The Mediterranean diet (MeDi) is a dietary pattern first described from observational studies which have reported low all-cause and CHD death rates in cohorts of the Mediterranean basin, with olive oil as the main fat(Reference Keys, Menotti and Karvonen1). There is no single MeDi but several definitions, since populations living in countries bordering the Mediterranean Sea have various dietary habits(Reference Simopoulos2, Reference Sofi3). Nevertheless, a high consumption of vegetables, legumes, fruits and cereals, a moderate-to-high intake of fish, a high MUFA:SFA ratio and a low intake of dairy products and meat, concomitant with a low-to-moderate intake of wine, define the common line of this dietary pattern(Reference Willett, Sacks and Trichopoulou4–Reference Bach, Serra-Majem and Carrasco6). The Mediterranean-style diet seems an optimal dietary strategy for health since a greater MeDi adherence, as evaluated by the MeDi score, has been associated with longer survival, reduced risk for cardiovascular mortality and cancer incidence and mortality(Reference Sofi, Cesari and Abbate7–Reference Sofi, Abbate and Gensini9). It has been hypothesised that n-3 PUFA and a balanced n-6:n-3 PUFA ratio associated with a high intake of antioxidants in the MeDi could be important determinants in influencing CVD(Reference Simopoulos2). Moreover, recent studies, the Washington Heights-Inwood Columbia Aging Project in the USA(Reference Scarmeas, Luchsinger and Schupf10) and the Three-City (3C) study in France(Reference Feart, Samieri and Rondeau11), have evidenced a decreased risk of Alzheimer's disease and a slower cognitive decline with greater MeDi adherence(Reference Feart, Samieri and Barberger-Gateau12). As for CVD protection, the MeDi combines several foods and nutrients which might protect against cognitive dysfunction or dementia such as fish, as the main provider of long-chain n-3 PUFA, olive oil, as the main source of MUFA, vitamins B12, folate, and antioxidants (vitamin E, carotenoids and flavonoids)(Reference Reynolds13–Reference Gillette Guyonnet, Abellan Van Kan and Andrieu20). In particular, there is a growing interest in the putative protective effects of fish and long-chain n-3 PUFA, notably DHA (22 : 6n-3), against age-related cognitive disorders(Reference Cunnane, Plourde and Pifferi21–Reference Yurko-Mauro, McCarthy and Rom23). However, computation of the MeDi score is based on dietary data, which may not accurately reflect the bioavailability of nutrients(Reference Trichopoulou, Costacou and Bamia5). Nutritional biomarkers are necessary to understand the mechanisms underlying the link between MeDi adherence and health outcomes. In particular, n-3 PUFA biomarkers are more precise risk predictors than dietary intake estimates(Reference Harris24). Plasma fatty acids have largely been used to validate nutrient intake but less frequently to characterise food patterns. Hence, data on the association between MeDi adherence and fatty acid status are scarce. A single observational study conducted in Greece has reported an association between MeDi adherence and plasma fatty acids in adults(Reference Panagiotakos, Kalogeropoulos and Pitsavos25). Nevertheless, several dietary patterns corresponding to a similar MeDi adherence may differ in other countries not close to the Mediterranean Sea(Reference Sofi3).
The present study examined whether spontaneous MeDi adherence was associated with plasma fatty acids in a large population-based sample of older people in France, included in the 3C study, where an association was evidenced between MeDi adherence and slower cognitive decline.
Subjects and methods
Participants
The data come from the Bordeaux sample of the 3C study, a prospective cohort study of vascular risk factors of dementia whose methodology has been described in detail elsewhere(26). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. The protocol of the 3C study was approved by the Consultative Committee for the Protection of Persons participating in Biomedical Research of the Kremlin-Bicêtre University Hospital (Paris, France). All participants signed a written consent. A sample of 2104 community dwellers aged 65 years and above was selected in 1999–2000 from the electoral rolls of Bordeaux. The baseline data collection included sociodemographic information, lifestyle, symptoms and complaints, medical history of CVD and stroke, blood pressure, past and present consumption of tobacco, alcohol and drug use, anthropometric data, neuropsychological testing, and blood sampling. The data were completed by a comprehensive dietary survey in the Bordeaux centre in 2001–2002. Bordeaux is also the only study centre where plasma fatty acid determination was performed.
Plasma lipid and fatty acid analysis
Fasting blood samples were obtained simultaneously from the baseline data collection in Bordeaux (n 1416). Blood was collected in heparinised vacutainers and centrifuged at 1000 g for 10 min. Plasma samples were divided into aliquots and frozen immediately at − 80°C. After 36 months of storage at − 80°C, aliquots were unfrozen for plasma fatty acid measurements, which were performed immediately. Total lipids were extracted from the plasma with 5 ml of hexane–isopropanol (3:2, v/v). Plasma fatty acid composition was determined from 2 ml of the lipid extract after transformation into isopropyl esters(Reference Peuchant, Wolff and Salles27). Separation of isopropyl esters was done on a gas chromatograph (Trace; Thermoelectron, Courtaboeuf Cedex, France) using a 25 m Carbowax capillary column (internal diameter 0·32 mm). Column conditions were 180°C for 5 min, increasing by 7·5°C/min to 220°C for 30 min. The injector was at 60°C, and the flame ionisation detector was at 250°C. Helium was used as the carrier gas (flow rate 2 ml/min). The peaks were identified by comparison with reference fatty acid esters (Sigma Chemical Company, Saint-Quentin Fallavier, France), and peak areas were measured with an automatic integrator (DP700; Fisons Instruments, Arcueil Cedex, France). The results of each fatty acid were expressed as percentage of total fatty acids. Total n-6 PUFA was the sum of linoleic acid (18 : 2n-6), γ-linolenic acid (18 : 3n-6) and arachidonic acid (AA, 20 : 4n-6) proportions, and total n-3 PUFA was the sum of α-linolenic acid (18 : 3n-3), EPA (20 : 5n-3), docosapentaenoic acid (22 : 5n-3) and DHA (22 : 6n-3) proportions. The sum of plasma 20 : 5n-3 and 22 : 6n-3 proportions was used as a proxy of the erythrocyte n-3 index(Reference Harris24). The MUFA:SFA, n-6:n-3 PUFA, linoleic acid:α-linolenic acid, AA:EPA, AA:DHA and AA:(EPA+DHA) ratios were calculated. Fatty acid composition was available only in the plasma.
Dietary assessment and Mediterranean diet score
In Bordeaux, participants (n 1660) were visited at home by a specifically trained dietitian who administered a FFQ and a 24 h dietary recall(Reference Feart, Jutand and Larrieu28, Reference Samieri, Jutand and Feart29). The 24 h recall was used to estimate nutrient intake in g/d, total energy intake in kJ/d and to compute the dietary MUFA:SFA ratio. Based on the FFQ, usual frequency of consumption in the previous year of forty categories of foods and beverages for each of the three main meals and three between-meal snacks was recorded in eleven classes. The food items were converted into number of servings per week and then aggregated into twenty food and beverage groups as described earlier(Reference Samieri, Jutand and Feart29). We computed mean number of servings per week for the food groups considered to be part of the MeDi: vegetables, fruits, legumes, cereals including bread, pasta and rice (whole and refined grains), fish, meat, dairy products and alcohol. The MeDi score was computed as follows: a value of 0 or 1 was assigned to each food group using sex-specific medians of the population as cut-offs (see e-Table 2 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). For beneficial components (vegetables, fruits, legumes, cereals and fish), individuals whose consumption was below the median were assigned a value of 0, compared with 1 for the others. For components presumed to be detrimental (meat and dairy products), individuals whose consumption was below the median were assigned 1, compared with 0 for the others. For alcohol, 1 point was given to mild-to-moderate consumers. The second quartile of the distribution of total alcohol was chosen as the cut-off in men and women separately. A score of 1 point was attributed for men if consumption was within 6–14 glasses/week (8·6–20 g/d) and for women if consumption was within 1–4 glasses/week (1·4–5·7 g/d). For the dietary MUFA:SFA ratio, ratios below the sex-specific median were given a value of 0, compared with 1 for those above the median. The MeDi score was generated by adding the scores (0 or 1 point) for each food category for each participant. Thus, the MeDi score could range from 0 to 9, with higher scores indicating greater MeDi adherence(Reference Trichopoulou, Costacou and Bamia5).
Other variables
Sociodemographic information recorded at baseline included age, sex, education, income and marital status. Height (m) and weight (kg) were measured by the interviewers at baseline. BMI was computed as the weight:height2 ratio. Biological data assessed at the same time were used to obtain information on the presence of chronic conditions including hypertension (blood pressure ≥ 140/90 mmHg or treated), diabetes (fasting blood glucose ≥ 7·2 mmol/l or treated) and hypercholesterolaemia (total serum cholesterol ≥ 6·2 mmol/l). The baseline blood sampling also included fasting plasma TAG measurement. Plasma TAG was analysed enzymatically using a multiparameter automated analyser (LX20; Coulter-Beckman, Paris, France) and used as a proxy of overall lipid status in our analysis. Smoking status and history of CVD or cerebrovascular disease were also considered as vascular risk factors(26). Genotyping was performed at the Lille Genopole (France), and apoE genotype was considered dichotomously: presence of at least one ɛ4 allele v. no ɛ4 allele(Reference Dufouil, Richard and Fievet30). The practice of physical exercise was assessed on a self-administered questionnaire(Reference Feart, Jutand and Larrieu28).
The diagnosis of dementia was based on a two-step procedure following the administration of the battery of neuropsychological tests(26). At each wave of the 3C study, the participants who were suspected of dementia on the basis of their present neuropsychological performances and declined relatively to a previous examination were examined by a neurologist. Then, an independent committee of neurologists reviewed all potential cases of dementia and analysed in depth the medical history of each participant to obtain a consensus on the diagnosis and aetiology according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition. In total, fifty-five subjects with dementia were identified and then excluded.
Statistical analyses
All statistical analyses were performed with the SAS Statistical package (version 9.1; SAS Institute, Cary, NC, USA). Participants were classified according to categories of the MeDi score. The MeDi categories (low, scores 0–3; middle, scores 4–5; high, scores 6–9) were defined so as to be nutritionally relevant, close to tertiles of the distribution of the MeDi score in our sample and similar to those used in previous studies(Reference Trichopoulou, Costacou and Bamia5, Reference Scarmeas, Luchsinger and Schupf10, Reference Feart, Samieri and Rondeau11). Demographic and clinical characteristics were compared between categories of the MeDi score using χ2 statistics for class variables and ANOVA, followed by 2 × 2 comparison post hoc tests for continuous variables (accepted significance at P < 0·05). The Pearson correlation coefficient was used to investigate the association between each plasma fatty acid or each fatty acid ratio and MeDi adherence as the continuous variable (scores 0–9). The association between each plasma fatty acid and each fatty acid ratio and MeDi adherence as the categorical variable was assessed using ANOVA. For each plasma fatty acid percentage or ratio, which was separately associated with MeDi adherence at P < 0·20 in Pearson's correlation analyses, multivariate linear regression was performed. Analyses of the association between plasma fatty acid percentages or ratios (entered into separate models as continuous variables) and MeDi adherence as the continuous variable were subsequently adjusted for age (continuous), sex, energy intake and physical activity in model 1, and for smoking status, BMI, apoE genotype and plasma TAG in addition to the previous covariates in model 2. The normality of residuals of each dependent variable (i.e. plasma fatty acid percentages or ratios) has been confirmed, and all hypotheses of each multivariate linear regression model have been satisfied in all statistical models. Statistical interactions between the MeDi score and apoE genotype were tested, and when a statistically significant interaction at P < 0·05 was detected, stratified analyses based on apoE genotype were conducted.
Results
Among the 1416 subjects with plasma fatty acid assessment, 1105 had a full dietary assessment. We excluded fifty-five participants with dementia. Thus, the study sample consisted of 1050 individuals, aged 75·9 years on average (range 67·8–94·9). Participants not included (n 366) in the present study were older, less educated and with lower income, had more often a medical history of stroke and were more often ex-smokers. They were more often ɛ4-allele carriers (24 v. 18·6 %, P = 0·02). Moreover, subjects not included had a different plasma fatty acid profile. They had higher mean plasma MUFA and 18 : 1n-9 proportions and significant higher AA:EPA, AA:(EPA+DHA) and total n-6:n-3 PUFA ratios than the included subjects (see e-Table 1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). Participants not included in the study had lower mean plasma total n-3 PUFA, 20 : 5n-3 and EPA+DHA index than the included participants. There were no significant differences for the other plasma fatty acids or ratios (P>0·05) (see e-Table 1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn).
The median of consumption of cereals was 23·0 servings per week for women v. 24·0 for men. The medians of consumption of dairy products, meat, vegetables, fruits, legumes and fish and of the MUFA:SFA ratio were very similar in men and women (see e-Table 2 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). The MeDi score ranged from 0 to 8 with less than 4 % of the total population in the extreme values (0 or 8). Of all the participants, 29 % had a low MeDi score (low category, scored as 0–3), 42 % had an MeDi score of 4 or 5 (middle category) and 29 % were considered high MeDi adherents (scored as 6–9). The mean MeDi score was 4·40 (sd 1·70, median 4·0), and its distribution was normal.
As expected, greater MeDi adherence was characterised by higher intake of vegetables, fruits, legumes, cereals and fish and by lower intake of meat and dairy products (Table 1).
Table 1 Number of servings per week for individual food categories, proportion of mild-to-moderate alcohol consumers and ratio of daily intake of MUFA:SFA by categories of the Mediterranean diet (MeDi) score among older persons living in Bordeaux, the Three-City study (2001–2002)
(Mean values and standard deviations, n 1050)
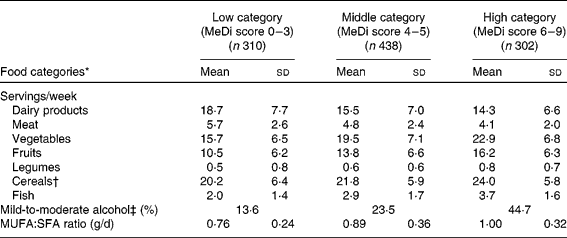
* All P values < 0·0001 for the ANOVA or for the χ2 test (proportion of mild-to-moderate alcohol consumers) among categories of the MeDi score.
† Cereals included consumption of bread, pasta and rice (whole and refined grains).
‡ For alcohol intake, we attributed a value of 1 for people whose consumption was mild to moderate, corresponding to the second quartile of the distribution of total alcohol intake. A score of 1 point was given for men if their consumption was within 6–14 glasses/week (8·6–20 g/d) and for women if their consumption was within 1–4 glasses/week (1·4–5·7 g/d).
MeDi adherence was not significantly associated with demographic or clinical characteristics (Table 2). Greater MeDi adherence was only associated with being married and never smoking status with borderline significance (P < 0·10).
Table 2 Demographic, clinical and dietary characteristics by categories of the Mediterranean diet (MeDi) score among older persons living in Bordeaux, the Three-City study (2001–2002)
(Mean values, standard deviations, number of subjects and percentages, n 1050)

* P value for the χ2 test or ANOVA among categories of the MeDi score.
Plasma fatty acids were mainly composed of SFA (more than 41 % of total plasma fatty acids), followed by PUFA (36·3 % on average), mainly n-6 PUFA, and MUFA (22·6 % on average (Table 3).
Table 3 Percentage of plasma fatty acids, fatty acid ratios and correlation analyses with Mediterranean diet (MeDi) adherence among older persons living in Bordeaux, the Three-City study (2001–2002)
(Mean values, standard deviations and Pearson's coefficients, n 1050)

LA, linolenic acid; ALA, α-linolenic acid; AA, arachidonic acid.
Pearson's correlation coefficients revealed a significant positive relationship between the MeDi score and plasma 22 : 6n-3, plasma EPA+DHA index and total n-3 PUFA (Table 3). Inverse correlations between the MeDi score and plasma total MUFA and 16 : 1n-7 were observed. There was no association between the MeDi score and plasma SFA or any of the n-6 PUFA. The directions of the correlations between the MeDi score and several plasma fatty acid ratios (n-6:n-3 PUFA, AA:EPA, AA:DHA and AA:(EPA+DHA)) were in full agreement with the positive association between n-3 PUFA and the MeDi score.
The associations between plasma fatty acids and fatty acid ratios and the MeDi score as a categorical variable are shown in Table 4. The percentages of each fatty acid in plasma did not differ across MeDi categories, except for 16 : 1n-7, 22 : 6n-3, EPA+DHA index and total n-3 PUFA. Individuals in the high MeDi category had a lower mean plasma 16 : 1n-7 and higher mean plasma 22 : 6n-3, EPA+DHA index and total n-3 PUFA than those in the lowest category. Moreover, mean AA:EPA, AA:DHA, AA:(EPA+DHA) and total n-6:n-3 PUFA ratios significantly decreased with increasing MeDi adherence. There was no association between the MeDi, as a categorical variable and plasma total SFA, total PUFA and n-6 PUFA or the MUFA:SFA and linoleic acid:α-linolenic acid ratios.
Table 4 Percentage of plasma fatty acids and fatty acid ratios by categories of the Mediterranean diet (MeDi) score among older persons living in Bordeaux, the Three-City study (2001–2002)
(Mean values and standard deviations, n 1050)

LA, linolenic acid; ALA, α-linolenic acid; AA, arachidonic acid.
* P value for the ANOVA among categories of the MeDi score.
† Mean values were significantly different for percentages of plasma fatty acids between categories of the MeDi score taking the lowest category as the reference group (2 × 2 significant comparisons): P < 0·05.
‡ P values were significant after Bonferroni's correction.
For each plasma fatty acid percentage or ratio, which was separately associated with MeDi adherence at P < 0·20 in Pearson's correlation analyses, multivariate linear regression was performed (Table 5). In models adjusted for age, sex, energy intake, physical activity, smoking status, BMI, plasma TAG and apoE genotype, plasma 16 : 1n-7 was significantly inversely associated with the MeDi score. The MeDi score was positively associated with plasma 22 : 6n-3, EPA+DHA index and plasma total n-3 PUFA. However, when considering the association between plasma 20 : 5n-3 and the MeDi score as continuous data, a significant statistical interaction between the MeDi score and apoE genotype was observed (P = 0·03). Stratified analyses were therefore conducted based on the apoE-ɛ4 status (Table 5). Plasma 20 : 5n-3 was significantly positively associated with the MeDi score only in apoE-ɛ4 non-carriers. Finally, the total n-6:n-3 PUFA, AA:EPA, AA:DHA and AA:(EPA+DHA) ratios significantly decreased when MeDi adherence increased in fully adjusted models (Table 5). No other significant interaction between the MeDi score and apoE-ɛ4 genotype was observed.
Table 5 Association between plasma fatty acids and fatty acid ratios (separated models) and adherence to the Mediterranean diet (MeDi) in multivariate linear regression models among older persons living in Bordeaux, the Three-City study (2001–2002)
(β Coefficients and P values)

AA, arachidonic acid.
* Adjusted for age, sex, energy intake and physical activity.
† Model 1 plus additional adjustment for smoking status, BMI, plasma TAG and apoE, allele ɛ4 status.
‡ Because of a significant interaction between the MeDi score and the apoE-ɛ4 status (P = 0·03), analyses of the association between plasma EPA and the MeDi score were stratified according to the apoE-ɛ4 status. Therefore, model 2 was not adjusted for apoE genotype.
Discussion
The present study shows a positive association between MeDi adherence and plasma 22 : 6n-3, EPA+DHA index and plasma total n-3 PUFA, and, conversely, an inverse association between MeDi adherence and plasma palmitoleic acid, and several n-6:n-3 PUFA ratios. Plasma 20 : 5n-3 was significantly positively associated with MeDi adherence only in apoE-ɛ4 non-carriers. These associations were independent of energy intake, BMI, physical activity and plasma TAG.
To date, few studies have investigated the association between MeDi adherence and plasma fatty acids, and interestingly, the present results are almost fully consistent with those previous studies. In 374 adults enrolled in the ATTICA study (Greece), spontaneous MeDi adherence was evaluated by a weighted MeDi score computed according to the frequency of consumption of eleven food groups (daily, weekly or monthly)(Reference Panagiotakos, Kalogeropoulos and Pitsavos25). In this small Greek sample, MeDi adherence was positively associated with plasma total n-3 PUFA, DHA, MUFA and MUFA:SFA ratio and inversely associated with plasma n-6 PUFA and the AA:EPA ratio in analyses adjusted for age, sex, physical activity and energy intake. However, traditionally distinct dietary habits in France and Greek could have induced discrepancies. Indeed, major characteristics of the Greek people are peculiar consumptions of vegetables and olive oil, whereas French people are greater consumers of alcohol, which would have influenced the country-specific MeDi adherence(Reference Sofi3). The association between a diet modelled on the traditional MeDi and blood fatty acid profile has already been investigated in several interventions studies which do not represent spontaneous dietary behaviours(Reference Djuric, Ren and Blythe31–Reference Urquiaga, Guasch and Marshall34). Most of these studies only involved men or women and were conducted on small samples. The largest study was the Lyon Heart Study (France) in which a modified Mediterranean-type diet was administered in a randomised secondary prevention trial in 605 patients recovering from myocardial infarction(Reference de Lorgeril, Renaud and Mamelle33). In this study, the protective effect of the dietary intervention on CHD was related to changes in plasma fatty acids. Indeed, the intervention diet was associated with an increase in plasma n-3 PUFA and 18 : 1n-9 and a decrease in 18 : 2n-6. However, the intervention group also benefited from a supplementation of 18 : 3n-3 in addition to dietary recommendations. Other dietary indices close to the MeDi have been correlated with nutritional biomarkers. In a French population living near the Mediterranean basin, a Diet Quality Index (Med-DQI) has been developed and correlated with biological markers such as plasma carotene, vitamin E and the percentage of 20 : 5n-3 and 22 : 6n-3 in erythrocyte membrane(Reference Gerber35).
The relationship between plasma phospholipid fatty acids and dietary intakes of major food groups has been analysed in the European Prospective Investigation into Cancer and Nutrition study(Reference Saadatian-Elahi, Slimani and Chajes36). Strong correlations have been observed between fish intake and long-chain n-3 PUFA, on the one hand, and olive oil and 18 : 1n-9, on the other hand. Considering major food groups of the MeDi score, fish, which has only recently been added in the MeDi score computation(Reference Trichopoulou, Costacou and Bamia5), is the main dietary source of long-chain n-3 PUFA. Previous reports have already shown a high correlation between fish intake and plasma n-3 PUFA(Reference Astorg, Bertrais and Laporte37–Reference Hodson, Skeaff and Fielding41). Fish consumption may in part explain the positive association between MeDi adherence and plasma long-chain n-3 PUFA, and the inverse association with several fatty acid ratios observed in the present study.
Consumption of alcohol, which is also a component of the MeDi score, might influence the metabolism of PUFA(Reference Pawlosky and Salem42) and has already been positively associated with plasma 20 : 5n-3, 22 : 6n-3 and EPA+DHA index, even after controlling for fish consumption(Reference Saadatian-Elahi, Slimani and Chajes36, Reference di Giuseppe, de Lorgeril and Salen43). Alcohol was also positively associated with plasma 16 : 0 and 16 : 1n-7 in Italian centres of the European Prospective Investigation into Cancer and Nutrition study(Reference Fusconi, Pala and Riboli44). Since only a mild-to-moderate intake of alcohol is considered as a beneficial component of the MeDi score, the proportion of heavy drinkers decreased when the MeDi score increased. Hence, alcohol consumption could partly explain the observed association between higher MeDi adherence and lower plasma 16 : 1n-7. Another explanation is the decrease in the endogenous synthesis of 16 : 1n-7 from SFA with increased MeDi adherence(Reference Nakamura and Nara45).
The hallmark of the traditional MeDi is a very high consumption of olive oil leading to a high dietary MUFA:SFA ratio(Reference Panza, Capurso and D'Introno46). However, in our sample, MeDi adherence was not associated with the plasma MUFA:SFA ratio or plasma SFA, and, surprisingly, inversely associated with plasma MUFA with borderline significance. These weaker associations with the MeDi score may be in part explained by the endogenous synthesis of these fatty acids, which may be a determinant compared with dietary contributions(Reference Hodson, Skeaff and Fielding41, Reference Nakamura and Nara45). Moreover, total plasma lipids appear not to be good at reflecting small changes in MUFA(Reference Hodson, Skeaff and Fielding41).
Few studies have analysed the interaction between dietary pattern or dietary fat and apoE genotype on lipid status and plasma fatty acids(Reference Anil47). We found a positive association between MeDi adherence and plasma 20 : 5n-3 only among the apoE-ɛ4 non-carriers. The plasma TAG-lowering effect of 20 : 5n-3 and 22 : 6n-3 is well known and may be in part mediated by apoE(Reference Balk, Lichtenstein and Chung48). As already suggested, after n-3 PUFA supplementation, incorporation of EPA and DHA into plasma TAG is decreased by the presence of apoE-ɛ4(Reference Plourde, Vohl and Vandal49). Why only the association between plasma 20 : 5n-3 and MeDi adherence was modified by apoE-ɛ4 polymorphism is difficult to interpret, and further research is needed to better understand this gene × diet interaction.
The overall association between MeDi adherence and plasma n-3 PUFA seems to be of particular interest regarding their respective protective effect on cognitive decline and dementia in older people(Reference Feart, Samieri and Barberger-Gateau12, Reference Cunnane, Plourde and Pifferi21, Reference Fotuhi, Mohassel and Yaffe22). In a previous analysis of the 3C study, we observed that higher MeDi adherence was significantly associated with better global cognitive performances over time(Reference Feart, Samieri and Rondeau11). We suggested that mechanisms by which the MeDi may exert its effect involved a decrease in oxidative stress, inflammation and vascular disease, which also participated in the pathophysiology of neurodegenerative disease(Reference Cummings50–Reference Razquin, Martinez and Martinez-Gonzalez52). Results from the present study reinforced these observations since mechanisms underlying the protective effect of long-chain n-3 PUFA on cognition could involve their anti-inflammatory or vascular properties in addition to their role in the composition and fluidity of neural membranes, and consequently in signal transduction and control of gene expression(Reference Cunnane, Plourde and Pifferi21, Reference Fotuhi, Mohassel and Yaffe22).
The present results should be interpreted with caution because of some methodological limitations. First, the MeDi score only included nine food groups and, therefore, did not consider the overall correlation between foods(Reference Jacques and Tucker53). The computation of the MeDi score based on sex-specific cut-off points does not measure adherence to a universal traditional MeDi pattern rather to a specific pattern, only characterising the study sample in which it was computed, which limits the generalisability of the present results(Reference Bach, Serra-Majem and Carrasco6). Moreover, similar MeDi scores could reflect different dietary patterns, among the nine food groups considered for its computation. In the same way, the FFQ used in the present study assessed the number of servings of each food group but not portion size. Although our analyses were adjusted for total energy intake, we cannot exclude that the quantity of presumed protective or deleterious nutrients consumed by men and women with a similar MeDi score may differ. Second, dietary assessments were recorded slightly later than plasma measurements, and a single total plasma measurement was available. If subjects modified their dietary habits, this could have introduced variability in our analysis. A commonly held view suggests that erythrocyte fatty acid composition better reflects long-term dietary intake than plasma fatty acid levels(Reference Arab54, Reference Sun, Ma and Campos55). Nevertheless, Hodson et al. (Reference Hodson, Skeaff and Fielding41) demonstrated that, at best, erythrocytes and plasma lipid fractions both reflect only recent (few weeks) rather than long-term dietary fatty acid intakes. Previous analyses have reported that serum PUFA are suitable biomarkers of usual dietary intake over a few years, although the association may weaken as the delay between dietary assessment and blood collection increases(Reference Thiebaut, Rotival and Gauthier38). Third, a selection bias cannot be dismissed and could have limited our ability to find additional or stronger associations. Indeed, plasma MUFA and 18 : 1n-9 were higher in not included subjects compared with those included, whereas plasma total n-3 PUFA and 20 : 5n-3 were significantly higher in subjects enrolled in the present study compared with others. Finally, competition between metabolic pathways may lead to changes in fatty acid composition not directly related to the diet and may partly explain the present results(Reference Simopoulos56). Other unknown potential confounding factors related to both plasma fatty acids and MeDi adherence, such as other food groups not considered in the computation of the MeDi score or a general healthier lifestyle of MeDi adherents, could also partly explain the present results.
Despite these limitations, the strengths of the present study are its size, the population-based design and the control for numerous potential confounders. In particular, we controlled our analysis for BMI, energy intake, plasma TAG, physical activity and smoking status(Reference Hodge, Simpson and Gibson57). Smoking was associated with MeDi adherence with borderline significance in this sample and may affect PUFA status(Reference Hodson, Skeaff and Fielding41, Reference Pawlosky, Hibbeln and Wegher58). Moreover, physical activity, through its action on the energy turnover, modulates the metabolism of fatty acids independently of nutrition(Reference Nikolaidis and Mougios59). Adjustment for plasma TAG allowed us to control for the overall lipid status since plasma fatty acids are expressed as percentage of total fatty acids. Demented subjects were excluded to achieve a better reliability of information about nutritional intake.
To conclude, the present cross-sectional study shows that MeDi adherence was positively associated with plasma total n-3 PUFA and 22 : 6n-3 and inversely associated with plasma 16 : 1n-7 and several n-6:n-3 PUFA ratios. Plasma 20 : 5n-3 was significantly positively associated with MeDi adherence only in apoE-ɛ4 non-carriers, although this gene × diet interaction deserves further research(Reference Simopoulos60). These findings may explain the protective effect of the MeDi on cognitive functioning evidenced by two previous studies, although the generalisation of these results to other populations needs to be investigated.
Acknowledgements
The 3C study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), Victor Segalen – Bordeaux2 University and the Sanofi-Synthélabo company. The Fondation pour la Recherche Médicale funded the preparation and beginning of the study. The 3C study is also sponsored by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, Ministry of Research-INSERM Program ‘Cohortes et collections de données biologiques’. Financial support for the 3C-COGINUT project was provided by the French National Research Agency (ANR-06-PNRA-005). C. F. was funded by the French National Research Agency (ANR-06-PNRA-005). C. S. was funded by the Institut Carnot LISA (Lipides pour l'Industrie et la santé, Lipids for Industry, Safety and Health). Fatty acid analyses performed by E. P. were sponsored by the Conseil Régional d'Aquitaine. P. B.-G. received fees for conferences from Danone, Lesieur, Bauch&Lomb and Aprifel, and benefits from research grants from Danone and Lesieur. None of the other authors had any financial or personal conflict of interest. The contribution of each author was as follows: P. B.-G. and A. P. S. designed and conducted the study. E. P. and P. B.-G. collected the data. P. B.-G. helped in the management of the data. C. F., M. J. M. T., M.-A. J., A. P. S. and P. B.-G. were responsible for the analysis and interpretation of the data. C. F. and P. B.-G. prepared the manuscript. C. F., M. J. M. T., C. S., M.-A. J., E. P., A. P. S. and P. B.-G. reviewed and approved the final version of the manuscript.







