes, Bosmans, Meltzer, Scharpe and Suy2–Reference Mikova, Yakimova, Bosmans, Kenis and Maes4 and changes in the acute phase protein response, notably enhancement of positive and diminution of negative acute phase proteins. Reference Sluzewska, Rybakowski, Bosmans, Sobieska, Berghmans and Maes5,Reference Berk, Wadee, Kuschke and O'Neill-Kerr6 C-reactive protein (CRP) is a positive acute response protein that marks systemic inflammation. Reference Pepys and Baltz7,Reference Deodhar8 Elevated levels of circulating CRP have been found in depression in both clinical and population studies. Reference Pikhart, Hubacek, Kubinova, Nicholson, Peasey and Capkova9–Reference Kop, Gottdiener, Tangen, Fried, McBurnie and Walston14 This literature, however, stems from cross-sectional association data that are unable to clarify whether systemic inflammation precedes the onset of depressive symptoms or occurs as part of the somatic manifestations of the depressive phenotype.
There is some evidence to suggest that systemic inflammation might be a risk factor for depression. In animal models, systemic administration of pyrogens to mice induces sickness behaviour analogous to depressive symptomatology in humans. Reference Bluthe, Michaud, Poli and Dantzer15 Depression is commonly reported among human patients following exposure to cytokine-based immunotherapy, as seen in the treatment with interleukin-2 of metastatic renal cell carcinoma and metastatic melanoma, Reference Dutcher16–Reference Dutcher, Logan, Gordon, Sosman, Weiss and Margolin18 and in the treatment with interferon-α of patients with hepatitis C. Reference Dell'Osso, Pini, Maggi, Rucci, Del Debbio and Carlini19 To date, findings from two prospective studies suggest that systemic inflammation might precede the development of depressive symptoms. Reference van den Biggelaar, Gussekloo, de Craen, Frolich, Stek and van der Mast20,Reference Milaneschi, Corsi, Penninx, Bandinelli, Guralnik and Ferrucci21 However, it is unclear to what extent these results can be generalised beyond the elderly population. Reference Janssen, Beekman, Comijs, Deeg and Heeren22
We hypothesised that circulating inflammatory markers might be associated with an increased risk for depression across the full adult-age spectrum. Although CRP levels are traditionally only elevated in severe inflammation, newer assays with improved sensitivity (high-sensitivity CRP, hsCRP) are able to measure CRP in healthy individuals, permitting an exploration of the posited association between subclinical systemic inflammation and risk of depression. We aimed to investigate serum hsCRP concentration as a risk marker for major depressive disorder in women, using longitudinal data extending over a decade.
Method
Study design and participants
This project was conducted as part of the Geelong Osteoporosis Study (GOS), a population-based study originally designed for monitoring the epidemiology of osteoporosis, but more recently expanded to examine psychiatric illness and other health disorders. The target population was the Barwon Statistical Division in south-eastern Australia and the sample was randomly selected from the Australian Commonwealth electoral roll. All women listed on the electoral roll for the study region fulfilled the inclusion criteria. Exclusion criteria included inability to provide informed consent, contact failure and death. Reference Henry, Pasco, Nicholson, Seeman and Kotowicz23 A cohort of 1494 women recruited into the GOS during the period 1994–7 has been prospectively followed for a decade. Collection of serum, questionnaire data and clinical measures was performed concurrently at baseline during the period 1994–7. During the period 2004–7, a total of 857 women participated in a psychiatric assessment and of these, 822 women also provided serum for biochemical analysis and were thus included in this retrospective cohort study. Written, informed consent was obtained from all participants. The Human Research Ethics Committee, Barwon Health, approved the study.
Measurement of exposure variables
Serum samples were collected in the morning following an overnight fast and stored at –80°C until batch analysis. Serum hsCRP was measured by the Roche immunoturbidimetric ‘CRP’ and ‘C-reactive protein (latex) high sensitivity’ methods. Specimens were initially analysed using the high-sensitivity assay, which has a range of 0.1–20 mg/l. Specimens with results above 20 mg/l were re-analysed using the CRP assay, which has a range of 3–480 mg/l. The long-term inter-assay coefficient of variation is <10% at 1 mg/l and <5% at 5 mg/l.
Height and weight were measured to the nearest 0.1 cm and 0.1 kg respectively, and body mass index (BMI) calculated in kg/m2. Waist circumference was measured in a horizontal plane with a narrow, non-elastic tape measure. Self-reported details of medication use and lifestyle were documented by questionnaire. Cigarette smoking was recorded as never, past or current. Alcohol consumption was recognised if alcohol was consumed daily. Activity level was described as very active or active if vigorous or light exercise was performed regularly; otherwise, individuals were classified as sedentary. Socioeconomic status was ascertained using Socio-Economic Indexes for Areas scores based on 1996 census data from the Australian Bureau of Statistics. These data were used to derive an index of relative socioeconomic disadvantage (IRSD) that was categorised into five groups, according to quintiles of IRSD for the study region. Medication use was classified as current if used regularly at the time of assessment for non-steroidal anti-inflammatory drugs (NSAIDs; including aspirin), oral glucocorticoids, antidepressants, oral contraceptives and hormone therapy. Exposure to diseases, including pernicious anaemia, cancer and systemic lupus erythematosus, was documented by self-report. Cases of rheumatoid arthritis were identified from specialist registers in the region.
Measurement of outcome
The Structured Clinical Interview for DSM–IV–TR Research Version, Non-patient edition (SCID–I/NP) Reference First, Spitzer, Gibbon and Williams24 was used to identify women with a lifetime history of major depressive disorder and to determine age at onset. Trained personnel who were masked to biochemical and questionnaire data conducted the psychiatric interviews.
Statistics
Standard descriptive statistics were used to characterise participants. Serum hsCRP concentrations were natural log transformed (ln-hsCRP) to normalise the data before statistical analysis. Participants were grouped into tertiles of hsCRP for descriptive purposes. Two major analyses were performed.
High-sensitivity CRP and rate of major depressive disorder
Based on data from all 822 women, Poisson regression models were used to estimate the rate of diagnosis for each standard deviation increase in ln-hsCRP after adjusting for age, anthropometry, and demographic, health and lifestyle factors. Models were also tested for interaction terms. Rate ratios (RR) are expressed together with 95% confidence intervals (CI).
High-sensitivity CRP and risk for de novo major depressive disorder
One hundred and seventy-eight individuals were not included in this analysis because they had experienced an episode of major depressive disorder prior to baseline or within the first year after baseline. The group of 644 eligible participants was followed from baseline until a first episode of depression or until the end of the 10-year follow-up period. The association between baseline serum hsCRP (expressed in standard deviation units) and the development of de novo major depressive disorder was examined using Cox proportional hazards regression models, using age as the time axis and adjusting for confounding variables. The advantage of using age as the time axis is that it permits the baseline hazard to change as a function of age, thereby accounting for any confounding effects of age. Reference Korn, Graubard and Midthune25 The proportional hazards assumptions were checked using Schoenfeld residuals before and after adjusting for confounders. Statistical analyses were performed using Stata (release 9.0, StataCorp, College Station, Texas, USA) and Minitab (version 15; Minitab, State College, Pennsylvania, USA) for Windows.
Results
High-sensitivity CRP and rate of major depressive disorder
The distribution of hsCRP in this population was positively skewed with a median value of 1.91 mg/l (interquartile range, IQR = 0.88–3.98). Few women (54/822, 6.6%) had hsCRP values greater than 10 mg/l, indicating an acute inflammatory response. Participant characteristics are listed in Table 1. Serum ln-hsCRP was weakly correlated with age (r = 0.1, P = 0.001), and moderately correlated with all indices of adiposity, including weight, BMI and waist circumference (r = 0.4, 0.5 and 0.5 respectively, all P<0.001), which persisted after adjusting for age (all P<0.001). In multivariable models adjusted for age and weight, lifestyle factors associated with increased mean ln-hsCRP included physical inactivity (sedentary 28% greater than active/very active) and use of oral contraceptives or hormone therapy (173% and 25% greater than non-users respectively) (all P<0.05). The mean ln-hsCRP for NSAID users was also greater than for non-users.
Table 1 Baseline characteristics for all participants
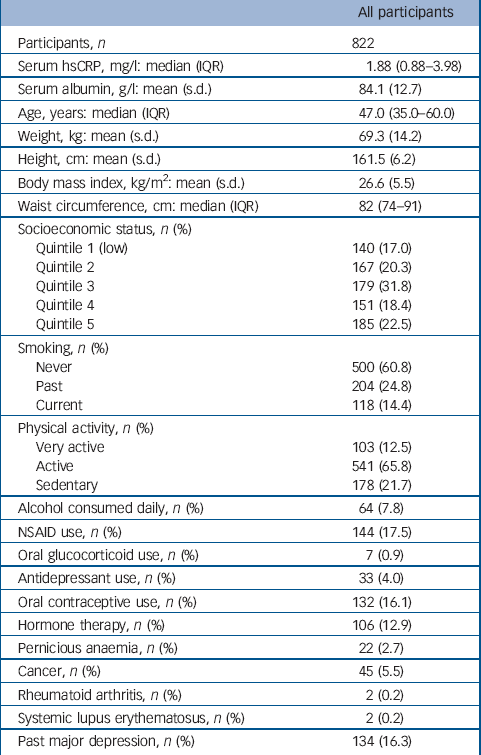
| All participants | |
|---|---|
| Participants, n | 822 |
| Serum hsCRP, mg/l: median (IQR) | 1.88 (0.88–3.98) |
| Serum albumin, g/l: mean (s.d.) | 84.1 (12.7) |
| Age, years: median (IQR) | 47.0 (35.0–60.0) |
| Weight, kg: mean (s.d.) | 69.3 (14.2) |
| Height, cm: mean (s.d.) | 161.5 (6.2) |
| Body mass index, kg/m2: mean (s.d.) | 26.6 (5.5) |
| Waist circumference, cm: median (IQR) | 82 (74–91) |
| Socioeconomic status, n (%) | |
| Quintile 1 (low) | 140 (17.0) |
| Quintile 2 | 167 (20.3) |
| Quintile 3 | 179 (31.8) |
| Quintile 4 | 151 (18.4) |
| Quintile 5 | 185 (22.5) |
| Smoking, n (%) | |
| Never | 500 (60.8) |
| Past | 204 (24.8) |
| Current | 118 (14.4) |
| Physical activity, n (%) | |
| Very active | 103 (12.5) |
| Active | 541 (65.8) |
| Sedentary | 178 (21.7) |
| Alcohol consumed daily, n (%) | 64 (7.8) |
| NSAID use, n (%) | 144 (17.5) |
| Oral glucocorticoid use, n (%) | 7 (0.9) |
| Antidepressant use, n (%) | 33 (4.0) |
| Oral contraceptive use, n (%) | 132 (16.1) |
| Hormone therapy, n (%) | 106 (12.9) |
| Pernicious anaemia, n (%) | 22 (2.7) |
| Cancer, n (%) | 45 (5.5) |
| Rheumatoid arthritis, n (%) | 2 (0.2) |
| Systemic lupus erythematosus, n (%) | 2 (0.2) |
| Past major depression, n (%) | 134 (16.3) |
In total, 151 women had at least one major depressive episode during 8090 person-years of follow-up and 671 remained depression-free. Those who experienced depression were younger (median 41.0 years, IQR = 33.0–52.0 v. 49.0 years, IQR = 36.0–62.0; P<0.001), more were current smokers (n = 35, 23.2% v. n = 83, 12.4%; P = 0.001) and users of antidepressants (n = 11, 7.3% v. n = 22, 3.3%; P = 0.023), and more had a history of major depressive disorder (n = 103, 68.2% v. n = 31, 4.6%; P<0.001). The rate of major depressive disorder was 18.7 per 1000 person-years (95% CI 15.9–21.9). For each standard deviation increase in ln-hsCRP, the rate of depression increased by 18%, after adjusting for age, weight, use of NSAIDs and past major depressive disorder (RR = 1.18, 95% CI 0.99–1.42, P = 0.069). None of the other anthropometric, demographic, medication or lifestyle factors were identified as confounders.
High-sensitivity CRP and risk for de novo major depressive disorder
Participant characteristics categorised into tertiles of hsCRP are shown in Table 2. The median concentration of hsCRP increased with weight, BMI and waist circumference, and those in the low tertile of hsCRP (<1.12 mg/l) were younger, more likely to be physically active and not to use oral contraceptives, and less likely to be socially disadvantaged. There were 48 cases of de novo major depressive disorder identified over 5827 person-years of follow-up. The unadjusted hazard ratio for major depressive disorder increased by 41% for each standard deviation increase in ln-hsCRP (HR = 1.41, 95% CI 1.07–1.86, P = 0.015). The Kaplan–Meier survival plot (Fig. 1) shows that the probability of remaining depression-free was lowest for women in the upper hsCRP tertile (>2.97 mg/l) and highest for those in the lowest tertile. Multivariable models developed sequentially by adjusting for potential confounders included contributions from ln-hsCRP, weight, current smoking and use of NSAIDs. Thus, for each standard deviation increase in ln-hsCRP there was an independent 44% increase in risk for major depressive disorder (adjusted HR = 1.44, 95% CI 1.04–1.99, P = 0.026). Further adjustment for lifestyle factors, comorbid disease and use of other medications (oral contraceptives or hormone therapy) failed to explain the observed increased risk for depression.
Table 2 Baseline characteristics for participants categorised into tertiles of high-sensitivity C-reactive protein (hsCRP)
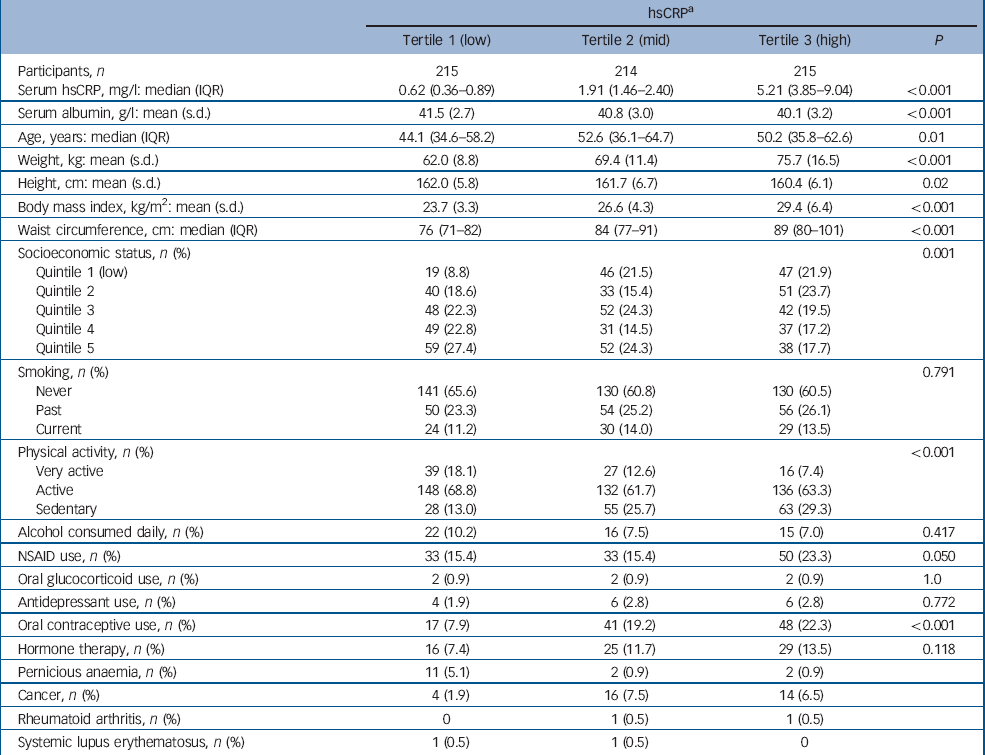
| hsCRPa | ||||
|---|---|---|---|---|
| Tertile 1 (low) | Tertile 2 (mid) | Tertile 3 (high) | P | |
| Participants, n | 215 | 214 | 215 | |
| Serum hsCRP, mg/l: median (IQR) | 0.62 (0.36–0.89) | 1.91 (1.46–2.40) | 5.21 (3.85–9.04) | < 0.001 |
| Serum albumin, g/l: mean (s.d.) | 41.5 (2.7) | 40.8 (3.0) | 40.1 (3.2) | < 0.001 |
| Age, years: median (IQR) | 44.1 (34.6–58.2) | 52.6 (36.1–64.7) | 50.2 (35.8–62.6) | 0.01 |
| Weight, kg: mean (s.d.) | 62.0 (8.8) | 69.4 (11.4) | 75.7 (16.5) | < 0.001 |
| Height, cm: mean (s.d.) | 162.0 (5.8) | 161.7 (6.7) | 160.4 (6.1) | 0.02 |
| Body mass index, kg/m2: mean (s.d.) | 23.7 (3.3) | 26.6 (4.3) | 29.4 (6.4) | < 0.001 |
| Waist circumference, cm: median (IQR) | 76 (71–82) | 84 (77–91) | 89 (80–101) | < 0.001 |
| Socioeconomic status, n (%) | 0.001 | |||
| Quintile 1 (low) | 19 (8.8) | 46 (21.5) | 47 (21.9) | |
| Quintile 2 | 40 (18.6) | 33 (15.4) | 51 (23.7) | |
| Quintile 3 | 48 (22.3) | 52 (24.3) | 42 (19.5) | |
| Quintile 4 | 49 (22.8) | 31 (14.5) | 37 (17.2) | |
| Quintile 5 | 59 (27.4) | 52 (24.3) | 38 (17.7) | |
| Smoking, n (%) | 0.791 | |||
| Never | 141 (65.6) | 130 (60.8) | 130 (60.5) | |
| Past | 50 (23.3) | 54 (25.2) | 56 (26.1) | |
| Current | 24 (11.2) | 30 (14.0) | 29 (13.5) | |
| Physical activity, n (%) | < 0.001 | |||
| Very active | 39 (18.1) | 27 (12.6) | 16 (7.4) | |
| Active | 148 (68.8) | 132 (61.7) | 136 (63.3) | |
| Sedentary | 28 (13.0) | 55 (25.7) | 63 (29.3) | |
| Alcohol consumed daily, n (%) | 22 (10.2) | 16 (7.5) | 15 (7.0) | 0.417 |
| NSAID use, n (%) | 33 (15.4) | 33 (15.4) | 50 (23.3) | 0.050 |
| Oral glucocorticoid use, n (%) | 2 (0.9) | 2 (0.9) | 2 (0.9) | 1.0 |
| Antidepressant use, n (%) | 4 (1.9) | 6 (2.8) | 6 (2.8) | 0.772 |
| Oral contraceptive use, n (%) | 17 (7.9) | 41 (19.2) | 48 (22.3) | < 0.001 |
| Hormone therapy, n (%) | 16 (7.4) | 25 (11.7) | 29 (13.5) | 0.118 |
| Pernicious anaemia, n (%) | 11 (5.1) | 2 (0.9) | 2 (0.9) | |
| Cancer, n (%) | 4 (1.9) | 16 (7.5) | 14 (6.5) | |
| Rheumatoid arthritis, n (%) | 0 | 1 (0.5) | 1 (0.5) | |
| Systemic lupus erythematosus, n (%) | 1 (0.5) | 1 (0.5) | 0 | |
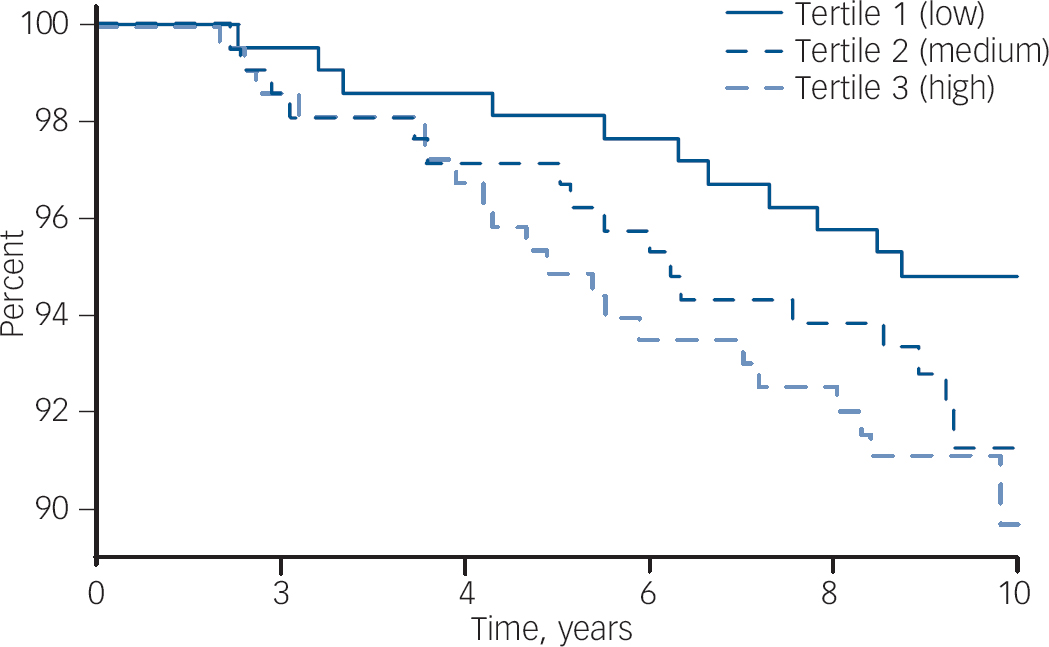
Fig. 1 Survival plot (Kaplan–Meier) showing the probability of remaining free of de novo major depressive disorder for women stratified into tertiles of hsCRP. The concentration of hsCRP in each tertile is: low, <1.12 mg/l; mid, 1.12–2.97 mg/l; and high, >2.97 mg/l.
Discussiong
To our knowledge, this is the first study to investigate hsCRP as a risk marker for de novo major depressive disorder in a population-based, longitudinal study spanning the full adult range. We report an apparent 44% increase in the risk for major depressive disorder for each standard deviation increase in ln-hsCRP among women who had never experienced a prior episode of depression. This association was not explained by differences in weight, lifestyle, medication use or comorbid disease. These findings were supported by a trend for a positive association between circulating hsCRP levels and the rate of major depression over the ensuing decade.
The moderate correlations we report between circulating CRP levels and indices of adiposity, both general and visceral, have been documented previously. Reference Visser, Bouter, McQuillan, Wener and Harris26–Reference Raitakari, Mansikkaniemi, Marniemi, Viikari and Raitakari30 Excess body fat enhances circulating CRP and may be considered a state of low-grade inflammation as adipose tissue produces interleukin-6 and tumor necrosis factor-α and is involved in the regulation of other cytokines. Reference Yudkin, Stehouwer, Emeis and Coppack28 Overweight and obesity are common in women with major depression. Reference Williams, Pasco, Henry, Jacka, Dodd and Nicholson31–Reference Kloiber, Ising, Reppermund, Horstmann, Dose and Majer33 The impact of obesity as a confounder in the relationship between hsCRP and major depressive disorder was thus identified and addressed by including weight as a covariate in the regression models. The association between hsCRP and depression was sustained, albeit attenuated, after adjusting for weight. Similar patterns were observed by substituting weight with other indices of adiposity such as BMI or waist circumference. Consequently, the relationship between hsCRP and major depression was deemed independent of adiposity.
C-reactive protein is regarded as a key indicator of an inflammatory process. Reference Reichenberg, Yirmiya, Schuld, Kraus, Haack and Morag34 Several cross-sectional studies have examined the association between CRP and depression in population-based samples. Most reported a positive association, Reference Ford and Erlinger10,Reference Panagiotakos, Pitsavos, Chrysohoou, Tsetsekou, Papageorgiou and Christodoulou11,Reference Penninx, Kritchevsky, Yaffe, Newman, Simonsick and Rubin13,Reference Kop, Gottdiener, Tangen, Fried, McBurnie and Walston14 one was negative, Reference Tiemeier, Hofman, van Tuijl, Kiliaan, Meijer and Breteler35 and another found only a gender-specific association in men. Reference Danner, Kasl, Abramson and Vaccarino12 Although a meta-analysis of these studies concluded that prospective trials are necessary to address issues of causality, Reference Kuo, Yen, Chang, Kuo, Chen and Sorond36 these data corroborate those from prospective studies of two elderly populations for which raised markers of systemic inflammation pre-dated the onset of depressive symptoms. Reference van den Biggelaar, Gussekloo, de Craen, Frolich, Stek and van der Mast20,Reference Milaneschi, Corsi, Penninx, Bandinelli, Guralnik and Ferrucci21
An acute phase immune response might operate to increase the risk of depression via multiple pathways. Both early-life stress (e.g. social isolation) and late-life stress (e.g. chronic mild stress) increase both peripheral and central levels of pro-inflammatory cytokines. The ability of stress to operate as a risk factor for depression is widely accepted. Inflammation increases oxidative stress, and oxidative stress is increasingly regarded as an operative mechanism in depression. Reference Berk, Ng, Dean, Dodd and Bush37,Reference Berk38 Oxidative stress disrupts lipid membranes via peroxidation, and proteins via carbonylation, which are described in depression. Reference Galecki, Szemraj, Bienkiewicz, Zboralski and Galecka39,Reference Wei, Zhou, He, Bai, Hui and Wang40 Pro-inflammatory cytokines also affect serotonin neurotransmission. Interferon gamma reduces the production of serotonin by converting its precursor, tryptophan, to tryptophan catabolites (TRYCATS) such as kynurine. Reference Barry, Clarke, Scully and Dinan41 These are potentially anxiogenic and neurotoxic.
Strengths and limitations
There are several strengths and potential weaknesses in our study. Although acknowledging that depression is a heterogeneous disorder, we utilised the reference-standard tool (SCID) for the clinical assessment of major depressive disorder. The longitudinal nature of the design and the length of the follow-up period are other key strengths. Given that elevated hsCRP is one of many potential operative risks for depression, such vulnerability may only be reliably demonstrated over an extended timeframe. However, recall limitations may have affected our ability to accurately diagnose the age at onset of depressive episodes. Moreover, as in all observational studies, there may be unrecognised confounding. Physical activity, smoking and alcohol use were explored as concomitant lifestyle factors with a potential for confounding because of their previously reported influence on both CRP concentration Reference Raitakari, Mansikkaniemi, Marniemi, Viikari and Raitakari30 and the risk for depression. Reference Pasco, Williams, Jacka, Ng, Henry and Nicholson42,Reference Hamalainen, Kaprio, Isometsa, Heikkinen, Poikolainen and Lindeman43 Our previous demonstration of an association between tobacco smoking and increased risk for major depressive disorder Reference Pasco, Williams, Jacka, Ng, Henry and Nicholson42 may have a multifactorial basis including dysregulation of the dopaminergic system Reference Malhi and Berk44 and offers further support to an inflammation-based aetiology, as smoking induces an inflammatory response. Reference Kangavari, Matetzky, Shah, Yano, Chyu and Fishbein45 Non-steroidal anti-inflammatory drugs and both oral contraceptives and oral hormone therapy have an impact on circulating levels of CRP Reference Raitakari, Mansikkaniemi, Marniemi, Viikari and Raitakari30,Reference Blumenfeld, Boulman, Leiba, Siegler, Shachar and Linn46 and our findings corroborate these reports. Childhood maltreatment predisposes to depression and may play a role in increasing the risk for inflammation in adulthood; Reference Danese, Pariante, Caspi, Taylor and Poulton47,Reference Danese, Moffitt, Pariante, Ambler, Poulton and Caspi48 this has not been entered into the models, as these data were not available.
We recognise that complex interactions are likely between inflammation, redox status and other factors modulating psychiatric well-being. Moreover, circulating hsCRP levels may be acting as a surrogate for unrecognised confounders that affect the risk for depressive disorders. However, within these constraints, we infer that circulating hsCRP is an independent prognostic marker for major depression in women. As these data pertain predominantly to White women, interpretation may not be generalisable to women of other ethnicities, or to men.
It is evident that diabetes, Reference Yuan, Zhou, Tang, Yang, Gu and Li49 cardiovascular disease Reference Danesh, Wheeler, Hirschfield, Eda, Eiriksdottir and Rumley50 and bone fragility Reference Pasco, Kotowicz, Henry, Nicholson, Spilsbury and Box51 are characterised by systemic inflammation. Depression is commonly reported among patients with these disorders and, although it is possible that hsCRP is simply a marker for these disorders, which in turn may increase the risk for depression, it is also plausible that inflammation could underpin this association. Replicatory studies measuring other cytokines are needed to confirm this notion. C-reactive protein has been shown to be a vascular risk factor in individuals with mental illness. Reference Nilsson, Gustafson and Hultberg52
Summary
Serum hsCRP appears to be an independent prognostic marker for de novo major depressive disorder risk in women. This supports an aetiological role for inflammatory activity in the genesis of depression. In addition, it provides a mechanistic link between depression and commonly comorbid medical disorders, and suggests potential intervention targets.
Acknowledgement
We thank Sharon Brennan for obtaining the socioeconomic data for the study.






eLetters
No eLetters have been published for this article.