Phyto-oestrogens are non-steroidal compounds found in many legumes and are particularly abundant in soya products. They have been reported to have a beneficial effect on body weight and adipose tissue deposition, mainly in postmenopausal women( Reference Christie, Grant and Darnell 1 , Reference Liu, Ho and Chen 2 ). Our group has recently reported that removing phyto-oestrogens from the diet of male adult rats induces obesity and diabetes, increasing energy intake through an orexigenic hypothalamic response mediated by neuropeptides( Reference Andreoli, Stoker and Rossetti 3 ). The arcuate nucleus in the hypothalamus is the central area involved in the control of food intake( Reference Schwartz, Woods and Porte 4 , Reference Valassi, Scacchi and Cavagnini 5 ). This nucleus contains two distinct populations of neurons: one produces orexigenic neuropeptides such as agouti-related protein (AgRP) and neuropeptide Y (NPY), whereas the other synthesises anorexigenic substances such as cocaine-amphetamine-related transcript (CART) and pro-opiomelanocortin (POMC)( Reference Schwartz, Woods and Porte 4 , Reference Valassi, Scacchi and Cavagnini 5 ).
The obesity pandemic can be prevented and managed by means of different dietary manipulations. At present, energy restriction is one of the most effective ways for individuals with obesity to lose weight and ameliorate-related metabolic diseases. Moreover, altering the macronutrient composition of the diet may be an effective way to improve food intake regulation and induce satiety.
Different protein types affect body weight and food intake: dairy proteins are known to suppress short-term food intake, increase subjective satiety and stimulate the mechanisms known to signal satiety( Reference Anderson, Aziz and Abou Samra 6 ). However, the contribution of major protein fractions such as whey to the control of food intake and the peripheral and central mechanisms involved have received little attention.
As whey protein is an abundant by-product in cheese production (10 litres of milk produces about 9 litres of whey during cheese manufacturing), it is considered an inexpensive source of high-quality protein that can be used in functional foods claiming health benefits( Reference Luhovyy, Akhavan and Anderson 7 ). Several studies conducted in humans have shown that whey supplementation affects appetite and reduces food intake( Reference Chungchunlam, Henare and Ganesh 8 – Reference MacKenzie-Shalders, Byrne and Slater 10 ). However, it is unclear whether the consumption of whey affects the central mechanisms involved in food intake control. Animal studies assessing how whey protein affects food intake have used different experimental models with diverse amounts of dietary proteins( Reference Pichon, Potier and Tome 11 – Reference Pezeshki, Fahim and Chelikani 14 ), as well as diverse experimental procedures (duration of the treatment, protein content, use of protein preloads, age and sex of the subjects, etc.), which make it difficult to draw a definitive conclusion about the effects of whey on appetite. Thus, further studies are needed to clarify the role of whey protein in the central control of energy intake.
Whey proteins have been demonstrated to improve glucose homoeostasis and insulin sensitivity in both rodent models and humans( Reference Nilsson, Holst and Bjorck 15 – Reference Shertzer, Woods and Krishan 17 ). In addition, whey proteins have been reported to diminish postprandial glycaemia( Reference Gunnerud, Holst and Ostman 18 , Reference Gunnerud, Ostman and Bjorck 19 ) through various inter-related mechanisms including enhancement of insulin and release of incretins( Reference Nilsson, Holst and Bjorck 15 , Reference Salehi, Gunnerud and Muhammed 20 ). These properties of whey proteins suggest that they can be used for the management of diabetes induced by different dietary treatments.
Given the possible beneficial effects of whey for overweight individuals, the aim of the present study was to evaluate whether a diet supplemented with whey protein concentrate can attenuate the susceptibility to obesity and diabetes in adult male rats previously exposed to a phyto-oestrogen-free (PF) diet, preventing or reversing alterations in central mechanisms of energy intake control. We consider that assessing the potential beneficial effects of whey intake on hyperphagic obese individuals would be relevant as whey could interfere in the obesogenic mechanisms of action of phyto-oestrogen deprivation.
Methods
Preparation of diets
The composition of diets was based on the American Institute of Nutrition Ad Hoc Committee recommendation for adult rodents (AIN-93M)( Reference Reeves, Nielsen and Fahey 21 ) and is shown in Table 1. All diets were prepared in-house using AIN 93 mineral and vitamin mixes, choline chloride, dl-methionine and α-cellulose from MP Biomedicals and using local sources of feed-grade sucrose, maize starch, soyabean oil and casein. A high-phyto-oestrogen (HP) diet was prepared with the addition of genistein and daidzein (Indofine Chemical Company). The amounts of phyto-oestrogens added were chosen to match those present in the regular laboratory chow previously used by our group( Reference Andreoli, Stoker and Rossetti 3 ). The PF was identical to the HP diet but without the addition of genistein and daidzein. The PF containing whey protein (PF-W) diet was a variation of the PF diet and contained whey protein concentrate instead of casein as a source of dietary protein. Whey protein concentrate (Lacprodan 80) was kindly provided by Arla Foods Ingredients. The three diets were isoenergetic, as can be seen in Table 1. The levels of phyto-oestrogens were quantified in the HP diet, and their absence in the PF and PF-W diets was confirmed using HPLC analysis.
Table 1 Composition of the experimental diets
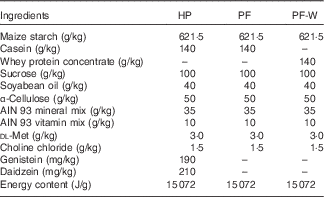
HP, high-phyto-oestrogen; PF, phyto-oestrogen-free; PF-W, PF diet supplemented with whey.
Animals and general protocol
All the procedures were approved by the Ethics Committee of the School of Biochemistry and Biological Sciences (University of Litoral), and were performed in accordance with the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals issued by the US National Academy of Sciences( 22 ). In all, thirty adult male Wistar rats were obtained from the Department of Human Physiology (University of Litoral). From conception until the beginning of the experiment, rats were housed under controlled conditions (23±2°C and 12 h light–12 h dark cycle) with free access to the HP diet. When the animals reached a weight of 250–300 g, they were randomly divided into two weight-matched groups and subjected to the following dietary treatments for 10 weeks. One group (n 12) was used as control and had ad libitum access to the same HP diet administered for 10 weeks (HP-10-wk group). To evaluate the effects of withdrawing phyto-oestrogens from the diet, the other group (n 18) was fed the PF diet administered for 10 weeks (PF-10-wk group). Body weight was recorded at the beginning and at the end of the experiment. Food intake was measured daily during the experimental period, calculating the weight difference between the offered and remaining food, adjusted for wastage by calculating food spillage. Energy intake was calculated by multiplying the amounts of ingested food with the respective energy contents. At the end of this treatment, six rats from each group were euthanised by decapitation. Trunk blood was collected, samples were centrifuged and serum was immediately used or frozen and stored at −80°C until analysis. Epididymal and perirenal fat pads were dissected and weighed. Immediately after euthanasia, the brain was removed from the skull, and the medio-basal hypothalamus was dissected (according to Paxinos atlas coordinates −1·0 to −4·5 mm from Bregma and 3 mm in depth)( Reference Paxinos and Watson 23 ), snap-frozen in liquid N2 and stored at −80°C until RNA isolation.
The remaining HP animals (six animals) continued receiving the HP diet administered for 16 weeks (HP-16 wk group), whereas the remaining PF rats (twelve rats) were divided into two groups (six rats each): one group was fed the PF diet administered for 16 weeks (PF-16 wk), and the other group received the PF-W diet to assess whether whey proteins could prevent or reverse the effects of phyto-oestrogen deprivation. Body weight and energy intake were also recorded in this period. At the end of the dietary treatments, the animals were euthanised and samples were collected as described above.
RNA isolation, reverse transcription and real-time quantitative PCR
Total RNA was isolated from the hypothalamus using TRIzol (Invitrogen) according to the manufacturer’s protocol. Reverse transcription and real-time quantitative PCR were performed as previously described using specific primer pairs for ribosomal protein L19 (housekeeping gene), Agrp, Pomc, Cart and Npy ( Reference Andreoli, Stoker and Rossetti 3 ). In brief, real-time quantitative PCR was carried out using a SYBR Green PCR master mix (HOTFIREPol EvaGreen qPCR Mix Plus (Solis BioDyne, Biocientífica)) and a real-time PCR system StepOne Cycler (Applied Biosystems Inc., Life Technologies). Product purity was confirmed by dissociation curves, and random samples were subjected to agarose gel electrophoresis. All reactions were carried out at least in triplicate. Fold change from HP values was determined using the delta–delta C t method( Reference Pfaffl 24 ).
Circulating glucose and hormone measurements
Fasting glucose was measured using commercially available kits (Wiener Lab). Serum insulin was assessed by RIA using an anti-rat insulin antibody (Sigma) and standard rat insulin provided by Laboratorios Beta. Thyroid-stimulating hormone was determined by competitive RIA according to the procedure outlined by Catalano et al.( Reference Catalano, Bonaventura and Silveyra 25 ) The levels of total thyroxine (T4), oestradiol (E2) and testosterone were measured using competitive RIA kits (Immunotech). Insulin resistance was evaluated according to the homoeostasis model assessment (HOMA). The HOMA index was calculated as (fasting insulin (IU/ml)×fasting glucose (mol/l))/22·5( Reference Matthews, Hosker and Rudenski 26 ).
Statistical analysis
Data corresponding to the HP-10-wk and PF-10-wk groups (expressed as means with their standard errors) were statistically analysed by Student’s t test. Data from the HP-16 wk, PF-16-wk and PF-W groups (also expressed as means with their standard errors) were analysed by one-way ANOVA. Post hoc multiple comparisons were made using Tukey’s critical range test. In all cases, differences were considered significant at P<0·05. For all the analyses, IBM SPSS Statistics 19 software (IBM Inc.) was used.
Results
Effects of 10 weeks of phyto-oestrogen deprivation
Phyto-oestrogen deprivation increased adipose tissue, body weight and energy intake
Rats fed the PF diet for 10 weeks significantly increased their body weights (+17 %) (Table 2). To determine whether there was a region-specific fat gain, selected fat deposits (epididymal and perirenal) were excised and weighed. Consistent with the changes in body weight, PF-10-wk animals had significantly greater amounts of epididymal and perirenal fat than HP-10-wk rats, expressed both in grams (absolute value) and as a percentage of body weight (Table 2). In close relation to this, food intake in animals fed the PF diet for 10 weeks was increased. This hyperphagia led to a significant increase in average energy intake compared with the HP-10-wk group (Table 2). Energy intake was higher in PF-10-wk v. HP-10-wk animals throughout the experiment, as can be seen in Fig. 1.
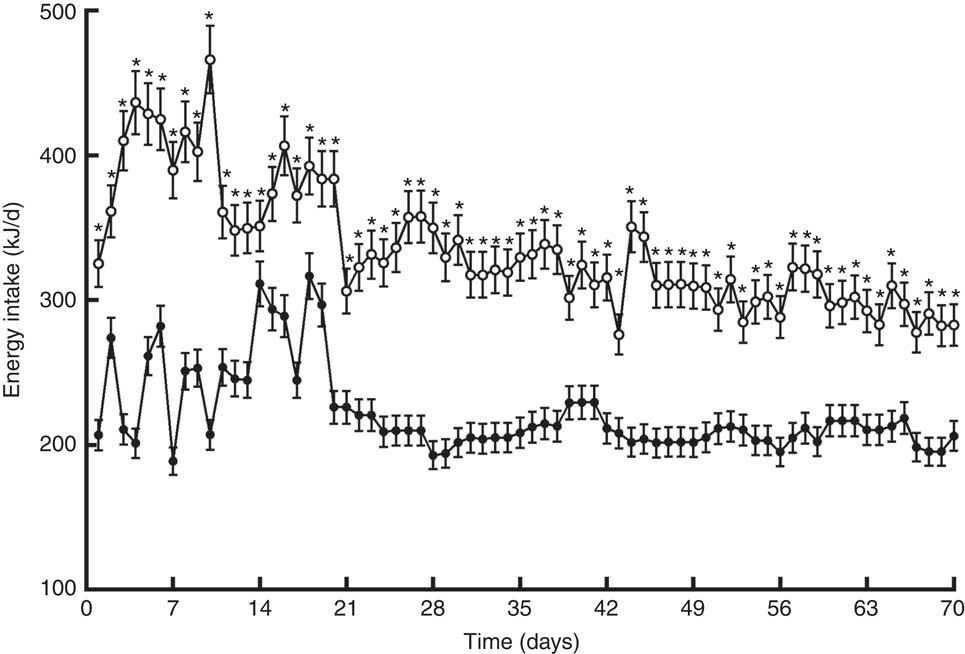
Fig. 1 Energy intake of rats fed high-phyto-oestrogen (HP-10 wk, ![]() ) or phyto-oestrogen-free (PF-10 wk,
) or phyto-oestrogen-free (PF-10 wk, ![]() ) diets for 10 weeks. Data shown are presented as means (n 6), with their standard errors. * Significant differences at P<0·05 (Student’s t test).
) diets for 10 weeks. Data shown are presented as means (n 6), with their standard errors. * Significant differences at P<0·05 (Student’s t test).
Table 2 Final body and adipose tissue weights and food and energy intake in animals fed high-phyto-oestrogen (HP) or phyto-oestrogen-free (PF) diet for 10 weeks (Mean values with their standard errors, n 6)
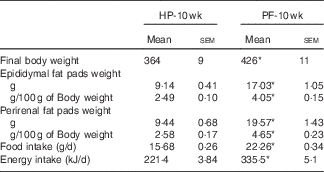
HP-10 wk, HP diet administered for 10 weeks; PF-10 wk, PF diet administered for 10 weeks.
* Significant differences at P<0·001 (Student’s t test).
Increased energy intake was associated with changes in the hypothalamic expressions of neuropeptides
In rats, 10 weeks of phyto-oestrogen deprivation induced an orexigenic response by significantly reducing anorexigenic POMC mRNA expression, increasing orexigenic AgRP and concomitantly decreasing NPY expression, as compared with the HP-10-wk group (P<0·05). CART mRNA levels were unchanged (Fig. 2).
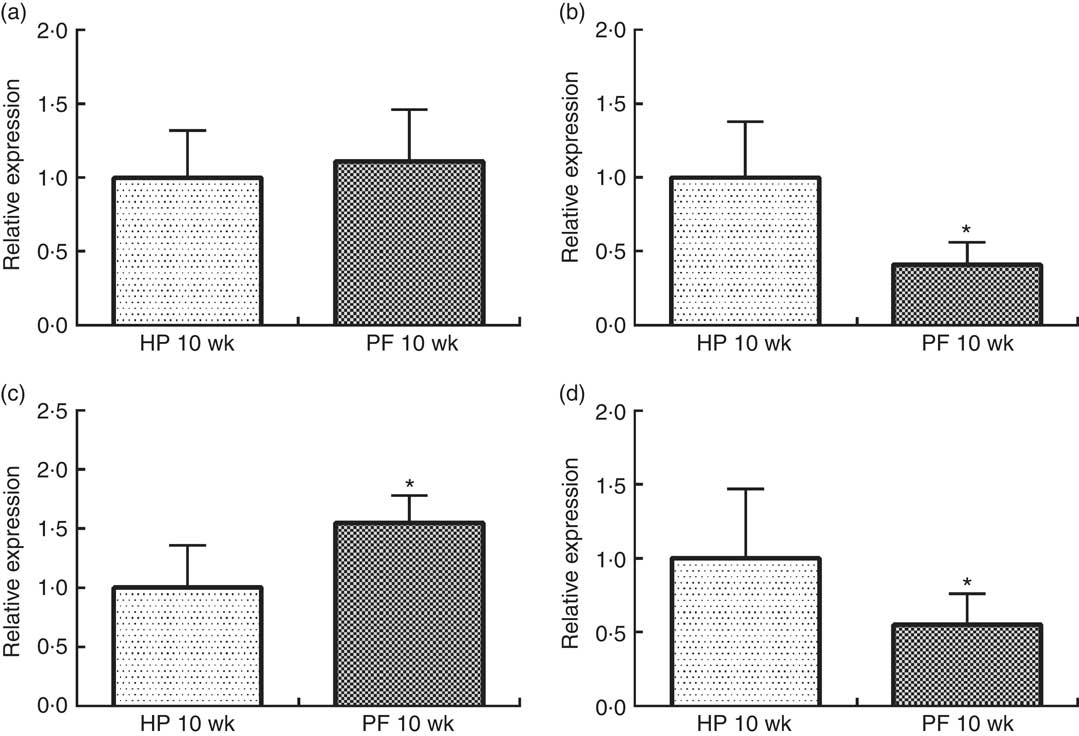
Fig. 2 Hypothalamic expression of (a) cocaine-amphetamine-related transcript, (b) pro-opiomelanocortin, (c) agouti-related protein and (d) neuropeptide Y in rats fed high-phyto-oestrogen (HP-10 wk) or phyto-oestrogen-free (PF-10 wk) diets for 10 weeks. Relative RNA expression was measured by quantitative real-time RT-PCR and is expressed as fold change from HP 10-wk values. Expression was normalised to that of ribosomal protein L19. Data shown are presented as means (n 6), with their standard errors. * Significant differences at P<0·05 (Student’s t test).
10 Weeks of phyto-oestrogen deprivation did not affect glucose metabolism
Phyto-oestrogen deprivation did not affect fasting glucose, insulin or the HOMA index (Table 3).
Table 3 Glucose metabolism parameters in animals-fed high-phyto-oestrogen (HP) or phyto-oestrogen-free (PF) diet for 10 weeks (Mean values with their standard errors, n 6)
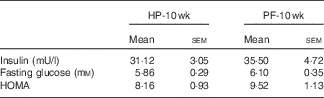
HP-10 wk, HP diet administered for 10 weeks; PF-10 wk, PF diet administered for 10 weeks; HOMA, homoeostasis model assessment.
Effects of whey administration
Whey administration reduced food and energy intake but did not affect adipose tissue or body weight
When the animals continued receiving the PF for 6 more weeks, their body weights remained higher compared with HP-fed rats. This was reflected in the final body weight (Table 4). PF-W rats showed a slight reduction in their final body weight that did not reach statistical significance v. PF-16-wk rats. Fat accretion observed in rats deprived of phyto-oestrogens for 10 weeks was exacerbated: compared with HP-16-wk rats, PF-16-wk rats had significantly greater amounts of epididymal (+50 %) and perirenal (+60 %) fat (Table 4). Consistent with the lack of changes in body weight, PF-W rats showed no changes in fat deposit weight (Table 4). As seen in the 10-week treatment, PF-16-wk rats were hyperphagic, and 6 weeks of whey administration reduced food and energy intake by 16 % (Table 4). This reduction was noticeable around day 10 of diet exposure, and remained until the end of the study (Fig. 3).
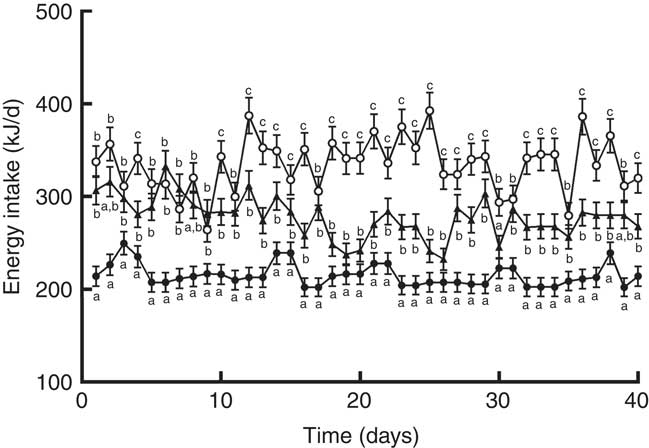
Fig. 3 Energy intake of rats fed high-phyto-oestrogen (HP-16 wk, ![]() ), phyto-oestrogen-free (PF-16 wk,
), phyto-oestrogen-free (PF-16 wk, ![]() ) or phyto-oestrogen-free+whey (PF-W,
) or phyto-oestrogen-free+whey (PF-W, ![]() ) diets. Data shown are presented as means (n 6), with their standard errors. a,b,c Unlike letters indicate significant differences (P<0·05 by Tukey’s test after one-way ANOVA).
) diets. Data shown are presented as means (n 6), with their standard errors. a,b,c Unlike letters indicate significant differences (P<0·05 by Tukey’s test after one-way ANOVA).
Table 4 Final body and adipose tissue weights and food and energy intake in animals-fed high-phyto-oestrogen (HP) diet, phyto-oestrogen-free (PF) diet or PF diet supplemented with whey (PF-W) (Mean values with their standard errors, n 6)
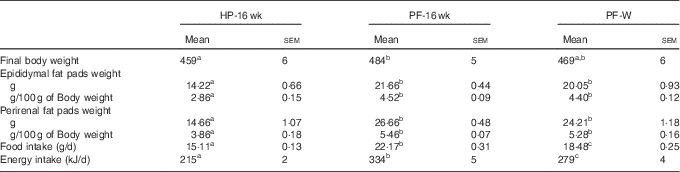
HP-16 wk, HP diet administered for 16 weeks; PF-16 wk, PF diet administered for 16 weeks.
a,b,c Mean values within a row with unlike superscript letters were significantly different at P<0·05 (Tukey’s test after one-way ANOVA).
The reduction in energy intake was associated with changes in the hypothalamic expression of neuropeptides
In line with the results observed in animals that were treated for 10 weeks, PF-16-wk rats showed an orexigenic response with reduced POMC and increased AgRP expression v. the HP-16-wk group (P<0·001) (Fig. 4). Nevertheless, NPY and CART mRNA levels were unchanged. The reduced energy intake observed in PF-W rats with respect to PF-16-wk rats was linked to an increase in POMC mRNA levels (P<0·001), although AgRP expression remained similar to that in PF-16-wk animals (P<0·001) (Fig. 2).
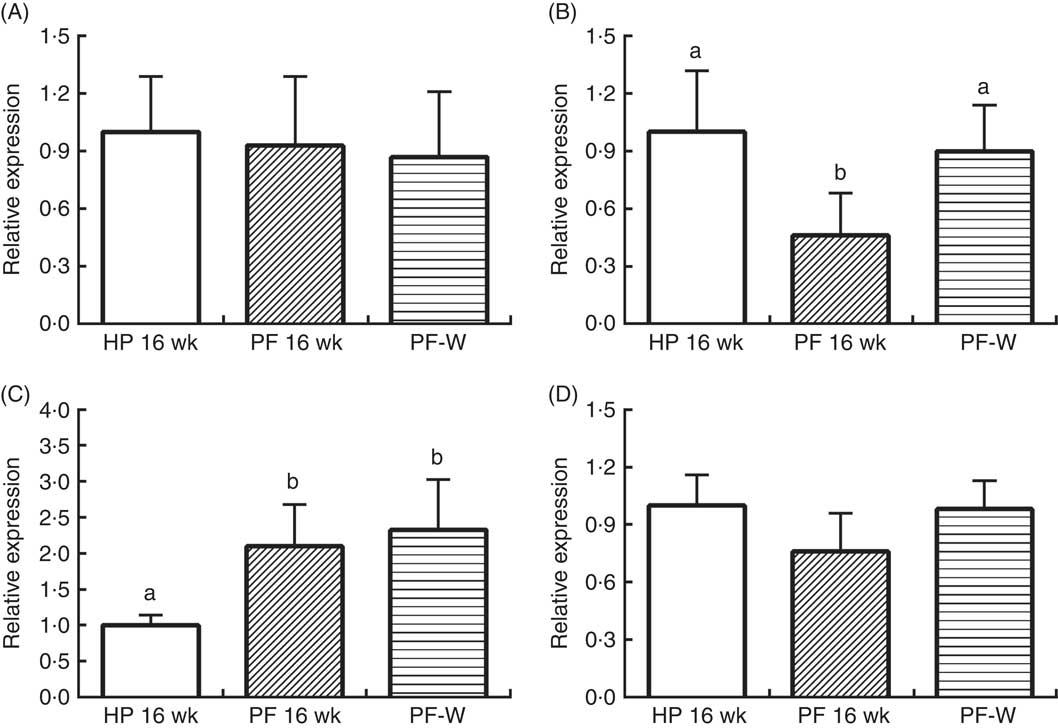
Fig. 4 Hypothalamic expression of (A) cocaine-amphetamine-related transcript, (B) pro-opiomelanocortin, (C) agouti-related protein and (D) neuropeptide Y in rats fed high-phyto-oestrogen (HP-16 wk), phyto-oestrogen-free (PF-16 wk) or phyto-oestrogen-free+whey (PF-W) diets. Relative RNA expression was measured by quantitative real-time RT-PCR and is expressed as fold change from HP-16 wk values. Expression was normalised to that of ribosomal protein L19. Data shown are presented as means (n 6), with their standard errors. a,b Mean values with unlike letters were significantly different at P<0·05 (Tukey’s test after one-way ANOVA).
Whey administration did not prevent hormonal changes induced by phyto-oestrogen deprivation
The blood hormone profile of the animals is shown in Table 5; 16 weeks of phyto-oestrogen deprivation increased the levels of total T4 and reduced levels of E2 compared with the HP-16-wk group. Whey administration did not prevent these changes. Thyroid-stimulating hormone and testosterone levels were not affected by the treatment.
Table 5 Blood hormones in animals fed high-phyto-oestrogen (HP) diet, phyto-oestrogen-free (HP) diet or PF diet supplemented with whey (PF-W) (Mean values with their standard errors, n 6)
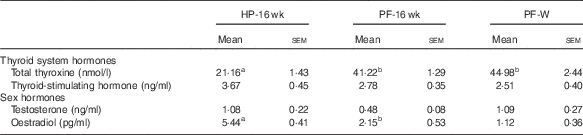
HP-16 wk, HP diet administered for 16 weeks; PF-16 wk, PF diet administered for 16 weeks.
a,b Mean values within a row with unlike superscript letters were significantly different at P<0·05 (Tukey’s test after one-way ANOVA).
Whey administration did not improve glucose metabolism impaired by 16 weeks of phyto-oestrogen deprivation
In this study, 16 weeks of phyto-oestrogen deprivation increased fasting blood glucose and insulin levels, leading to a higher HOMA index (Table 6), indicating insulin resistance. PF-W rats showed similar fasting glucose and reduced insulin concentrations (v. PF-16-wk rats), although this reduction was not sufficient to affect the HOMA index (v. PF-16-wk rats).
Table 6 Glucose metabolism parameters in animals in animals fed high-phyto-oestrogen (HP) diet, phyto-oestrogen-free (PF) diet or PF diet supplemented with whey (PF-W) (Mean values with their standard errors, n 6)

HP-16 wk, HP diet administered for 16 weeks; PF-16 wk, PF diet administered for 16 weeks; HOMA, homoeostasis model assessment.
a,b,c Mean values within a row with unlike superscript letters were significantly different at P<0·05 (Tukey’s test after one-way ANOVA).
Discussion
This study demonstrates for the first time that obese phyto-oestrogen-deprived male rats fed whey display a food intake reduction and an alteration in the gene expressions of food intake-regulating hypothalamic neuropeptides. However, the dietary treatment with whey for 6 weeks was unable to prevent obesity induced by phyto-oestrogen deprivation in the adult stage of life. We consider that this rat model is suitable for the study of human obesity, because it evaluates the effects of whey in obese individuals, when energy intake is increased and hypothalamic neuropeptides are already altered, reflecting an actual background of chronic obesity.
We have recently reported that if male rats consume phyto-oestrogens until they reach adult life, and if the feed is replaced by a PF diet, rats develop an obese phenotype with diabetes and alterations in energy intake mediated by a hypothalamic orexigenic neuropeptide response. These results were achieved with a 15-week dietary treatment( Reference Andreoli, Stoker and Rossetti 3 ). We now showed that 10 weeks of phyto-oestrogen deprivation is enough to cause obesity with increased adiposity and body weight, but not to induce diabetes. Replacing the HP diet by a PF diet resulted in obesity due to an anabolic process, with changes in food intake mediating the weight gain at least in part. However, we cannot rule out that alteration in other mechanisms such as energy expenditure may also be involved. As previously seen( Reference Andreoli, Stoker and Rossetti 3 ), hypothalamic neuropeptide signals mediate this process; phyto-oestrogen deprivation for 10 weeks significantly decreased POMC and increased AgRP mRNA, implying that energy intake is increased at least partially through these systems. Regarding the paradoxical increase in NPY gene expression, it has been reported that the Agrp/Npy neurons primarily sense energy deficits, whereas the Pomc/Cart neurons primarily sense energy excess, as reported by Barsh & Schwartz( Reference Barsh and Schwartz 27 ). These authors also indicated that Npy mRNA levels are up-regulated by starvation but are not always affected by energy excess.
The current results on the effects of 10 or 16 weeks of phyto-oestrogen deprivation in adult male rats were obtained by feeding animals a semi-synthetic AIN-based diet. The formulation of this diet made possible to manipulate the macronutrient composition, allowing the replacement of casein (the source of dietary proteins recommended by the American Institute of Nutrition for rodent diets( Reference Reeves, Nielsen and Fahey 21 )) by whey protein concentrates to assess their potential beneficial effects. The amount of phyto-oestrogens added to obtain the HP diet was similar to that present in regular laboratory chow, in which soya is used as the main source of protein( Reference Brown and Setchell 28 ).
Feeding the PF diet for 16 weeks exacerbated the accretion of fat tissue and the body weight gain observed in animals treated for 10 weeks. Hyperphagia and excessive energy intake also persisted, but with the same magnitude as that observed in PF-10-wk v. HP-10-wk rats. However, orexigenic hypothalamic signalling was more prominent: AgRP mRNA expression was 1·55-fold higher in PF-10-wk rats than in HP-10-wk rats and 2·1-fold higher in PF-16-wk rats than in HP-16-wk rats. These results show that as the dietary treatment progresses, AgRP expression increases to maintain the hyperphagic behaviour.
The switch to PF diet in adulthood generates obesity and glucose homoeostasis impairment. In this study, we aimed to investigate whether these effects could be attenuated using whey as a source of dietary protein, as it has numerous well-known health benefits. We observed that whey administration for 6 weeks was not enough to affect body weight or epididymal or perirenal fat pad weight. Zhou et al.( Reference Zhou, Keenan and Losso 12 ) reported that whey administration reduced body fat and body weight gain in Sprague–Dawley rats, but the dietary treatment was longer (10 weeks) and whey was provided in the context of a high-protein diet, which is known to promote weight loss( Reference Astrp, Raben and Geiker 29 ). Pezeshki et al. ( Reference Pezeshki, Fahim and Chelikani 14 ) showed that whey significantly reduced fat mass in obese rats, but the animals were obesity-prone rats previously fed a high-fat diet. These authors also reported that whey reduced energy expenditure in these animals, indicating that this mechanism plays a role in the anti-obesity effect of whey proteins. Therefore, the strain of the animals, their preceding obese or lean status, the amount of dietary proteins and the duration of the experimental protocol may affect the ability of whey to alter body weight and adiposity. Besides, the number of animals in each group in our study could have been insufficient to reach statistical significance for the parameters evaluated.
Our results show that whey administration significantly reduced food and energy intake. This was associated with an increase in the anorexigenic signal of POMC, although AgRP expression remained unchanged compared with PF-16-wk animals. It was also noticeable that energy intake in PF-W remained higher than in HP animals. This could suggest involvement of other neuropeptides such as orexins or changes in hedonic mechanisms of food intake control. The greater satiating effect of whey protein is in agreement with results by other authors( Reference MacKenzie-Shalders, Byrne and Slater 10 , Reference Pichon, Potier and Tome 11 , Reference Hall, Millward and Long 30 ); however, the mechanisms involved in food intake are still unknown. Pezeshki et al.( Reference Pezeshki, Fahim and Chelikani 14 ) showed that the satiating effect was related to a reduction in taste preference. Hall et al.( Reference Hall, Millward and Long 30 ), reported an increased satiety response to whey with respect to casein in human subjects and stated that this response was mediated by plasma amino acids and gastrointestinal hormones. The influx of amino acids following ingestion of whey proteins is also an important factor in the effect of whey on food intake. It is widely known that different amino acids and proteins affect peripheral and central mechanisms involved in food intake control( Reference Fromentin, Darcel and Chaumontet 31 ). Whey proteins contain a high concentration of branched-chain amino acids( Reference Walzem, Dillard and German 32 ), with l-leucine representing about 10 % of the total amino acids of whey( Reference Luhovyy, Akhavan and Anderson 7 ). The importance of leucine in the hypothalamic food intake regulatory system has been reported, as its intracerebroventricular injection has been shown to suppress food intake. This effect has been related to increased mammalian target of rapamycin (mTOR) activity in the hypothalamus, which leads to the stimulation of POMC expression( Reference Ropelle, Pauli and Fernandes 33 ), in agreement with our results.
It has been previously reported that phyto-oestrogen deprivation modifies endocrine homoeostasis( Reference Andreoli, Stoker and Rossetti 3 , Reference Ruhlen, Howdeshell and Mao 34 , Reference Cederroth and Nef 35 ). Phyto-oestrogen-deprived rats showed higher circulating total T4 and slightly (but not significant) decreased thyroid-stimulating hormone, probably as a consequence of negative feedback from T4. Other authors have reported that phyto-oestrogens, particularly genistein, reduce circulating thyroid hormone levels, as they affect their synthesis, metabolism and thyroid hormone transport proteins( Reference Marini, Polito and Adamo 36 ). In our study, the presence of whey in the diet was unable to prevent these alterations. The potential effects of whey on the thyroid system remain unknown. It has been recently reported that 36 weeks of treatment with whey did not interfere with thyroid status in overweight human subjects( Reference Wright, Craddock and Weinheimer-Haus 37 ), in agreement with our results.
A plausible mechanism by which phyto-oestrogen deprivation may exert several effects could be through a reduction in circulating E2 levels( Reference Andreoli, Stoker and Rossetti 3 ). Our present results also showed decreased serum E2 concentrations in phyto-oestrogen-deprived rats, which could not be prevented by dietary supplementation with whey.
One of the most remarkable alterations induced by phyto-oestrogen deprivation is the impairment of glucose homoeostasis – a hallmark of insulin resistance and diabetes( Reference Andreoli, Stoker and Rossetti 3 , Reference Cederroth and Nef 35 , Reference Cederroth, Vinciguerra and Gjinovci 38 ). In the present study, the exacerbation of adiposity observed in PF-16-wk rats was concomitant with the appearance of diabetes and insulin resistance. This was reflected by high levels of fasting glucose combined with increased levels of circulating insulin and the consequent high HOMA index, in agreement with previous results( Reference Andreoli, Stoker and Rossetti 3 ). The marked fat accretion observed in the last 6 weeks of treatment appeared to be essential in the onset of diabetes in phyto-oestrogen-deprived male rats, and whey administration did not prevent the increase in fat depots or the high levels of fasting glucose. Whey proteins have been reported to improve glucose homoeostasis( Reference Nilsson, Holst and Bjorck 15 – Reference Shertzer, Woods and Krishan 17 ). Our results showed that, although whey administration did not reduce circulating glucose, circulating insulin levels were reduced when compared with PF-16-wk rats, which could be considered an improvement in insulin resistance. This result is in agreement with that found by Tong et al.( Reference Tong, Li and Xu 39 ), who showed that administration of whey proteins for 8 weeks decreased circulating insulin in rats previously fed a high-fat diet, and attributed this effect to the high concentration of leucine in whey proteins. Moreover, Belobrajdic et al.( Reference Belobrajdic, McIntosh and Owens 40 ) reported that dietary whey reduces plasma insulin concentration compared with red meat, but only when provided along with a high-protein diet.
The present study demonstrated that whey administration reduces food intake and affects hypothalamic gene expression in obese phyto-oestrogen-deprived male rats. An increase in POMC expression was the main anorexigenic hypothalamic signal affected by dietary whey. On the other hand, 6 weeks of treatment was not enough to affect body weight or fat pad weights. Phyto-oestrogen deprivation modified endocrine homoeostasis, affecting total T4 and E2, and whey administration could not prevent these changes. High levels of circulating glucose were not prevented by whey administration; however, the reduction in fasting insulin is a promising result for a possible beneficial effect of whey with a longer treatment. Our results should encourage further studies considering the potential of whey proteins to reduce food intake and circulating insulin.
Acknowledgements
The authors thank Juan Grant and Juan C. Villarreal for their technical assistance and animal care. The authors also acknowledge Arla Foods Ingredients for the kind donation of Lacprodan 80 whey protein concentrate. The authors thank the Mathematics and Statistics Department of Facultad de Bioquímica y Ciencias Biológicas, Universidad Nacional del Litoral, for their kind assistance in statistical analysis.
This work was supported by the Universidad Nacional del Litoral CAI+D (grant nos 50120110100423LI and 50120110100167) and the Argentine National Agency for the Promotion of Science and Technology (APFCyT) (PICT grants nos 1715 and 0145). These funding agencies had no involvement in the study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
The authors’ contributions were as follows: M. F. A., E. H. L. and J. G. R. contributed to the experimental design; M. F. A., C. S., G. P. L. and G. C. performed the experiment and data analysis; M. F. A. and J. G. R. wrote the paper.
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the study reported.













