Introduction
The composition and abundance of nutrients (C, N and P) in runoff derived from glacial ecosystems has important implications for the ecology and biogeochemistry of supraglacial streams. Recent research has demonstrated convincingly that glacier ecosystems are biogeochemically active environments that can affect the dynamics and export of nutrients to downstream ecosystems (Reference HodsonHodson and others, 2008). For example, Reference Hodson, Roberts, Engvall, Holmén and MumfordHodson and others (2010) showed that glacial ecosystems in the High Arctic can actively cycle N and thus attenuate the export of episodic atmospheric N pollution from source areas in Europe at times when terrestrial soil ecosystems are frozen and unresponsive.
Within glacial ecosystems, subglacial environments have been shown to contain abundant and active microbial communities (Reference Skidmore, Foght and SharpSkidmore and others, 2000; Reference Bhatia, Sharp and FoghtBhatia and others, 2006) that can strongly influence the biogeochemistry of glacial runoff (e.g. Reference Bottrell and TranterBottrell and Tranter, 2002; Reference Tranter, Sharp, Lamb, Brown, Hubbard and WillisTranter and others, 2002; Reference Wadham, Bottrell, Tranter and RaiswellWadham and others, 2004; Reference Skidmore, Anderson, Sharp, Foght and LanoilSkidmore and others, 2005; Reference Wynn, Hodson and HeatonWynn and others, 2006). Supraglacial environments have also been shown to be biogeochemically active. Glacial snow covers, which may contain high concentrations of algae and cyanobacteria, can actively retain and cycle both N and P (Reference JonesJones, 1999; Reference Hodson, Mumford, Kohler and WynnHodson and others, 2005; Reference HodsonHodson, 2006). In addition, supraglacial cryoconite holes have been shown to contain a diversity of autotrophic and heterotrophic bacteria (Reference Säwström, Mumford, Marshall, Hodson and Laybourn-ParrySäwström and others, 2002; Reference Kaštovská, Elster, Stibal and ŠantréčkováKaštovská and others, 2005) capable of substantial C fixation (Reference HodsonHodson and others, 2008; Reference Anesio, Hodson, Fritz, Psenner and SattlerAnesio and others, 2009) and the metabolism of organic C (Reference TranterTranter and others, 2004).
Streams in supraglacial environments are important conduits for surface meltwater on both temperate and polar glaciers. On temperate glaciers, supraglacial streams form annually below the glacier snowline during the melt season. These streams acquire solutes from melting ice and snow as well as external sources such as aeolian dust (Reference Tranter, Brown, Raiswell, Sharp and GurnellTranter and others, 1993), transporting them to englacial and subglacial flow paths that drain to the glacier terminus. Despite the importance of supraglacial streams as hydrological conduits within glacial ecosystems, relatively little is known about their ecology (Reference HodsonHodson and others, 2008). A few studies have examined fluxes of water and solutes through supraglacial streams (e.g. Reference Hodson, Tranter, Gurnell, Clark and HagenHodson and others, 2002; Reference Fortner, Tranter, Fountain, Lyons and WelchFortner and others, 2005). These observations, combined with evidence for active microbial communities within other supraglacial environments (e.g. cryoconite holes), hint at the potential for biogeochemically active supraglacial streams.
Supraglacial streams are hydrologically unique compared to other stream systems. In most running waters, the stream– sediment interface is a zone of active biogeochemical processing with substrate available for the development of attached biotic communities (e.g. algal mats). As solutes are transported to the sediment–water interface, nutrients and organic matter are used for both photosynthetic and heterotrophic processes (Reference McClainMcClain and others, 2003) on the stream bed and into the stream bed along subsurface flow paths. Supraglacial streams differ in that the stream bottom is generally devoid of large quantities of rocks and sediment where biofilms develop; instead, they run over the glacial ice. The lack of exchange across the ice–water interface not only reduces contact with microbial communities, but also reduces the hydrologic residence time during transport. As a result, the potential for nutrient and carbon retention is relatively low, and limited to regions of the supraglacial network that have higher hydrologic residence time (e.g. wide and low gradient stream reaches).
In this study, we conducted a tracer experiment in order to assess the retention of inorganic nutrients (N and P) and organic carbon within a supraglacial stream on Mendenhall Glacier, southeastern Alaska, USA. A conservative solute (Cl−) was co-injected with NH4 + , PO4 3– and labile dissolved organic carbon (DOC) into a supraglacial stream to quantify nutrient removal. Our overall goal was to evaluate the extent to which microbial processes in supraglacial streams influence the biogeochemistry of glacial meltwater transported through them.
Methods
Stream tracer methodology
The tracer injection experiment was conducted on a supraglacial stream on Mendenhall Glacier near Juneau, Alaska (Fig. 1). A 100 m study reach was selected, which consisted of an initial 15 m narrow reach for mixing, followed by a shallow and wide 45 m reach (together reach 1), and a 40 m narrow, steep reach (reach 2). Samples were collected above the injection site, and at the beginning and end of each reach (e.g. 15, 60 and 100 m downstream from injection). The 45 m section of reach 1 had observable regions of slow-moving water (e.g. pools with abundant ‘surface storage’), with some visible sediment and organic material (wind-blown leaves). Reach 2 was devoid of any bottom substrate other than glacial ice.

Fig. 1. Location of Mendenhall Glacier in southeast Alaska. The study stream, which was located 2 km from the glacier terminus, is shown in the photograph on the right.
The stream tracer experiment consisted of co-injecting conservative and non-conservative solutes. NaCl was injected into the supraglacial stream at a rate of 59 mLmin−1 for 3 hours. A second solution of labile carbon (dextrose) was added in conjunction with a third solution containing a mixture of HNa2PO4 and NH4Cl. The objective was to increase background concentrations of DOC from <15 to 125 μm, PO4 3– from <1 to 2 μm, and NH4 + from <1 to 5 μm in order to determine the potential uptake within this environment. Since only one nutrient addition experiment was possible due to logistics, we acknowledge that our results will be an underestimate of the actual uptake rate within our study stream (Reference MulhollandMulholland and others, 2002). This is because nutrient uptake rate is only directly proportional to small perturbations of the in-stream nutrient concentrations under strongly nutrient-limiting conditions, and becomes nutrient-saturated at higher concentrations (thus following a nutrient saturation model (e.g. Michaelis–Menten)).
Sampling for the stream tracer experiment was performed in three phases. For the first 60 min after the beginning of the injection, samples for Cl− were collected every 30 s to 5 min to capture the rise of and shoulder of the Cl− breakthrough curve at each site. From 60 to 180 min during the plateau of the stream injection, samples were collected every 15 min for nutrients and Cl−. At 180 min, the injection was turned off, and samples were collected frequently (from 30s to 5 min) to capture the decline and tail of the breakthrough curve at each site. The conservative solute breakthrough curve facilitates quantification of advection, dispersion and transient storage within the surface pools. Lateral inflow is quantified using observations during the plateau portion of the breakthrough curve. Synoptic samples were collected during the plateau every 10 m along the experimental reach. Samples for anions and nutrients were immediately filtered through 0.45 μm into 20 mL scintillation HDPE vials. Anion samples were stored in the dark, and were analyzed within 30 days using ion chromatography (Dionex 8000). Nutrient samples were stored at 0°C and analyzed within 45 days, and were measured using standard colorimetric techniques on a SEAL flow injection analyzer. The standard deviations of check standards analyzed throughout the analyses were 0.1 μm NH4 + , 0.1μm NO3 −, 0.01 μm PO4 2– and 18μm DOC. DOC samples were filtered through 0.45 μm filters into pre-combusted amber glass vials, and DOC was measured on a Shimadzu TOC-V carbon analyzer within 48 hours. To examine non-conservative nutrient behavior, the ratio of nutrient/chloride along the experimental reach normalized to a value of 1 at x = 0m was calculated using
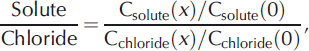
where x is the distance downstream.
Modeling
The conservative solute (Cl−) was used to calibrate a simple one-dimensional advection–dispersion model with transient storage (OTIS; Reference RunkelRunkel, 1998).
Transport within the stream was modeled using OTIS:
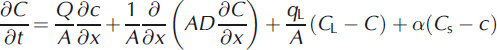
where Q is discharge (m3 s−1), A is stream area (m2), D is the dispersion coefficient (m2 s−1), qL is lateral volumetric inflow rate (m3s−1 m−1), CL is solute concentration in lateral inflow (μM), C is stream solute concentration, Cs is transient storage zone concentration, As is cross-sectional area of the transient storage zone, α is the transient storage zone exchange coefficient, x is distance downstream (m) and t is time (s). The partial differential equations (2) and (3) are numerically approximated within OTIS. Chloride observations at the end of each reach were used to calibrate the model using an inverse modeling approach following Reference Scott, Gooseff, Bencala and RunkelScott and others (2003). The calibration approach linked UCODE (Universal Computer Code (Groundwater Modeling Systems)) to the advection-dispersion model OTIS to arrive at parameters for dispersion (D), lateral inflow (q), stream and transient storage area (A and As) and transient storage exchange (a). Stream discharge at the injection site was measured using the salt dilution method,
where Q stream is stream discharge (m3s−1), Qinjection is injectate flow rate (m3s−1), Clinjection is injectate concentration (μM), and Clstream is chloride concentration at any downstream location during plateau (μM).
Transport of the non-conservative solutes (NH4 + , NO3 −, PO43-, DOC) was modeled using the calibrated advection-dispersion model. For each solute, the incoming lateral inflow was fixed to the solute’s background concentrations. For DOC, observations at the beginning of reach 1 were used as the input to the experimental reaches to assess the potential for carbon retention due to a variation in the pump injection rate that occurred over the experiment. For each reactive solute that exhibited non-conservative behavior, a first-order decay term, λ, was incorporated into both the main water column and transient storage zone:
where L(C) and S(Cs) represent physical processes in the main water column and transient storage zone (right-hand side of Equations (2) and (3)), λ is the first-order decay coefficient in the main water column (s−1) and λ s is the first-order decay in the transient storage zone (s−1).
Results and Discussion
Stream discharge
During the experiment, stream discharge increased by 66% (from 10.5 to 17.4 L s−1) down the 100 m study reach (Fig. 2). Measuring the change in stream discharge over the study reach was important for determining if the added reactive nutrients were simply diluted by inputs of melting ice or if they were retained during downstream transport. Most of the streamflow increase occurred in reach 1, across which streamflow increased by ∼45%. Along reach 2, stream discharge increased at a lower rate, presumably due to the steeper gradient and smaller contributing area.

Fig. 2. Discharge within the supraglacial stream increased throughout the experimental reach, most notably within the zone of the highest-contributing area.
The chloride measurements over the 3 hour injection period also suggested variable streamflow in response to solar inputs, as seen by the increasing Cl– concentrations towards the end of the 180min (Fig. 3). During the last 60 min of the injection period (from 120 to 180 min), the glacier was no longer receiving direct sunlight, which began to decrease lateral inputs of water into the channel, thereby increasing Cl– concentrations.

Fig. 3. Chloride observations were used to model the physical transport processes within the stream reach using a one-dimensional advection–dispersion model. The modeled fit closely matched the observations over the breakthrough curves within reaches 1 and 2.
Conservative solute modeling
The chloride observations at the end of the two study reaches provide the ability to calculate travel time and estimate the physical parameters of D, q L, A, As and α for the OTIS model. Using the inverse modeling approach, we estimated a unique set of parameters for both stream reaches (Table 1). Although streamflow began to decrease at the end of the injection period, we modeled the stream using a constant discharge assumption since the decrease was only ∼8% over the injection period. We found that the first reach’s mean travel time was ∼13min, and the second reach’s travel time was only 2–3 min. The calculated velocity using the model parameters in the first reach was only 0.04ms−1, in contrast to 0.34 m s−1 in reach 2. This result is consistent with the channel geomorphology, and suggests that there is more opportunity for nutrient transformations in the first reach.
Table 1. Optimized model parameters and physical characteristics of the supraglacial stream
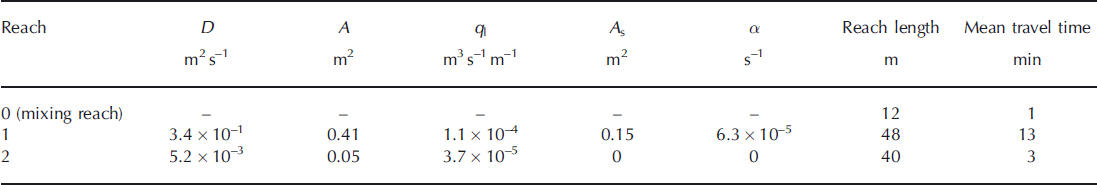
Our modeling results also confirm differences in transient storage between the first and second reaches. We found that inclusion of transient storage within reach 1 provided a statistically more robust model to explain the observed Cl− tailing in the breakthrough curve. This result is consistent with the field observations of a pre-tracer dye used to plan for the injection. Water within the first reach was transported slowly, mixing with zones of much slower surface water that were acting as surface dead zones (Reference Bencala and WaltersBencala and Walters, 1983). Although transient storage within forested streams consists of both surface and subsurface storage, supraglacial streams are not expected to have subsurface storage owing to the frozen stream bed. In our study, reach 2 had neither surface nor subsurface transient storage due to the lack of surface water pools and the frozen stream bed.
Nutrient fate
There are relatively few reports documenting biogeochemical processes within supraglacial streams (Reference HodsonHodson and others, 2008). Supraglacial streams can be expected to have very low levels of C, N and P, given that the meltwater does not come into contact with substantial nutrient pools and thus largely reflects inputs via precipitation. These streams are in sharp contrast to streams within forested watersheds, where water entering the stream has likely been in contact with upland and riparian soils that can act as a source of organic and inorganic nutrients. Background nutrient measurements within our study stream were all near the instrument detection level (Table 2), with the exception of NO3 − (0.7 μm), which was similar in concentration to the NO3 − concentration measured in a supraglacial stream on Canada Glacier, Antarctica (Reference Fortner, Tranter, Fountain, Lyons and WelchFortner and others, 2005). In addition, the background supraglacial stream water at our site was so dilute that even concentrations of Cl− were below instrument detection limits.
Table 2. Background and targeted input concentrations for the supraglacial stream. Only Cl−, NH4 + , PO4 3– and labile DOM were added to the stream

The nutrient injection was designed to determine if there was any short-term nutrient removal within the supraglacial stream environment. The NH4 + and PO4 3– injection resulted in steady-state concentrations of 18 μm NH4 + and 4.4 μm PO4 3– at the beginning of reach 1 (Table 2). DOC concentrations entering reach 1 were at a maximum value at 60 min (190 μm), and decreased in response to the change in the carbon injection input over the course of the experiment.
We found that the added NH4 + and PO4 3– were generally conservative over the course of the short-term experiment, as seen by the NH4 +/Cl− and PO4 3–/Cl− ratios (Fig. 4). Our analysis shows that the nutrient addition did not stimulate growth, implying that there is limited net uptake of P and NH4 + and likely little primary production. Because supraglacial streams frequently disappear within tens of meters into moulins or crevasses, the potential for measurable nutrient uptake is already limited by short travel times. In our study, the first experimental reach was specifically chosen because of the observed surficial transient storage zones, lower stream gradient and some observable sediment accumulation zones. The second reach was more typical of other observed supraglacial streams, with a narrow profile, steeper gradient and no bed sediments. Even within the first reach where calculated velocities were on the order of 0.04 ms−1, there was no detectable removal of either NH4 + or P. Thus it appears that the short travel times, combined with stream temperatures at or near 0°C and limited surface area for colonization, inhibit in-stream nutrient uptake.

Fig. 4. Normalized NH4 + , PO4 3– and NO3 − to chloride ratios illustrate whether a solute is conservative, increasing or decreasing along the stream reach.
Although we are not aware of any previous efforts to quantify nutrient uptake within supraglacial streams, other extreme environments have been shown to support algal communities. For example, freeze-dried algal mats within Antarctic streams respond within days of receiving melting glacial water from upstream after being dry for >40 weeks (Reference McKnight, Niyogi, Alger, Bomblies, Conovitz and TateMcKnight and others, 1999). One large difference with these Antarctic streams is their relative stability over time in contrast to supraglacial streams, which frequently appear in new locations on the glacier surface each season. As a result, supraglacial streams lack the potential for accumulating a supportive substrate for long-term colonization by algal communities.
Other studies have found evidence for primary production within cryoconite holes on glacier surfaces (Reference Anesio, Hodson, Fritz, Psenner and SattlerAnesio and others, 2009). However, the higher hydrologic retention times as well as the presence of fine sediments (Reference Kaštovská, Elster, Stibal and ŠantréčkováKaštovská and others, 2005) within cryoconite holes make them more suitable for algal growth. Although algal communities within cryoconite holes were observed at our study site, the study stream had little fine sediment to support algal communities and no net N and P uptake. Our findings and observations suggest that biological activity in cryoconite holes likely has little influence on nutrient concentrations in supraglacial streams, except in circumstances where cryoconite holes are directly hydrologically connected to the stream.
Interestingly, nitrate increased through the experimental reach (as seen in the upward trend in NO3 −/Cl− ratios; Fig. 4), even though nitrate was not injected into the stream. Concentrations more than doubled by the end of the reach to 2.4 mm NO3 −, even though streamflow nearly doubled over the same distance. Prior to the NH4 + addition, background NO3 − concentrations were 0.6 and 1.1 mm at the beginning and end of the first reach respectively (NH4 + was non-detectable). Assuming conservative transport, the incoming lateral inflow into reach 1 would have needed concentrations of >2.7 μm NO3 − to account for the observed increase in mass flux. An alternate assumption is that the incoming lateral inflow had an average concentration of 0.85 p.m, the average in-stream concentration prior to the experiment, and that nitrification produced NO3 − within the stream. This assumption results in an estimated aerial stream-bed nitrification rate of 1.6 (μmoles m−2 min−1, which is at the lower end of the reported range for forested streams (0.1–6 (μmoles m−2 min- 1 ) (e.g. Reference PetersonPeterson and others, 2001).
Previous studies have also documented ‘excess’ NO3 − as evidence of nitrification in subglacial and ice-marginal environments in a variety of glacial ecosystems (Reference Tockner, Malard, Uehlinger and WardTockner and others, 2002; Reference Hodson, Mumford, Kohler and WynnHodson and others, 2005; Reference HodsonHodson 2006; Reference Williams, Knauf, Cory, Caine and LiuWilliams and others, 2007). Because there was no net NH4 + removal along the reach, the organic matter mineralization rate would need to have compensated for any loss of NH4 + through nitrification. These findings suggest that supraglacial streams have some resident nitrifying and heterotrophic bacteria, but further examination is needed to elucidate coupled N reactions.
A further indication of heterotrophic activity is that the added bioavailable dissolved organic matter (DOM) was not conservatively transported (Fig. 5). Although DOC/Cl ratios could not be used due to the absence of a plateau, the fate of DOM was analyzed within OTIS. Within reach 1, the conservative simulation initially matched the observations prior to the observed spike between 75 and 90 min, then over-predicted DOC concentrations through 165 min. We applied a first-order decay term in both the main water column and transient storage zone within reach 1 to account for the DOC overestimation from 90 to 165 min, and found the decay variable significant only in the main water column. The estimated decay, A, from the inverse modeling was 2 × 10–4s−1, translating to a mass transfer coefficient from the water to the sediment-water interface of 10 μm s−1. At this rate, it would take a travel time of nearly 1 hour for the concentration to decrease 50%. Although there was no detectable decay in reach 2, the travel time was <3 min, which at the estimated decay rate would only result in a decrease of ∼4μm, which is within the uncertainty of the DOC measurements. Other studies measuring the removal of labile organic carbon (e.g. sucrose, acetate, glucose) have found mass transfer coefficients ranging from 9 to 146 μm s−1 in forested streams (Reference Munn and MeyerMunn and Meyer, 1990; Reference Hall and MeyerHall and Meyer, 1998; Reference Wiegner, Kaplan, Newbold and OstromWiegner and others, 2005). Our measured mass transfer coefficient is on the low side of this range, suggesting lower heterotrophic metabolism in comparison to forested streams.

Fig. 5. DOC was modeled in reach 1 under two scenarios: conservative transport (solid curve) and transport including first-order decay (dotted curve).
The observed NO3 − production and DOM removal, particularly within reach 1 , suggest that resident populations of chemotrophs and heterotrophs respond rapidly to short-term nutrient perturbations. These populations likely reside in the limited stream substrate, which was only visually identifiable in reach 1 . The measured DOM removal in the supraglacial stream we studied was likely due to mineralization of the added carbon by heterotrophic organisms. Several recent studies have documented the export of labile, protein-rich DOM from glaciers in a variety of environments (Reference Barker, Sharp, Fitzsimons and TurnerBarker and others, 2006; Reference HoodHood and others, 2009; Reference Bhatia, Das, Longnecker, Charette and KujawinskiBhatia and others, 2010). Other research has documented the presence of heterotrophic species at the glacier–sediment interface (e.g. Reference Skidmore, Foght and SharpSkidmore and others, 2000). Taken together, these findings suggest that there may be both production and consumption of organic matter along hydrologic flow paths within glaciers.
Conclusions
Supraglacial streams form annually on glaciers, transporting a portion of the season’s snowfall to subsurface glacial flow paths and to the stream at the glacier terminus. Our study was designed to measure the potential for nutrient uptake and DOM removal. Our observations conclude that nutrient uptake is limited within supraglacial streams, although surprisingly we found evidence of some rapid biogeochemical processing. Both nitrification and mineralization were detectable within the channel, even along this short-residence-time supraglacial channel with little habitat for microbial colonization. Thus, we suspect that supraglacial streams with longer retention times may also support autotrophic communities. In summary, our findings suggest the presence of both heterotrophic and nitrifying microbial communities that alter nutrient fate and transport within these supraglacial streams.
Acknowledgements
Support for this project was from the US National Science Foundation (EAR 0838497 and EAR 0838587). We thank Northstar Trekking in Juneau for helicopter support, and S. Schreiber, S. Perry and A. Jeffrey for field assistance. Two anonymous reviewers and A. Hodson provided valuable feedback that improved the manuscript.









