Introduction
The first nordite species was discovered in Pegmatite No. 65 located in the Chinglusuai river valley, Lovozero alkaline complex, Kola Peninsula, Russia (Gerasimovsky, Reference Gerasimovsky1941). The original description showed the sample to be rich in Mn without Zn and with La being the dominating lanthanide. Semenov (Reference Semenov1961) described a nordite from Pegmatite No. 66 in the Motchisuai river valley, Sengischorr Mt., Lovozero, containing 3.90 wt.% ZnO. In the same publication, the original nordite-(La) material from Gerasimovsky (Reference Gerasimovsky1941) was reanalysed and revealed to contain 4.22 wt.% ZnO (Semenov, Reference Semenov1961). This means that the original analysis by Gerasimovsky (Reference Gerasimovsky1941) was a Zn-dominated nordite rather than Mn-dominated as originally described. Pekov et al. (Reference Pekov, Chukanov, Kononkova, Belakovsky, Pushcharovsky and Vinogradova1998) described ferronordite-(Ce) as a new species from Pegmatite No 65 located in the Chinglusuai river valley and from Mt. Karnasurt, and manganonordite-(Ce) as a new species from Pegmatite No. 60, Mt Karnasurt and from Mt. Kedykverpakhk – all localities are within Lovozero. Ferronordite-(La) was described from the Bolshoi Punkaruaiv Mountain, Lovozero by Pekov et al. (Reference Pekov, Chukanov, Turchkova and Grishin2001). In the Khibiny alkaline complex, Kola peninsula nordite-(La) was described from Kirovskii apatite mine, Mt. Kukisvumchorr (Khomyakov, Reference Khomyakov1990) and Mt. Eveslogchorr (Pekov, Reference Pekov1997). Outside the Lovozero and Khibiny complexes, nordite-supergroup minerals are rare and only nordite-(Ce) has been described from Igdlutalik [now spelled Illutalik], Gardar Province, South Greenland (Upton et al., Reference Upton, Macdonald, Hill, Jefferies and Ford1976); Mont Saint-Hilaire, Québec, Canada (Wight and Chao, Reference Wight and Chao1995); and Dara-i-Pioz, Tajikistan (Pekov et al., Reference Pekov, Chukanov, Kononkova, Belakovsky, Pushcharovsky and Vinogradova1998).
The first crystal structure refinement on a nordite-group mineral was performed by Bakakin et al. (Reference Bakakin, Belov, Borisov and Solovyeva1970) on a nordite-(La) from Lovozero, showing that nordite-(La) is orthorhombic (Pcca) with a = 14.27(3), b = 5.16(1), c = 19.45(15) and Z = 4. Subsequently, the crystal structures of ferronordite-(Ce) and manganonordite-(Ce) were reported by Pushcharovsky et al. (Reference Pushcharovskii, Pekov, Pluth, Smith, Ferraris, Vinogradova, Arakcheeva, Soboleva and Semenov1999), confirming the previous structural model established by Bakakin et al. (Reference Bakakin, Belov, Borisov and Solovyeva1970). A new mineral isostructural to nordite, and free from rare earth elements (REE) was published Yang et al. (Reference Yang, Gu, Downs, Evans, Van Nieuwenhuizen, Lavinsky and Xie2019), namely meieranite, ideally Na2Sr3MgSi6O17, from the Wessels Mine in the Kalahari manganese field, South Africa.
During a study of nordite-group minerals from the Ilímaussaq alkaline complex, South Greenland (Gulbransen, Reference Gulbransen2020) it became apparent that the nordite-(Ce) from Illutalik (South Greenland) published by Upton et al. (Reference Upton, Macdonald, Hill, Jefferies and Ford1976) should be re-investigated as the chemical data results in an empirical formula with significant deviation from the ideal composition. Furthermore, it was realised that there is no published structure solution for nordite-(Ce). Consequently, new chemical and crystallographic investigations are presented on nordite-(Ce) from Illutalik, South Greenland.
In addition, we present the newly approved nomenclature for the nordite-supergroup minerals, including nordite-(La), nordite-(Ce), ferronordite-(La), ferronordite-(Ce), manganonordite-(Ce) and meieranite. This nomenclature scheme has been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) (Miyawaki et al., Reference Miyawaki, Hatert, Pasero and Mills2021).
Crystal chemistry of nordite-supergroup minerals
Six species are currently approved in the nordite supergroup. They can be described as silicates with the general formula A 2BXYZT 6O17, where [6–8]A = Na; [6]B = Na, Ca; [8]X = Ca, Sr, Ba; [8]Y = Sr, REE 3+; [4]Z = Mg, Mn2+, Fe2+, Zn and [4]T = Si (Tables 1 and 2). In all described species, the A and B sites are dominated by Na and the X site by Sr. The Y site is either dominated by La or Ce in the nordite-group minerals, or by Sr in meieranite. In the nordite-group minerals the Z site is either dominated by Mn2+, Fe2+ or Zn, whereas in meieranite Mg is the dominant cation on the Z site.
Table 1. List of the valid nordite-supergroup minerals and their unit-cell parameters.
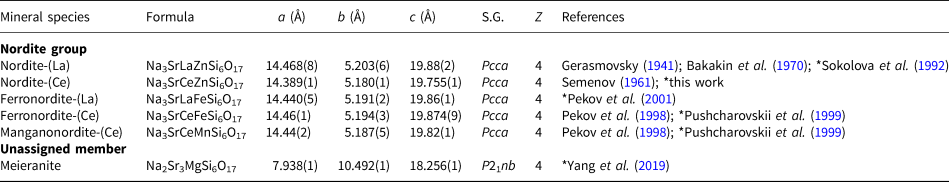
S.G. = Space Group. *Unit-cell parameters are taken from this reference.
Table 2. Cationic distribution in the crystal structure of the approved and hypothetical new end-members of the nordite supergroup.

* In italic: hypothetical new end-member compositions.
Nordite-group minerals are all isostructural, orthorhombic, space group Pcca (#54); Z = 4; a ≈ 14.4, b ≈ 5.2 and c ≈ 19.8 Å. Meieranite, Na2Sr3MgSi6O17, is orthorhombic, space group P21nb (#33); Z = 4; a = 17.938(1), b = 10.492(1) and c = 18.256(1) Å. In the structure of meieranite the A site is split into two distinct sites, A and A’ (Table 2). Starting from a nordite composition, meieranite may be obtained through the coupled heterovalent substitution ANa+ + YREE 3+ ↔ A’Sr2+ + YSr2+ (Yang et al., Reference Yang, Gu, Downs, Evans, Van Nieuwenhuizen, Lavinsky and Xie2019).
Nordite-supergroup minerals are classified as inosilicates with ring-branched 12-periodic single chains. The silicate chains (T sites) are interconnected through the tetrahedrally coordinated Z site forming layers consisting of 4-, 5- and 8-membered rings perpendicular to [010] (Fig. 1a). The T1 and T2 sites share one corner with the Z sites and two corners with other T sites. The T3 site is tri-coordinated with the other T sites. The remaining apical oxygen atoms are shared with the A, A’, B, X and Y sites, that form heteropolyhedral layers (Fig. 1b). These sites are connected through edge and face sharing. In the species belonging to the nordite group the A site has a 7+1 square antiprism coordination and is typically occupied by Na. In the case of meieranite, the complete ordering of Na and Sr on the A site leads to the splitting of the A site into the A and A’ positions; the A position is 7-coordinated and is occupied by Na, while the A’ position is 8-fold coordinated and occupied by Sr. The B site has an octahedral coordination and is occupied by Na. The X and Y sites have an 8-fold square antiprism coordination; the X site is populated by large divalent cations such as Sr and Ba, while the Y site is occupied by REE (nordite group) or by large divalent cations such as Sr in the case of meieranite (Table 2).
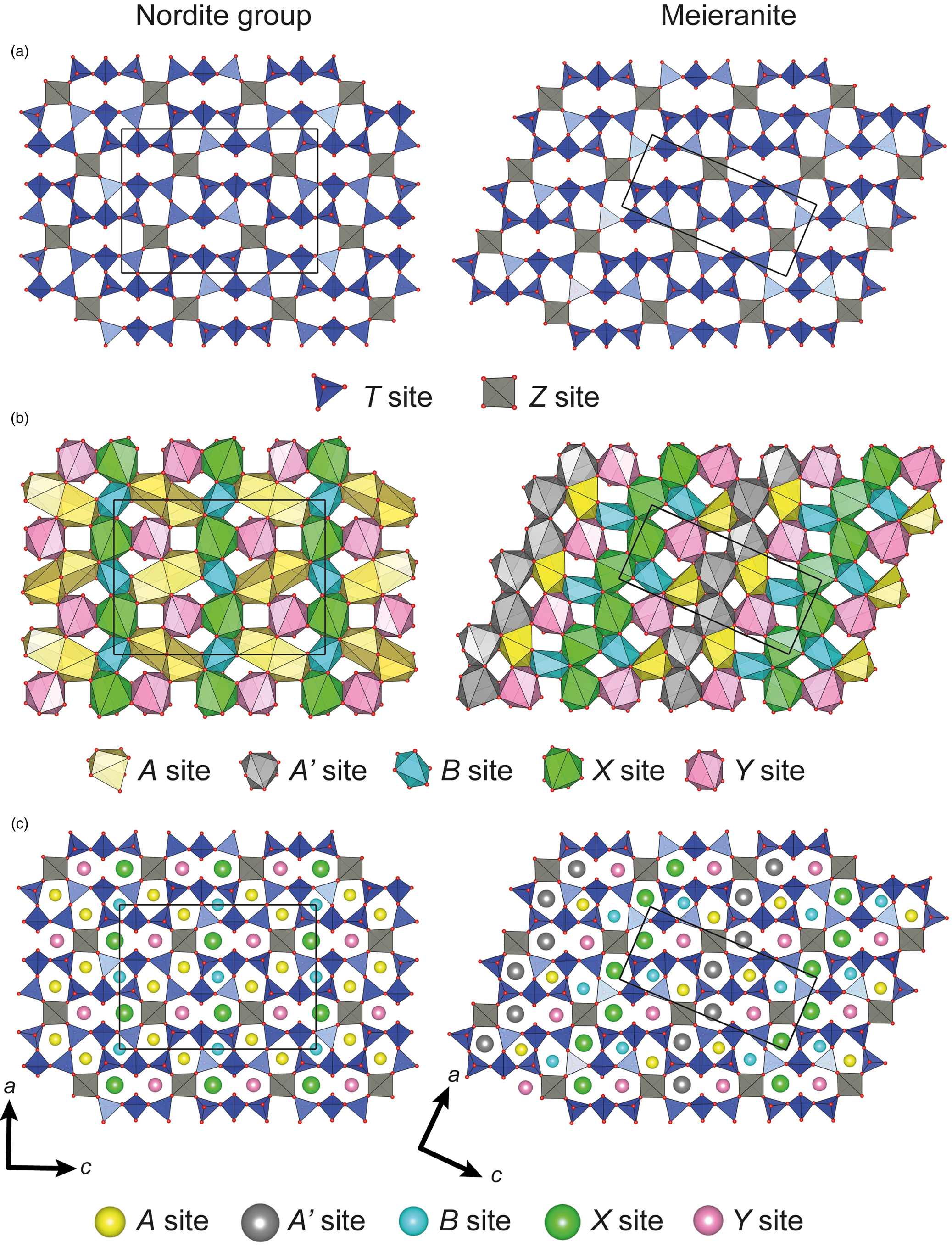
Fig. 1. View along the b axis of (a) tetrahedral layers, (b) heteropolyhedral layers and (c) stacking of both layers. The solid lines show one unit-cell.
The stacking of the tetrahedral and heteropolyhedral layers shows that the [8]X and [8]Y sites are located above and below the 8-membered rings, while the [8]A and [6]B sites occur above and below the 5- and 4-membered rings, respectively (Fig. 1c). In meieranite, the situation is more complex due to change from the centrosymmetric space group Pcca to the non-centrosymmetric space group P21nb. In that regard, in meieranite the successive tetrahedral layers are oriented in a different way, and therefore the [7]A and [6]B sites sit above and below the 4- and 5- membered rings, the [8]A’ and [8]X sites sit above and below the 5- and 8-membered rings, and the [8]Y sites are located solely above and below the 8-membered rings.
The nodal net describing the tetrahedral layer of nordite-group minerals is unique among silicate minerals and is given by [(4.5.8)8(52.8)4(5.8.5.8)2]2 (Hawthorne et al., Reference Hawthorne, Uvarova and Sokolova2019). However, as shown in Fig. 2, the tetrahedral layer in the nordite-group minerals and meieranite has a different symmetry, and consequently a different topology. In both nordite-group species and meieranite, the tetrahedral layer can be reconstructed from the same tetrahedral chains (Fig. 2a), in which the basic unit is one 5-membered ring pointing upwards (green), one 5-membered ring pointing downwards (red), and one 4-membered ring (blue). Simplified, in the nordite-group minerals the adjacent chains are symmetrically related through a mirror plane perpendicular to the a axis and passing through the Z site positions (Fig. 2b). In meieranite the chains are related through a 2-fold axis parallel to the b axis and going through the Z site positions (Fig. 2c).
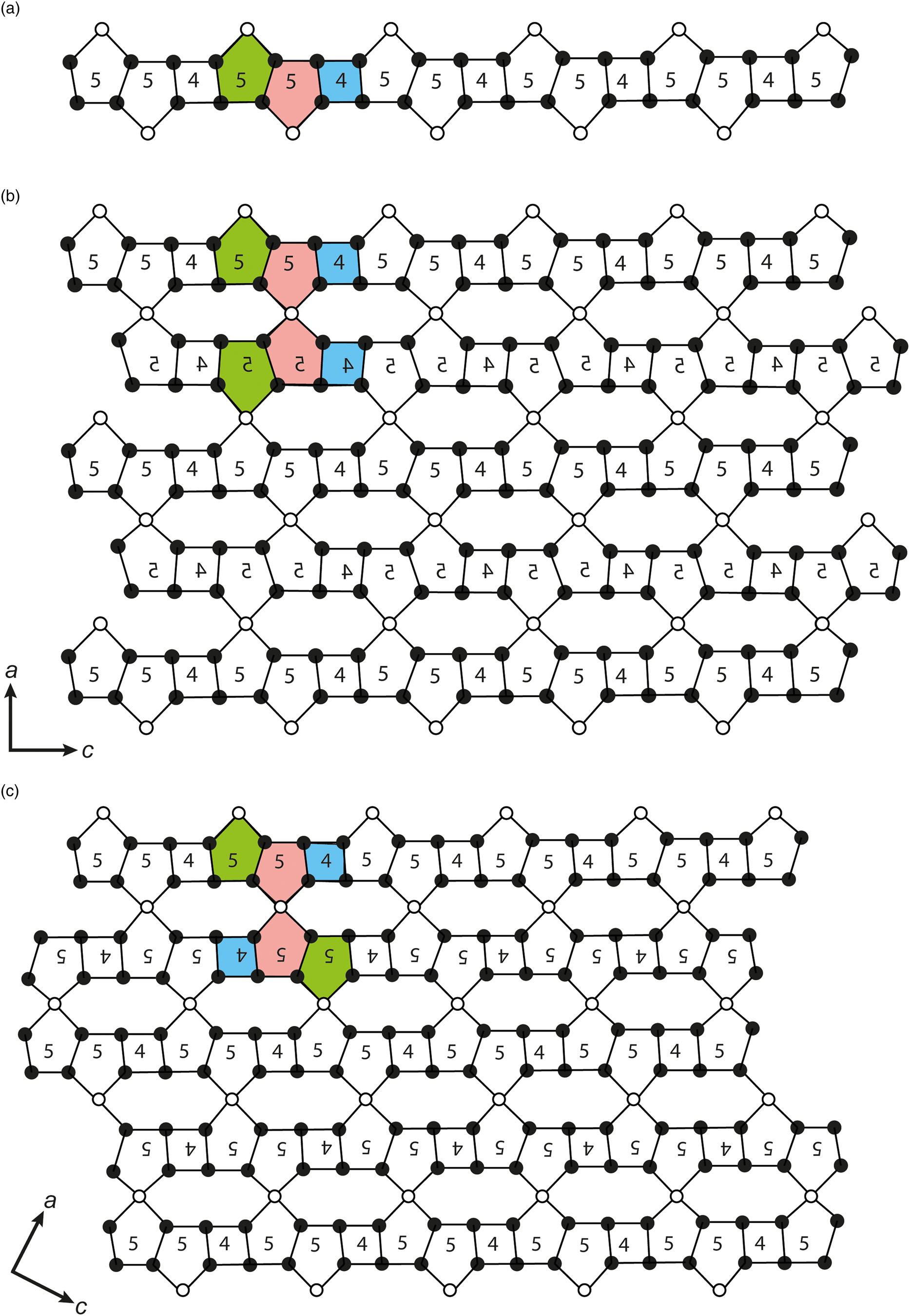
Fig. 2. Idealised topological representation of (a) tetrahedral chains occurring in the layers of (b) a nordite-group mineral and (c) meieranite. The solid and open circles represent the T and Z sites, respectively. For clarity, the numbers show the type of rings.
Methodology
A sample from the collection of NHM in Oslo (KNR 44277) was used for the study of nordite-(Ce). The sample is from the same locality studied by Upton et al. (Reference Upton, Macdonald, Hill, Jefferies and Ford1976), which is a trachyte dyke on the island of Illutalik (formerly spelled as Igdlutalik), South Greenland. Electron probe microanalyses (EPMA) were undertaken with a CAMECA SX100 housed in the Department of Geosciences, University of Oslo. The instrument is equipped with five spectrometers and was operated in wavelength dispersive mode. The instrument conditions were an acceleration voltage of 15 kV, beam current of 15 nA and a beam size of 5 μm. The following natural and synthetic standards were used: wollastonite (Si and Ca), albite (Na), pyrophanite (Mn), MgO (Mg), BaSO4 (Ba), Sr-glass (Sr) and pure metal (Fe). The intensity data were corrected for inter-element overlaps and for matrix effects using the PAP procedure (Pouchou and Pichoir, Reference Pouchou and Pichoir1984). Because of elemental overlaps that could not be successfully resolved, an Aurora Elite M90 ICPMS equipped with a Cetax LSX-213 G2+ laser (LA-ICPMS) housed at Department of Geosciences, University of Oslo was used for the following elements: Si, Ca, Zn, Rb, Y, Zr, Nb, Cs, REE 3+, Hf, Ta, Th and U. Instrument drift was monitored using NIST610 and BCR2G while Si from EPMA was used as internal standard. Raw data were reduced using the Glitter program (Griffin et al., Reference Griffin, Powell, Pearson, O'Reilly and Sylvester2008), using a linear fit to standards within a session. The formula calculated based on 17 anions is presented in Table 3.
Table 3. Chemical composition of nordite-(Ce) from Illutalik.
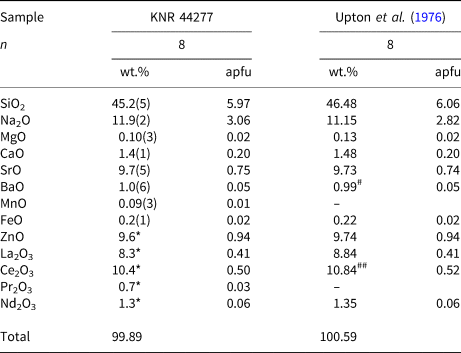
* From LA-ICPMS. # is given as 8.99 in Upton et al. (Reference Upton, Macdonald, Hill, Jefferies and Ford1976), but the 8 is a typo for zero. – Not determined. ## has been converted from CeO2.
For the single-crystal investigation, intensity data were collected at room temperature with monochromated MoKα radiation (50 kV and 1 mA) on a Rigaku Synergy-S diffractometer equipped with a HyPix-6000He detector housed at NHM in Oslo. The instrument has Kappa geometry and both data collection and subsequent data reduction, and face based absorption corrections were carried out using the Rigaku CrysAlis Pro software. The structure was solved by direct methods and refined by SHELXL (Sheldrick, Reference Sheldrick2008) using neutral atom scattering factors and the WinGX interface (Farrugia, Reference Farrugia2012). Table 4 contains details about data collection and refinement of nordite-(Ce). The atomic coordinates, anisotropic atomic displacement parameters and the bond distances are given in Tables 5–6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Data collection and structure refinement details for nordite-(Ce) from Illutalik.

P = ((F o)2 + 2(F c)2)/3)
Table 5. Site population, atomic coordinates atomic equivalent isotropic and anisotropic displacement parameters (Å2) for nordite-(Ce).

Table 6. Selected bond distances (d in Å) for nordite-(Ce).

Results
Nordite-(Ce) from Illutalik occurs as single euhedral, colourless-to-white crystals up to 150 μm in size or as aggregates up to a 300 μm in size. The mineral has been found in the part of the locality that also contains narsarsukite, but not in direct contact with emeleusite. The matrix is a fine-grained aegirine and albite dyke of trachytic composition (Upton et al., Reference Upton, Macdonald, Hill, Jefferies and Ford1976, Reference Upton, Hill, Johnsen and Petersen1978) and accessory minerals include pectolite, a britholite-group mineral, calcite and zircon. The empirical formula for nordite-(Ce) is Na3.06(Sr0.75Ca0.20Ba0.05)Σ1.00(Ce0.50La0.41Nd0.06Pr0.03)Σ1.00(Zn0.94Fe0.02Mg0.02Mn0.01)Σ0.99Si5.97O17, which is similar to that reported by Upton et al. (Reference Upton, Macdonald, Hill, Jefferies and Ford1976). However, the new data does provide a better stoichiometry than that previously reported despite Na being slightly high and Si slightly low (Table 3). The new chemical data confirms that the 8.99 wt.% BaO reported by Upton et al. (Reference Upton, Macdonald, Hill, Jefferies and Ford1976) was a typo and that the correct value was 0.99 wt.% BaO. The values for Zn, La, Ce, Nd and Pr are taken from LA-ICPMS analyses. The other elements analysed by LA-ICPMS are only present in trace amounts.
The refinement of the site scattering factors shows that the A and B sites are fully occupied by Na, and the Y site by REE. The X site has a low scattering factor (35.8 e –) in comparison to a full occupancy by Sr, which is explained by the incorporation of 0.2 Ca atoms per formula unit (apfu). The incorporation of significant amount of Ca on the X site is also reported in nordite-(La) (Bakakin et al., Reference Bakakin, Belov, Borisov and Solovyeva1970). The Z site shows only a minor deviation (28.9 e –) from the ideal value of 30, which is attributed to the incorporation of small amounts of Fe, Mg and Mn. The detailed cationic distribution on the different crystallographic sites is provided in Table 7 and is in good agreement with the chemical data and the different structural parameters. The bond-valence sums (BVS) calculated according to the cationic distribution are presented in Table 8.
Table 7. Cationic distribution in the crystal structure of nordite-(Ce).
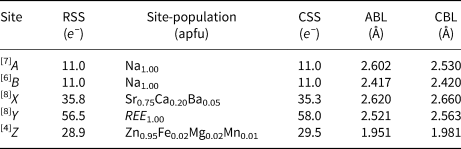
RSS: Refined site scattering factor; CSS: Calculated site scattering factor; ABL: average observed bond lengths; CBL: calculated bond lengths; Ideal bond distances are calculated using the ionic radii of Shannon (Reference Shannon1976). Ce3+ is used as a proxy for all REE.
Table 8. Bond-valence sums (valence units) for nordite-(Ce).

Note: bond-valence parameters are recalculated according to the site occupancies (see Table 5) and taken from Brown and Altermatt (Reference Brown and Altermatt1985) for the all the cations apart Si, for which the parameters from Gagné and Hawthorne (Reference Gagné and Hawthorne2015) have been used. VS: valence sums calculated from the site popluation.
Nomenclature scheme
Following the recommendations of Mills et al. (Reference Mills, Hatert, Nickel and Ferraris2009) on the standardisation of mineral group hierarchies we propose the establishment of the nordite supergroup, subdivided into the nordite group containing the following five mineral species: nordite-(La), nordite-(Ce), ferronordite-(La), ferronordite-(Ce) and manganonordite-(Ce) (Table 1). Due to structural and compositional differences meieranite is considered as an unassigned member of the nordite supergroup and it will be the first mineral of the potential meieranite group if a related species is reported. The supergroup is named according to the first described species, nordite-(La) (Gerasimovsky, Reference Gerasimovsky1941).
Members of the nordite supergroup are classified according to the following rules. The rootname is dependent on the dominant cation located on the X site (Table 2). In the nordite group, the nordite rootname is applied to species with X = Sr, and new rootnames will be applied to species with X ≠ Sr. In the potential meieranite group, the meieranite rootname is applied to species with X = Sr and new rootnames are required for species with X ≠ Sr. In the case of the species belonging to the nordite group the Levinson suffix (Bayliss and Levinson, Reference Bayliss and Levinson1988) is added to the names to indicate the dominant REE on the crystallographic Y site.
In both nordite and meieranite groups a prefix is added according to the dominant chemical composition of the tetrahedral Z site; magnesio (Mg), mangano (Mn2+), ferro (Fe2+) and zinco (Zn). The first described nordite-group mineral, nordite-(La), has Z = Zn, and consequently the prefix zinco is not used in approved or new species belonging to the nordite group. The prefix rule is the same in the potential meieranite group when Z = Mg. The first described species (meieranite) has Z = Mg, therefore the prefix magnesio is not added.
The published data on nordite-group minerals indicate that only limited cationic substitutions occur on the A and B sites. Although, in the case of end-members with A ≠ Na or B ≠ Na a new rootname must be used and the rules given above have to be applied. Meieranite has so far been reported from only one locality, and therefore it is challenging to predict all potential new members. The nomenclature scheme proposed herein provides to the mineralogical community a tool for the classification of nordite-supergroup minerals according to their crystal-chemical properties.
Acknowledgements
We thank Principal Editor Stuart J. Mills and Irina Galuskina for handling the manuscript as well as Igor V. Pekov and two anonymous reviewers for their helpful suggestions and comments that improved the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.42












