Monochorionic twin pregnancies are at increased risk for adverse outcome compared to dichorionic twin pregnancies and singletons. This is primarily due to the fact that almost all monochorionic twins share a single placenta, with inter-twin anastomoses allowing blood to flow bidirectionally between the two fetuses. Unbalanced net inter-twin blood transfusion may lead to various complications, including TTTS and twin anemia polycythemia sequence (TAPS). TTTS was first described in the 19th century and results from imbalanced inter-twin blood flow causing hypovolemia and oligohydramnios in the donor and hypervolemia and polyhydramnios in the recipient twin, the so-called TOPS. TAPS is a newly described form of chronic and slow inter-twin blood transfusion characterized by large inter-twin Hb differences without signs of TOPS. The pathogenesis of TAPS is based on the presence of a few minuscule vascular anastomoses. TAPS may occur spontaneously in monochorionic twin pregnancies or may occur in TTTS cases after incomplete laser surgery due to a few small residual anastomoses. The post-laser form of TAPS was first described in 2006 (Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006), whereas the spontaneous form of TAPS, as well as the acronym TAPS, was first described in 2007 (Lopriore, Middeldorp et al., Reference Lopriore, Middeldorp, Oepkes, Kanhai, Walther and Vandenbussche2007). In the past decade, over 100 studies have been published on TAPS, and our knowledge and awareness have gradually increased. This review focuses on the epidemiology, pathogenesis, diagnostic criteria, management options, and short- and long-term outcome in TAPS, and summarizes the data gathered since the first description of TAPS a decade ago.
Epidemiology
TAPS may occur spontaneously (spontaneous TAPS) or after laser treatment for TTTS (post-laser TAPS). Spontaneous TAPS occurs in 3–5% of monochorionic twin pregnancies (Casanova et al., Reference Casanova, Paiva, Carvalho and Cunha2014; Gucciardo et al., Reference Gucciardo, Lewi, Vaast, Debska, De Catte, Van Mieghem and Deprest2010; Lewi et al., Reference Lewi, Jani, Blickstein, Huber, Gucciardo, Van Mieghem and Deprest2008; Lopriore et al., Reference Lopriore, Slaghekke, Middeldorp, Klumper, Oepkes and Vandenbussche2009; Yokouchi et al., Reference Yokouchi, Murakoshi, Mishima, Yano, Ohashi, Suzuki and Torii2015), whereas post-laser TAPS occurs in 2–16% of TTTS cases after incomplete laser treatment (Habli et al., Reference Habli, Bombrys, Lewis, Lim, Polzin, Maxwell and Crombleholme2009; Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006; Slaghekke, Lewi et al., Reference Slaghekke, Lewi, Middeldorp, Weingertner, Klumper, Dekoninck and Lopriore2014). The wide range in incidence rate in post-laser TAPS can be explained by the use of different laser surgical techniques and/or the existence of different definitions and criteria for TAPS (see sections below).
Pathogenesis
Placental Characteristics
The pathogenesis of TAPS is based on the unique angioarchitecture of the placenta, characterized by the presence of only few minuscule anastomoses. These few small anastomoses shared between the two placentas allow a chronic and slow transfusion of blood from the donor to the recipient twin (Figure 1). The actual blood flow through these small anastomoses ranges from 5 to 15 mL per 24 hours (Lopriore, Middeldorp et al., Reference Lopriore, Middeldorp, Oepkes, Kanhai, Walther and Vandenbussche2007; Lopriore, van den Wijngaard et al., Reference Lopriore, van den Wijngaard, Middeldorp, Oepkes, Walther, van Gemert and Vandenbussche2007). This process gradually leads to highly discordant Hb levels, causing the donor twin to become anemic and the recipient twin to become polycythemic. In monochorionic placentas, three types of anastomoses have been described: arterio-venous (AV), arterio-arterial (AA), and veno-venous (VV) anastomoses. AV anastomoses are unidirectional whereas AA and VV anastomoses are bidirectional. TAPS placentas are characterized by the presence of a few very small AV anastomoses, with a diameter <1 mm. TAPS placentas have on average 3 to 4 anastomoses compared to an average of 8 anastomoses in normal monochorionic placentas (Zhao et al., Reference Zhao, de Villiers, Slaghekke, Walther, Middeldorp, Oepkes and Lopriore2013). AA anastomoses are rare in TAPS cases and occur in 10–20% of TAPS placentas. AA anastomoses are considered to protect against the development of TTTS or TAPS because of the bidirectional blood flow, allowing inter-twin equilibration of blood volumes (de Villiers et al., Reference de Villiers, Slaghekke, Middeldorp, Walther, Oepkes and Lopriore2012; Lopriore, van den Wijngaard et al., Reference Lopriore, van den Wijngaard, Middeldorp, Oepkes, Walther, van Gemert and Vandenbussche2007; Suzuki, Reference Suzuki2010; van Meir et al., Reference van Meir, Slaghekke, Lopriore and van Wijngaarden2010).The size of AA-anastomoses in TAPS appear to be significantly smaller (diameter <1 mm) compared to AA anastomoses in TTTS cases or in uncomplicated monochorionic twins (Zhao et al., Reference Zhao, de Villiers, Slaghekke, Walther, Middeldorp, Oepkes and Lopriore2013). Spontaneous TAPS placentas differ from post-laser TAPS placentas. Spontaneous TAPS placentas have a higher total number of anastomoses compared to post-laser TAPS placentas, 4 versus 2, respectively. Miniscule AA anastomoses were detected sporadically in both groups, but the rate of AA anastomoses was slightly higher in the spontaneous group (de Villiers et al., Reference de Villiers, Slaghekke, Middeldorp, Walther, Oepkes and Lopriore2013).

FIGURE 1 A spontaneous TAPS placenta injected with color dye. Blue and green dye was used for the arteries and yellow and pink for the veins, showing the presence of only a few very small anastomoses.
TAPS Versus TTTS
The absence of oligohydramnios and polyhydramnios is an essential element in the diagnosis of TAPS. The presence of TOPS is only pathognomonic for TTTS. Importantly, 2–8% of TTTS cases may have pre-operative signs of severe fetal anemia in the donor and polycythemia in the recipient (Donepudi et al., Reference Donepudi, Papanna, Snowise, Johnson, Bebbington and Moise2015; Van Winden et al., Reference Van Winden, Quintero, Kontopoulos, Korst, Llanes and Chmait2015)
The mechanism preventing the donor twin to develop oligohydramnios and the recipient twin to develop polyhydramnios in TAPS cases is not entirely clear. The most probable explanation is that due to the chronic character of TAPS, slow inter-twin blood transfusion allows more time for the hemodynamic compensatory mechanisms to take place (Lopriore, Deprest et al., Reference Lopriore, Deprest, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Lewi2008). While TTTS results from large, unbalanced inter-twin blood transfusion in combination with unbalanced hormonal regulation with rapid deterioration, TAPS probably results from slow inter-twin blood transfusion without hormonal imbalance and a slow deterioration (van den Wijngaard et al., Reference van den Wijngaard, Lewi, Lopriore, Robyr, Middeldorp, Vandenbussche and van Gemert2007). In TTTS, donor twins show high renin levels, presumably as a result of renal hypoperfusion (Mahieu-Caputo et al., Reference Mahieu-Caputo, Dommergues, Delezoide, Lacoste, Cai, Narcy and Gubler2000), and recipients have absent renin protein, presumably because of down-regulation due to hypervolemia. This hormonal discordance has not been described in TAPS pregnancies.
Interestingly, in post-laser TAPS, it is usually the former recipient who becomes anemic, whereas the former donor becomes polycythemic (Lewi et al., Reference Lewi, Jani, Cannie, Robyr, Ville, Hecher and Deprest2006; Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2005, Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006; Yamamoto et al., Reference Yamamoto, El Murr, Robyr, Leleu, Takahashi and Ville2005). A possible explanation could be that the colloid osmotic pressure of the ex-recipient is strongly increased prior to and shortly after laser therapy, which attracts excess fluid from the maternal blood to the recipient's fetal blood. This source of increased fetal plasma volume followed by amniotic fluid production may delay the development of oligohydramnios in the ex-recipient who becomes the TAPS donor (van den Wijngaard et al. Reference van den Wijngaard, Lewi, Lopriore, Robyr, Middeldorp, Vandenbussche and van Gemert2007).
TAPS Versus Uncomplicated Monochorionic Twins
In addition to the small anastomoses that characterize TAPS placentas, TAPS placentas also show a remarkable difference in placental shares. In uncomplicated monochorionic twins, the placental share is the principal element affecting fetal growth and final birth weight, that is, the smaller twin usually has a relatively smaller placental share (Lewi et al., Reference Lewi, Cannie, Blickstein, Jani, Huber, Hecher and Deprest2007). In TAPS twins, the donor twin is usually smaller than the recipient twin but often has a paradoxically larger placental share compared to its co-twin. In contrast to uncomplicated monochorionic twins, fetal growth in TAPS appears to be primarily determined by inter-twin blood transfusion rather than placental share. A relatively larger placental share may enable the fetal survival of the anemic twin in TAPS (Zhao et al. Reference Zhao, Slaghekke, Middeldorp, Duan, Oepkes and Lopriore2014). In addition, TAPS donor twins also have hypoalbuminemia and hypoproteinemia due to loss of not only blood cells, but also proteins and nutrients, which may partly also affect their fetal growth (Verbeek et al., Reference Verbeek, Slaghekke, Hulzebos, Oepkes, Walther and Lopriore2013).
Diagnosis
TAPS can be diagnosed either antenatally or post-natally. TAPS is characterized by Hb differences with the absence of antenatal ultrasound signs of polyhydramnios in the recipient and oligohydramnios in the donor. Antenatal diagnosis is based on ultrasound abnormalities whereas the post-natal diagnosis is derived from large inter-twin hematologic discordances and characteristic placental angioarchitecture.
Antenatal Criteria
Antenatal diagnosis of TAPS is based on Doppler ultrasound abnormalities. MCA-PSV measurement, a non-invasive test, has become the standard test for the prediction of fetal anemia in singletons in a variety of fetal diseases. In TAPS, this test will show an increased MCA-PSV in the donor twin, suggestive of fetal anemia, and decreased velocities in the MCA-PSV in the recipient, suggestive of polycythemia. During the past decade, different MCA-PSV values for the diagnosis of TAPS have been proposed. Robyr et al. (Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006) initially suggested the use of a MCA-PSV >1.5 MoM for the donor twin and <0.8 MoM for the recipient twin. However, Slaghekke et al. (Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010) showed that a MCA-PSV between 0.8 and 1.0 MoM in the recipient was also frequently found in post-natally diagnosed TAPS cases and suggested therefore the currently used cut-off of MCA-PSV <1.0 MoM in the recipient twin and >1.5 MoM in the donor twin. This cut-off level is characterized by high sensitivity and specificity of MCA-PSV for both anemia and polycythemia, confirming the clinical usefulness of this non-invasive test (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010). The MCA-PSV measurement has a high diagnostic accuracy for predicting abnormal Hb levels in fetuses with TAPS (Slaghekke, Pasman et al., Reference Slaghekke, Pasman, Veujoz, Middeldorp, Lewi, Devlieger and Oepkes2015; Veujoz et al. Reference Veujoz, Sananes, Severac, Meyer, Weingertner, Kohler and Favre2015). However, the high predictive value of MCA-PSV measurements reported in the study from Slaghekke et al. (Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010) was determined in a highly selected group of TAPS-only cases followed in a specialized fetal therapy center. In a recent study, Fishel-Bartal et al. (Reference Fishel-Bartal, Weisz, Mazaki-Tovi, Ashwal, Chayen, Lipitz and Yinon2015) did not find a similar predictive value of MCA-PSV for the detection of polycythemia in monochorionic twins. MCA-PSV measurement in polycythemic twins did not differ compared to normal twins. Fishel-Bartal et al. (Reference Fishel-Bartal, Weisz, Mazaki-Tovi, Ashwal, Chayen, Lipitz and Yinon2015) did find a significant correlation between a large inter-twin difference in MCA-PSV (delta MCA-PSV) and large inter-twin hematocrit difference and proposed the alternative use of a delta MCA-PSV >0.5 MoM instead of the currently used fixed cut-off levels of <1.0 MoM and >1.5 MoM for the pre-natal diagnosis of TAPS.
In some TAPS cases, additional ultrasound findings have been reported. In several cases of spontaneous TAPS, a striking difference in placental thickness and echodensity on ultrasound examination was detected (Figure 2; Movva & Rijhsinghani, Reference Movva and Rijhsinghani2014; Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010; Stritzke et al., Reference Stritzke, Thomas and Somerset2014). This difference can be explained by the hydropic and echogenic character of the anemic placental share in contrast to the normal appearance of the polycythemic placental share. Another ultrasound finding described in TAPS is the so-called ‘starry sky liver’ (Figure 3; Soundararajan & Howe, Reference Soundararajan and Howe2014). Starry sky appearance refers to a sonographic pattern of the liver, characterized by clearly identified portal venules (stars) and diminished parenchymal echogenicity (sky) that accentuates the portal venule walls. Starry sky liver is usually reported in acute hepatitis, but other conditions like heart failure can lead to this typical finding as well. More studies are needed to further investigate the validity and significance of these antenatal ultrasound findings for the diagnosis of TAPS.

FIGURE 2 Ultrasound image of a TAPS placenta showing a difference in placental thickness and echodensity. On the left side of the image the hydropic and echogenic placental share of the anemic donor and on the right side the normal aspect of the placenta of the recipient is depicted.

FIGURE 3 Ultrasound image showing a starry sky liver in a TAPS recipient with clearly identified portal venules (stars) and diminished parenchymal echogenicity (sky) that accentuates the portal venule walls.
Since diagnosis of TAPS at an earlier gestational age is associated with more favorable outcomes (Rossi & Prefumo, Reference Rossi and Prefumo2014), we recommend the routine use (at least biweekly) of MCA-PSV Doppler ultrasound measurements in all monochorionic pregnancies to timely detect TAPS.
Postnatal Criteria
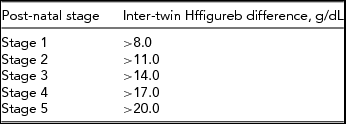
In 40–63% of the cases, TAPS is not detected antenatally, but is only diagnosed after birth (de Villiers et al., Reference de Villiers, Slaghekke, Middeldorp, Walther, Oepkes and Lopriore2013; Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010). Therefore, post-natal diagnostic criteria have been proposed. These criteria are based on the presence of (chronic) anemia in the donor and polycythemia in the recipient twin (Figure 4) and the characteristic angioarchitecture of the placenta. Different cut-off levels for anemia and polycythemia have been used since the first description of TAPS in the last decade. Lewi et al. (Reference Lewi, Jani, Blickstein, Huber, Gucciardo, Van Mieghem and Deprest2008) first defined TAPS as the presence of an Hb level <11g/dL in the anemic twin and >20g/dL in the polycythemic co-twin. However, absolute Hb levels do not take the positive association between Hb levels and higher gestational age into account. Fetal Hb concentrations are known to increase linearly with gestation (Jopling et al., Reference Jopling, Henry, Wiedmeier and Christensen2009; Lubin, Reference Lubin1978; Nicolaides et al., Reference Nicolaides, Thilaganathan and Mibashan1989). Lopriore, Middeldorp et al. (Reference Lopriore, Middeldorp, Oepkes, Kanhai, Walther and Vandenbussche2007) used gestational-age-dependent cut-off levels to define anemia in the donor (Hb <5th centile) and polycythemia in the recipient (hematocrit >65%). However, this contained practical disadvantages since it required the use of specific normograms related to gestational age. Several normograms have been published and differ slightly from one another (Jopling et al., Reference Jopling, Henry, Wiedmeier and Christensen2009; Nicolaides et al., Reference Nicolaides, Thilaganathan and Mibashan1989). Since a fixed inter-twin Hb difference is a more logical and pragmatic criterion, nowadays an inter-twin Hb difference >8 g/dL is commonly used, based on a case-control study were all TAPS cases had an inter-twin Hb difference >8 g/dL (Lopriore et al., Reference Lopriore, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Walther2010).
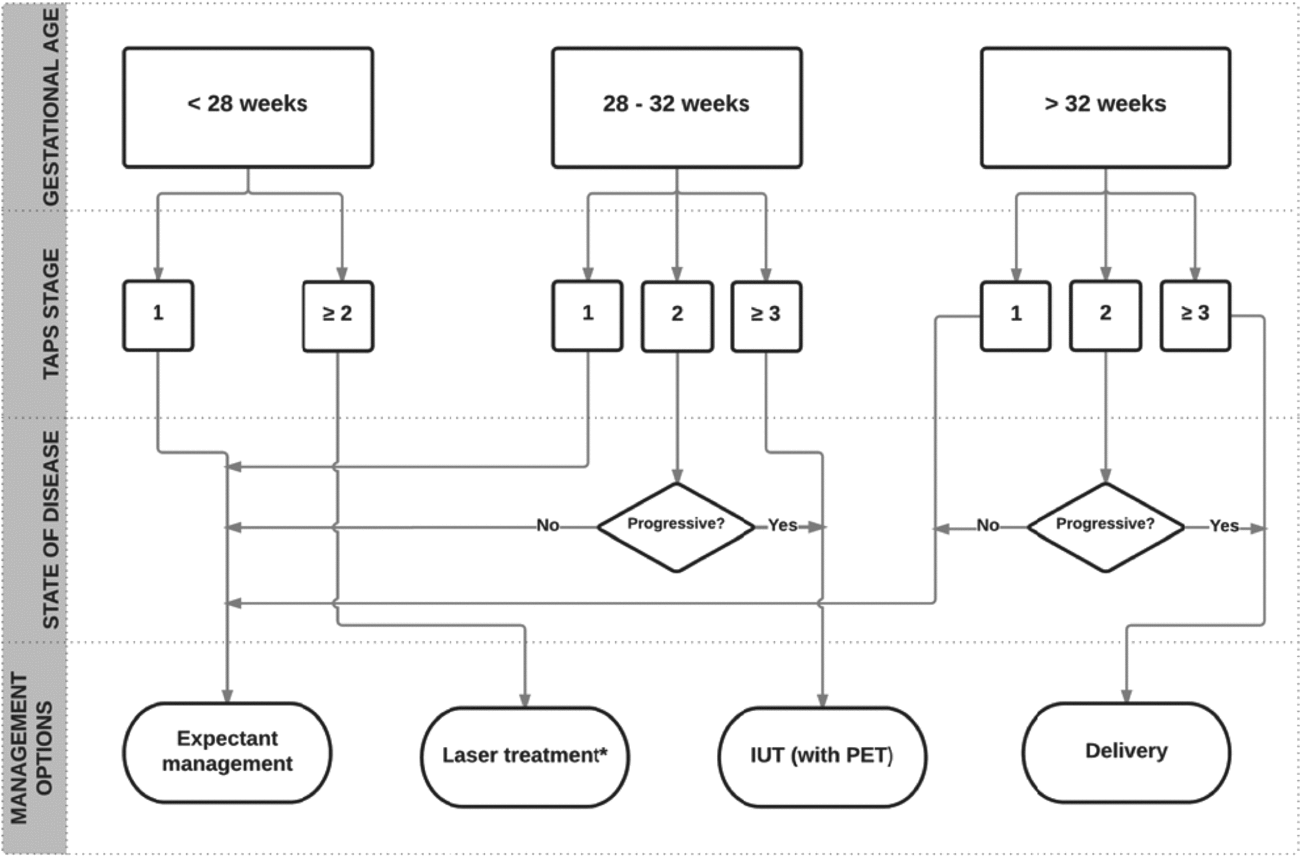
FIGURE 4 Flowchart with antenatal management options for TAPS.
A large Hb difference (>8 g/dL) at birth is also detected in case of acute peripartum TTTS. Distinction between these two clinical pictures (TAPS and acute peripartum TTTS) is important, as they require a different therapeutic neonatal management. Two additional criteria are required to distinguish TAPS from acute peripartum TTTS (Lopriore et al., Reference Lopriore, Sueters, Middeldorp, Vandenbussche and Walther2005). The first criterion is an increased reticulocyte count measured in the TAPS donor (as a result of increased erythropoiesis due to chronic anemia). An inter-twin reticulocyt count ratio >1.7 is pathognomonic for TAPS (Lopriore et al., Reference Lopriore, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Walther2010). This ratio is measured by dividing the reticulocyte count of the donor by the reticulocyte count of the recipient. The second criterion for the post-natal diagnosis of TAPS is the presence of small residual anastomoses (diameter <1 mm) at the placental surface (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010), detected through color dye injection of the placenta (Lopriore et al., Reference Lopriore, Slaghekke, Middeldorp, Klumper, van Lith, Walther and Oepkes2011). In acute peripartum TTTS, blood transfusion from the donor twin to the recipients occurs rapidly and reticulocyte count in the donor is typically still low. Acute anemia will eventually lead to increased erythropoiesis, but the increased reticulocyte production is not detected in the acute phase. In addition, in contrast to TAPS, the pathogenesis of acute peripartum TTTS is based on large placental AA or VV anastomoses with low resistance, allowing large amount of blood to flow directly from the donor to the recipient (Lopriore et al., Reference Lopriore, Sueters, Middeldorp, Vandenbussche and Walther2005).
Since reticulocyte count is not always measured and placental injection is difficult to perform, we recently studied a new additional criterion for post-natal diagnosis of TAPS. In analogy with the difference in skin color of the TAPS twins at birth (the anemic donor is pale and the polycythemic recipient is plethoric), the maternal side of the TAPS placenta also shows a striking color difference (Figure 5 & Figure 6). We developed a new quick and easy tool to determine the color difference ratio (CDR) between the two placental shares using digital pictures (Tollenaar et al., Reference Tollenaar, Zhao, Middeldorp, Slaghekke, Oepkes and Lopriore2016). We found that TAPS placentas had a significantly higher CDR (>1.5) compared to uncomplicated monochorionic twin placentas. Whether this method can eventually be added to the list of post-natal criteria requires further investigations in larger series of placentas with and without TAPS to determine the sensitivity and specificity of the test. Importantly, CDR measurements should also be investigated in placentas with acute TTTS to determine whether this method can help distinguish TAPS cases from cases with acute peripartum TTTS, since in both situations large inter-twin Hb differences are present at birth.

FIGURE 5 Spontaneous TAPS twins at birth. On the left, the plethoric polycythemic recipient and on the right the pale anemic donor.

FIGURE 6 Maternal side of the TAPS placenta showing the difference in color between the plethoric share of the recipient (left side of the placenta) and the anemic share of the donor (right side of the placenta).
Classification
Since TAPS is a heterogeneous disease, a staging system can be helpful to discriminate between the various forms. In addition, a staging system may also prove to be useful in the future to compare and analyze TAPS cases (including effect of treatment) between the various centers. We, therefore, recently proposed both an antenatal and post-natal classification system (Tables 1 and 2). Whether this classification has an additional value to adequately stage and treat TAPS requires further investigation.
TABLE 1 Antenatal TAPS Classification

TABLE 2 Post-natal TAPS Classification
Perinatal Management and Outcome
The optimal perinatal management for TAPS is not clear. Options include expectant management, induction of labor, IUTin the donor, with or without PET in the recipient, selective feticide, and (repeat) fetoscopic laser surgery.
Expectant management consists of close monitoring with ultrasound including Doppler measurements of MCA-PSV. Close monitoring can be considered in less severe cases of TAPS, such as stage 1 and 2. Whether close monitoring is safe enough in these TAPS stages needs to be evaluated in combination with validating the staging system. When TAPS stage 1 quickly progresses to stage 2 or stage ≥3, intra-uterine intervention or termination of pregnancy should be considered. However, both management options may lead to pre-mature delivery and its associated risks of perinatal morbidity and mortality.
Intrauterine Blood Transfusion
Treatment with IUT in the donor can be performed either intravascularly or intraperitoneal. Intraperitoneal IUT is preferred, since intraperitoneal transfusion may allow slower absorption of red blood cells into the fetal circulation, preventing rapid loss of transfused blood in the circulation of the recipient twin (Herway et al., Reference Herway, Johnson, Moise and Moise2009). Although treatment with IUT has often been reported, it is not a causal treatment and is only a temporary solution. Furthermore, a potential side effect of IUT treatment is worsening of the polycythemia hyperviscosity syndrome in the recipient. Robyr et al. (Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006) reported skin necrosis of the leg in the recipient twin of a TAPS case treated with several IUTs. To reduce the risk of increasing polycythemia hyperviscosity, a combination procedure of IUT in the donor and PET in the recipient can be of additional value. The rationale behind this therapy is that PET may help to decrease the viscosity of the blood of the polycythemic recipient. Genova et al. (Reference Genova, Slaghekke, Klumper, Middeldorp, Steggerda, Oepkes and Lopriore2013) reported on three different TAPS cases treated with IUT with PET. We recently developed a computational model to evaluate the effect of IUT with and without PET in post-laser TAPS cases, and showed the beneficial effect of PET (Slaghekke, van den Wijngaard et al., Reference Slaghekke, van den Wijngaard, Akkermans, van Gemert, Middeldorp, Klumper and Lopriore2015).
Since TAPS twins share their blood circulation and therefore have exactly the same blood cell characteristics, it may be of additional value to transfuse the anemic twin with the recipient's whole blood as a donor source instead of foreign donor blood. Recently, Yarci et al. (Reference Yarci, Alyamac Dizdar, Oncel, Kose Cetinkaya, Derme, Canpolat and Dilmen2014) reported a case of TAPS in which the anemic donor was successfully transfused after delivery with blood obtained from the polycythemic co-twin during PET. The main advantage of this new therapeutic method is avoidance of donor exposure and of the risk of blood product infections (Yarci et al., Reference Yarci, Alyamac Dizdar, Oncel, Kose Cetinkaya, Derme, Canpolat and Dilmen2014). Whether this new approach may lead to decreased morbidity in TAPS pregnancies requires further investigation.
Fetoscopic Laser Coagulation
The only causal treatment of TAPS is (repeated) fetoscopic laser coagulation of the (residual) anastomoses at the vascular equator of the placental. Fetoscopic laser coagulation in TAPS is more challenging than in TTTS, since the absence of oligo-polyhydramnios sequences and therefore a wavering inter-twin membrane makes the visualization of the vascular equator more difficult (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010). Moreover, placental anastomoses in TAPS are known to be only few and minuscule and may therefore be missed during fetoscopy (Slaghekke et al., Reference Slaghekke, Kist, Oepkes, Pasman, Middeldorp, Klumper and Lopriore2010). Different case reports show the feasibility of fetoscopic laser coagulation in TAPS placentas (Abdel-Sattar et al., Reference Abdel-Sattar, Platt, DeVore, Porto, Benirschke and Chmait2014; Assaf et al., Reference Assaf, Benirschke and Chmait2011; Diehl et al., Reference Diehl, Glosemeyer, Tavares De Sousa, Hollwitz, Ortmeyer and Hecher2013; Groussolles et al., Reference Groussolles, Sartor, Connan and Vayssiere2012; Ishii et al., Reference Ishii, Hayashi, Mabuchi, Taguchi, Yamamoto, Murata and Mitsuda2014). In a retrospective study where laser treatment for antenatally detected TAPS is compared to IUT or expectant management, laser therapy appeared to improve perinatal outcome by prolonging pregnancy and reducing respiratory distress syndrome (Slaghekke, Favre et al., Reference Slaghekke, Favre, Peeters, Middeldorp, Weingertner, van Zwet and Lopriore2014). The median time between diagnosis and birth was 11 weeks in the laser group compared to 5 weeks after intrauterine transfusion, and 8 weeks after expectant management. In the laser group, no residual anastomoses were found after color dye injection. Larger, adequately randomized controlled studies are required to determine the optimal management and to evaluate the possible additional value of fetoscopic laser coagulation for the treatment of TAPS. When performing laser coagulation in TAPS placentas, we recommend using the Solomon technique to reduce the risk of residual anastomoses and recurrent TAPS (Slaghekke, Lewi et al., Reference Slaghekke, Lewi, Middeldorp, Weingertner, Klumper, Dekoninck and Lopriore2014). In the Solomon technique, a line is drawn from one placenta margin to the other, connecting the individual laser spots (Slaghekke, Lopriore et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014).
In unique circumstances, spontaneous resolution of antenatal TAPS may also occur. Spontaneous resolution has been reported once (Lopriore, Hecher et al., Reference Lopriore, Hecher, Vandenbussche, van den Wijngaard, Klumper and Oepkes2008) and was presumably caused by thrombosis of the residual AV-anastomosis. Whether expectant management would lead to spontaneous resolution in other TAPS cases is unknown and should be considered unlikely.
Proposal for Antenatal Management
In the absence of evidence on optimal management, we suggest that management decisions should be made after careful evaluation of different factors, including TAPS stage, gestational age, and the feasibility of the different types of intra-uterine intervention. TAPS stage 1 and possibly stage 2 can be observed with close monitoring. In case TAPS progresses quickly to stage 2 or in case of stage ≥3, intervention should be considered. If gestational age is below 28 weeks and laser treatment is feasible, laser treatment should be considered, since this is the only causal treatment for TAPS and is known to prolong the pregnancy (Slaghekke, Favre et al., Reference Slaghekke, Favre, Peeters, Middeldorp, Weingertner, van Zwet and Lopriore2014). When laser treatment is not feasible and gestational age is below 30 to 32 weeks of pregnancy, intra-uterine transfusion should be considered. When repeated intra-uterine transfusions are expected or in case of severe polycythemia in the recipient, PET of the recipient should be envisaged. A management proposal for antenatal TAPS is presented in the flowchart in Figure 4. Whether this flowchart is useful in current practice and will improve outcome still needs to be validated.
Prevention of Post-Laser TAPS
Post-laser TAPS is caused by residual anastomoses at the placental surface after fetoscopic laser surgery for TTTS. In order to reduce the number of residual anastomoses, the Solomon technique was introduced. The Solomon randomized trial by Slaghekke, Lewi et al. (Reference Slaghekke, Lewi, Middeldorp, Weingertner, Klumper, Dekoninck and Lopriore2014) showed a significant reduction of post-laser TAPS of 16% in the standard treatment group to 3% in the Solomon group. The Solomon technique did not appear to be associated with an increase in any identifiable short-term adverse outcome or complications. A study investigating the neurodevelopmental outcome at 2 years in TAPS survivors randomized for the Solomon trail showed no difference in the risk of neurodevelopmental impairment between the groups treated with the Solomon technique and the standard laser technique (van Klink et al., Reference van Klink, Slaghekke, Balestriero, Scelsa, Introvini, Rustico and Lopriore2015). The Solomon technique should therefore be used in all TTTS cases to reduce the risks of residual anastomoses and prevent the occurrence of post-laser TAPS.
Neonatal and Pediatric Outcome
Data of perinatal mortality and morbidity rates in TAPS are scarce and mostly based on case reports and small series. The neonatal outcome in TAPS may vary from isolated large inter-twin Hb differences to severe neonatal morbidity, including cerebral injury and neonatal death (Luminoso et al., Reference Luminoso, Figueira, Marins and Peralta2013).
Short-Term Neonatal Outcome
Hematological complications are commonly seen in TAPS donors and recipients, requiring blood transfusion or PET, respectively. TAPS recipients may develop polycythemia hyperviscosity syndrome, which may possibly lead to necrosis of the skin and multiple limb ischemia (Robyr et al., Reference Robyr, Lewi, Salomon, Yamamoto, Bernard, Deprest and Ville2006; Stranak et al., Reference Stranak, Korcek, Hympanova, Kyncl and Krofta2015). In addition, recipients are at increased risk of thrombocytopenia, probably due to impaired production secondary to tissue hypoxia and slow spleen blood flow (Lopriore et al., Reference Lopriore, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Walther2010; Sarkar & Rosenkrantz, Reference Sarkar and Rosenkrantz2008). Platelet count at birth was inversely related to the severity of polycythemia in recipients (Lopriore et al., Reference Lopriore, Slaghekke, Oepkes, Middeldorp, Vandenbussche and Walther2010). In addition to lower Hb levels, donor twins with TAPS also have significantly lower albumin and total protein levels compared to recipient twins, suggesting that the inter-twin transfusion process does not only concern red blood cells but also proteins and albumin (Verbeek et al., Reference Verbeek, Slaghekke, Hulzebos, Oepkes, Walther and Lopriore2013). Chronic inter-twin transfusion in TAPS may also cause short-term renal dysfunction: Verbeek et al. (Reference Verbeek, Slaghekke, Favre, Vieujoz, Cavigioli, Lista and Lopriore2015) found that donor twins with TAPS have higher creatinine levels than recipients, probably due to chronic renal hypoperfusion. Whether donor twins may also have permanent renal damage and long-term renal complications is not known. Chronic severe anemia in donor twins and polycythemia in recipient twins may theoretically also lead to cerebral injury. Several small case studies report on severe cerebral injury leading to fatal outcome in TAPS (Genova et al., Reference Genova, Slaghekke, Klumper, Middeldorp, Steggerda, Oepkes and Lopriore2013; Lopriore et al., Reference Lopriore, Slaghekke, Kersbergen, de Vries, Drogtrop, Middeldorp and Benders2013). Genova et al. (Reference Genova, Slaghekke, Klumper, Middeldorp, Steggerda, Oepkes and Lopriore2013) described a TAPS case, in which despite treatment with IUT with PET, the anemic twin died of extensive cerebral injury including numerous large cysts in the basal ganglia, bilateral white matter injury, and multiple microbleeds. Lopriore et al. (Reference Lopriore, Slaghekke, Kersbergen, de Vries, Drogtrop, Middeldorp and Benders2013) reported on a spontaneous TAPS case delivered after an emergency cesarean section at 33 weeks gestation. The recipient twin suffered from severe cerebral injury due to massive hemorrhage and infarctions and died on day 3 after withdrawal of intensive care.
Long-Term Neurodevelopmental Outcome
The long-term neurodevelopmental outcome in surviving TAPS infants is not well known and data is based on small uncontrolled case series. Severe long-term morbidity such as bilateral deafness and spastic paralysis has recently reported (Taniguchi et al., Reference Taniguchi, Sumie, Sugibayashi, Wada, Matsuoka and Sago2015). In another recent study on long-term neurodevelopmental outcome in post-laser TAPS, Slaghekke, van Klink et al. (Reference Slaghekke, van Klink, Koopman, Middeldorp, Oepkes and Lopriore2014) detected neurodevelopmental impairment or mild to moderate cognitive delay in 9% and 17% of TAPS survivors, respectively. No difference in impairment was found between donors and recipients. The rate of impairment in TAPS seems to be comparable to the rate of impairment in children with TTTS after laser surgery. Risk factors for decreased cognitive scores in the study from Slaghekke et al. were low gestational age at birth and low birth weight, as well as intrauterine transfusion. In a recent small study, neonatal outcome in monochorionic twins affected by TAPS appeared to be comparable to gestational age-matched uncomplicated monochorionic twins. However, only 10 TAPS cases were included with mild TAPS (stage 1 and 2), limiting the conclusions (Ashwal et al., Reference Ashwal, Yinon, Fishel-Bartal, Tsur, Chayen, Weisz and Lipitz2015). To date, there are no studies reporting on neurological, motor, and cognitive outcomes of TAPS twins in childhood and adolescence. Data from these studies could provide us with a more complete view of the long-term consequences of TAPS.
Conclusion
TAPS is recently described form of feto-fetal transfusion through small (diameter <1 mm) anastomoses that may occur in monochorionic twins spontaneously or in TTTS cases after laser surgery (post-laser TAPS). In the past decade, our knowledge on pathogenesis, diagnostic criteria, management options, and short- and long-term outcome has greatly increased. However, further studies are required to determine the optimal diagnostic criteria. Whether a delta MCA-PSV >0.5 (comparable to a delta Hb postnatally) is a better alternative criterion than the currently used fixed cut-off levels of <1.0 and >1.5 MoM of MCA-PSV requires further investigation. In addition, we recently introduced a new diagnostic criterion based on the color difference of the maternal side of the placenta. Whether this criterion can eventually be added to the list of post-natal criteria requires further investigations in larger series of placentas with and without TAPS.
Although different management options have been proposed, optimal treatment for TAPS is still unclear and remains a challenging problem due to lack of randomized trials. In this review, we proposed a stage and gestational age-based flow chart for the treatment of TAPS. Large randomized controlled trials are needed to test the clinical usefulness of this proposed flowchart.
Studies on long-term outcome in TAPS survivors show that neurodevelopmental outcome is similar to TTTS twins, but data on neurological, cognitive, and motor function for childhood and adulthood are still not available. Long-term follow-up studies comparing TAPS to TTTS and uncomplicated monochorionic twins are required to determine whether TAPS twins have an increased risk for developing adverse long-term neurodevelopmental outcome at an older age.
Since TAPS is a rare disease, collaboration between international fetal therapy centers is of utmost importance to increase sample size and quality of the studies. To facilitate this purpose, we have recently created a web-based registry (www.TAPSregistry.org) to gather information on the short- and long-term outcome in TAPS. This information will provide us crucial information to set up well-designed studies and investigate the optimal management in the future.










