There are many studies relating to the low incidence of breast cancer in Asian women and the link to the high intake of dietary soya foods, particularly during the early years of life(Reference Lamartiniere1, Reference Cotroneo, Wang and Fritz2). Epidemiological studies in humans as well as animals have shown that the period of adolescence or pre-puberty is a sensitive period for the later occurrence of breast cancer(Reference van't Veer, Kok and Hermus3). Dietary supplements, many with unproven effectiveness, have become popular remedies against many forms of cancer. In post-menopausal women, soya is often recommended as an alternative to hormone replacement therapy. Contrary to the protective benefits of soya products, research has linked dietary isoflavones found in soya to oxidative DNA damage, thereby promoting tumour development in oestrogen-sensitive organs such as the uterus(Reference Murata, Midorikawa and Koh4). For example, genistein, the major isoflavone in soya, increases the incidence of hormone-dependent tumours in ovariectomised rats(Reference Allred, Allred and Ju5). Genistein is believed to enhance tumour development by increasing the rate of epithelial proliferation in the normal human breast in both pre- and post-menopausal women. Additionally, stimulation of breast secretion and growth of hyperplastic epithelial cells have been noted after soya intake(Reference Petrakis, Barnes and King6). An example of the contradictory nature of studies done using soya isoflavones can be seen below. Thus, the effects of soya and isoflavones on breast tissue are not well defined(Reference Messina and Wu7).
Another line of research focuses on the consumption of dairy products and its relationship to breast cancer. In animal models, dairy products, including whey proteins, are protective against chemically induced mammary cancers. Reports have shown that dietary intake of whey or protein hydrolysates during the period of pre-pubertal development decreased the incidence of chemically induced mammary cancers in rats(Reference Bounous, Batist and Gold8). One hypothesis is that components in whey protein hydrolysates stimulate precocious maturation of the regions of the mammary gland that are most susceptible to neoplastic development(Reference Hakkak, Korourian and Shelnutt9). In 1996, a longitudinal study followed up human subjects, demonstrating that those participants who regularly consumed milk as children had decreased incidence of breast cancer(Reference Knekt, Jarvinen and Seppanen10). Milk consumption as a child was negatively correlated with subsequent breast cancer in young women 34–39 years of age(Reference Hjartaker, Laake and Lund11). In vitro studies using the whey milk protein α-lactalbumin (α-LA) have supported these findings, showing that the protein can modulate activity at the cellular and tissue levels(Reference Thompson, Gutkind and Robbins12–Reference Hakansson, Andreasson and Zhivotovsky14) and decrease cell division in normal and transformed cell lines. Based on these results, we initially examined skimmed milk supplementation to the diets of newly weaned (day 21) female rats. At day 45, the chemical carcinogen 7,12-dimethylbenz(a)anthracene was administered, and the tumour incidence was compared with those of rats fed a soya and a control diet, with casein as its protein source. We observed that rats fed skimmed milk had significantly fewer tumours than the control or the soya-fed groups (EJ Hudson and B Alston-Mills, unpublished results). These results have been corroborated by more recent studies(Reference Nielsen, Khan and Davis15).
The undifferentiated terminal ductal lobular unit in women, called the lobulaveolar unit in mice, is believed to include the stem cells that give rise to cancers, thus making this tissue of potential importance for determining susceptibility to tumorigenesis(Reference Cardiff and Wellings16, Reference Russo, Lynch and Russo17). Overall cell activity depends not only on cell–cell associations but also on cell–stromal interactions(Reference Wiseman and Werb18). Cells removed from an organism and cultured on plastic became morphologically and functionally undifferentiated, implying an important relationship or cross-talk between cell development and the surrounding extracellular matrix (ECM). This cross-talk is through signalling within the ECM, which in turn coordinates signalling among the epithelial cells(Reference Bissell and Radisky19). While the ECM mediates normal cell behaviour, disruptions of the matrix have been implicated as a major factor in tumour development and progression(Reference Alford and Taylor-Papadimitriou20, Reference Maffini, Soto and Calabro21). It is well known that hormones and other local regulators such as epidermal growth factors (EGF) can modulate the activity of the ECM. As the ECM is altered, it facilitates changes in the mammary epithelial cells(Reference Weaver and Gilbert22). In theory, stabilising the ECM may contraindicate epithelial changes, leading to tumour development.
The mouse mammary tumour virus-neu/ErbB-2 (Her2/neu) transgenic mouse develops spontaneous mammary tumours with a long latency (approximately 5–6 months) because of overexpression of the neu proto-oncogene(Reference Guy, Webster and Schaller23). Overexpression of neu/ErbB has been reported to occur in 20–40 % of human cancers(Reference Revillion, Bonneterre and Peyrat24). Studies have shown that in the mouse mammary tumour virus-neu mouse, soya isoflavones can delay tumour onset, without affecting tumour number or size(Reference Jin and MacDonald25). Therefore, this mouse model offers an opportunity to examine mammary gland development in response to dietary intervention. We hypothesise that the protein components present in diets fed to pre-pubertal mice can alter the morphology of the gland and the regulatory ECM proteins by perturbing normal glandular growth patterns.
Materials and methods
Expt 1
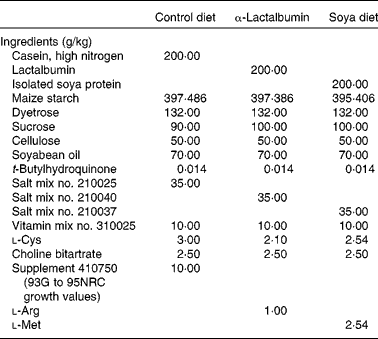
Female CD1 mice (Charles River Laboratories, Wilmington, MA, USA) were weaned at 21 d of age and housed individually in standard cages with dimensions of 29·21 × 19·05 × 12·70 cm fitted with wire lids and a substrate of sawdust on the floor of the cage. All mice were provided ad libitum access to drinking-water. The light–dark cycle was maintained at 12–12 h throughout the experiment, and the room temperature was regulated at 22°C. Animals were weighed before (day 21) and after (day 28) the treatment. All procedures were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were reviewed and approved by the North Carolina State University Animal Care and Use Committee. Treatment diets were formulated to be isoenergetic and with equal protein (Dyets, Inc., Bethlehem, PA, USA) (Table 1). At weaning, female pups from the normal strain of CD1 mice were randomly assigned to one of the four treatment groups: (1) normal casein-based chow as a negative control (n 30), (2) normal chow and daily subcutaneous injections of 2·5 μg oestradiol benzoate (Sigma, St Louis, MO, USA) in 20 μl maize oil (n 30), (3) chow with an α-LA supplement (n 30) and (4) chow with a soya protein supplement (n 30). The left or right inguinal mammary glands were excised and fixed for whole-mount preparations, while abdominal glands were used for evaluations of total DNA and proteins. Whole glands were spread onto slides and fixed in Carnoy's fixative, hydrated through a series of alcohols followed by carmine-alum staining and dehydrated through a series of alcohols which is a slight modification of published procedures(Reference Kenney, Bowman and Korach26). Mammary fat pad invasion was determined by the longitudinal growth from the nipple with respect to the lymph node using a double-blind scoring system by two evaluators. The standard used for comparison was the pattern for mammary fat pad invasion and ductal branching typical for that of a 4-week-old mouse from our colony fed a normal diet and not from the experimental groups. The grid used to assess the development was developed using a typical 4-week-old mouse from our colony to use as a scoring template: 1, longitudinal growth from the nipple not yet reaching the lymph node; 2, growth reaching the medial edge of the lymph node; 3, growth midway past the lymph node; 4, growth to the lateral edge of the lymph node; 5, growth beyond the lymph node. To validate the use of our method of evaluation, duct lengths were measured from the nipple to the ends of the ducts.
Table 1 Dietary constituents
The average ductal length from each gland was determined, and the means were combined and averaged per treatment and found to be in agreement with our methodology (Fig. 1).

Fig. 1 Whole-mount evaluations were done by two investigators as a double-blind scoring trial, measuring outgrowth from the nipple to the lymph node. The grid used to assess the development was developed using a typical 4-week-old mouse from our colony to use as a scoring template. 1, Longitudinal growth from the nipple not yet reaching the lymph node; 2, growth reaching the medial edge of the lymph node; 3, growth midway past the lymph node; 4, growth to the lateral edge of the lymph node; 5, growth beyond the lymph node. Scale bar, 0·5 cm.
DNA was extracted from approximately 100 mg of tissue using a Mini-Prep Kit (Qiagen, Valencia, CA, USA) and measured at a wavelength of 260/280 mm on a Spectromax 250 (Molecular Device, Inc., Sunnyvale, CA, USA).
Epidermal growth factor
Quantification of EGF in the serum was performed by an enzyme-linked immunoassay with the use of kit no. BT-720 (Biomedical Technologies, Stoughton, MA, USA). For the analysis of tissue EGF, previously published procedures were followed (Kenney et al.)(Reference Kenney, Bowman and Korach26). A total of 150 mg of tissue taken from the abdominal gland was homogenised in 2·5 × 12·1 g Tris base, 3·7 g Na2EDTA.2H2O (pH 7·4) and precipitated in 1·25 ml of 10 % tricarboxylic acid. Following centrifugation, the protein pellet was resuspended in 0·5 ml of buffer with protease inhibitors, and assayed as before.
Serum oestradiol
The serum concentrations of oestradiol were measured using RIA (kit no. TKE21; Diagnostic Products Corporation, Los Angeles, CA, USA) following the manufacturer's instructions.
Expt 2
As a result of our findings in the wild-type strain, we examined the Her2/neu transgenic strain. The same treatments and experimental design were provided, with twelve animals for each treatment group.
Statistical analyses
For each dependent variable and experiment, individual one-way ANOVA were used and significant F ratios were followed by comparisons of the group means using Tukey–Kramer multiple comparison post hoc tests. The analyses used Graph Pad Prism®, Instat® (GraphPad Software, Inc., La Jolla, CA, USA) and SAS® (SAS Institute, Cary, NC, USA) software, and statistical significance was set at the P < 0·05 level.
Results
Expt 1
There were no significant differences observed in body weights among the control and experimental groups.
Whole-mount evaluation
In the control group fed casein, most of the longitudinal ducts did not reach the lymph node (score 1). The glands of the oestradiol-treated group displayed similar longitudinal growth as observed in the control group, but there was increased collateral branching compared with the growth pattern of the control group. The most significant longitudinal growth was evident in the soya-fed group, with development well beyond the lymph node (score 5) and significantly greater than all other groups (P < 0·05). The group fed α-LA showed longitudinal development and extensive collateral branching, with a score of 3. The variance within each treatment group was low (Table 2). Overall growth was reflected in the total DNA concentration with the highest concentration of DNA in soya or α-LA (Fig. 2(A)). The number of terminal end buds (TEB) as well as the sparse cell population of the ECM contributed to these values (Fig. 3(A)).
Table 2 Mammary fat pad invasion for the wild-type (n 30) and mutant (n 12) strains*
(Indices with their standard errors)

a,b,c Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Measurements were based on the grid from Fig. 1. Comparisons are made within the strain.

Fig. 2 (A) Mean concentrations of total DNA from mammary tissue (ng/μl) for all treatment groups (n 30) for the CD1 strain in Expt 1. (B) Mean concentration for total DNA for the Her2/neu strain (n 12). DNA was extracted and absorbance read at 260/280 nm. Values are means, with their standard errors represented by vertical bars. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). C, casein control; O, oestrogen; L, α-lactalbumin; S, soya.

Fig. 3 Prominent buds were counted per gland and designated as terminal end buds (TEB). Counting was done by two investigators as a double-blind trial. Values are means of total TEB for (A) wild type and (B) mutant mice from all treatment groups, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05). C, casein control; O, oestrogen; L, α-lactalbumin; S, soya.
Expt 2
The Her2/neu mice were generally smaller in body weight and in gland size compared with the wild type mice. There were no differences in weights among the treatment groups either at the onset or at the completion of the experiment. Even so, the same criteria for evaluation were used. The groups treated with soya (score 4) or oestrogen (score 4) had significantly (P < 0·05) more growth than the control (score 2·3). α-LA had the greatest longitudinal growth (score 5; Table 2). Overall growth was reflected in the total DNA concentration with the highest concentration of DNA in soya or α-LA.
In the wild-type mice, obvious TEB were evaluated and showed the following: the groups fed soya and α-LA provided significantly increased numbers of TEB compared with the casein-fed controls or the group treated with oestradiol. A similar pattern was observed with the group fed α-LA. When compared with the controls, the complex branching pattern from the oestradiol treatment resulted in a greater number of TEB. In Her2/neu mice, all treatments resulted in significantly increased (P < 0·001) TEB when compared with the control. The highest numbers were found in the oestrogen group followed by the soya group, which in turn was followed by the α-LA group (Fig. 3(B)).
Extracellular matrix
Our interest was in relative values rather than absolute values for each protein. Therefore, using the absolute values for the control group, we set up ratios of matrix metalloproteinase-2 (MMP2):tissue inhibitor of MMP2 (TIMP2) to be equal to 1 and compared all experimental values with the control. A ratio greater than 1 suggested evidence that there was a treatment effect that disrupted the balance between the two proteins. As indicated by the results (Table 3 and Fig. 4), the α-LA- and soya-fed groups had the greatest differences (P < 0·05) when compared with the control or the oestradiol treatment groups in favour of degradation. The more dramatic overall effects were observed in the mutants compared with the wild types, although the trends were similar.
Table 3 Ratios of matrix metalloproteinase-2 (MMP2):tissue inhibitor of MMP2 (TIMP2) for all groups based on raw absorbance mean values of the respective immunoassays*

a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* The control for MMP2 and TIMP2 values, were set to equal 1, such that MMP2:TIMP2 = 1. Calculations were done compared with that ratio and are given in absolute values.

Fig. 4 Quantification of matrix metalloproteinase-2 (MMP2) and tissue inhibitor of MMP2 (TIMP2) as determined by immunoassay. Total proteins were extracted from 150 mg of tissue from the abdominal glands using tricarboxylic acid precipitation. Specific proteins were evaluated using manufacturers' guidelines as described in Methods (ng/ml). Each sample was plated in triplicate. Values are mean concentrations, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05). (A) Wild type (n 30) and (B) mutant. C, casein control; O, oestrogen; L, α-lactalbumin; S, soya.
Oestrogen
No significant differences were observed in the serum concentrations of oestradiol in the groups fed casein (control), α-LA or soya. In the oestradiol-treated group, values were significantly less (P < 0·05) by approximately 35 % when compared with the other three groups in both the wild-type and mutant strains (Fig. 5(A) and (B)).

Fig. 5 RIA was done to measure serum oestradiol (pg/ml). Some samples from each group/strain were pooled to assure sufficient quantities. Concentrations of oestradiol in the oestradiol benzoate-treated group for both (A) wild type (n 10) and (B) mutant (n 8) were significantly less than all other groups (control (C), α-lactalbumin (L) and soya (S)) by 35 %. Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05). O, oestrogen.
Epidermal growth factor
It is well documented that the interaction of oestrogen and EGF is dependent on the developmental state of the mammary gland. Because soya has oestrogenic effects, the question was whether concentrations of EGF in the tissue could be influenced by treatment other than oestrogen. Our results demonstrate that concentrations of EGF in the wild-type mammary tissue are not only increased by oestrogen and soya, but to a greater extent by α-LA (P < 0·01) when compared with the control (Fig. 6(A)). In the mutant strain, soya had little effect when compared with the control (Fig. 6(B)). However, both oestrogen and α-LA significantly increased the tissue concentration of EGF (P < 0·05).

Fig. 6 Quantification of epidermal growth factor (EGF) concentrations (μg/ml) in mammary tissues. Total proteins were extracted from 150 mg of tissue from the abdominal glands using tricarboxylic acid precipitation. There was no separation of epithelia and stroma. Individual samples were plated in triplicate. The standard used was the mouse EGF extracted from serum and commercially available. Values are means, with their standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05). (A) Wild type (n 10) and (B) mutant (n 8). C, casein control; O, oestrogen; L, α-lactalbumin; S, soya.
Discussion
As in agreement with other studies(Reference Jin and MacDonald25), we found that soya as a treatment did not alter body weights in either strain. There were also no effects of our other treatments at the end of 7 d. The critical period for the effects of the administration of chemical carcinogens in rodents is between days 45 and 52 of age. It is at this time the TEB divide actively and thereby are most susceptible to transformation(Reference Hakansson, Andreasson and Zhivotovsky14). Dietary soya is most effective in decreasing mammary cancer incidence if consumed during pre-pubertal development(Reference Nielsen, Khan and Davis15). The premise is that if the gland can structurally differentiate, fewer mitotic cells are available for transformation. Similar findings using milk and its derivatives have been demonstrated in human longitudinal studies. In the present study, we administered a short-term (7 d) treatment regimen to weaned female mice, before puberty, to examine changes in gland morphology and key ECM proteins using two separate mouse models. From weaning day 21 to termination day 28, there should be little endogenous oestrogen to confound the results. As demonstrated by other dietary studies(Reference Lamartiniere1, Reference Cotroneo, Wang and Fritz2, Reference Nielsen, Khan and Davis15), we found that soya and α-LA can affect growth and maturation of the pre-pubertal mammary gland. In order for ductal elongation to occur, there has to be deconstruction and reconstruction of the ECM to allow growth and support, respectively. The scaffolding that supports the mammary gland was also affected by treatment. Collagens are the major components of the ECM, and degradation of the ECM by MMP is necessary for ductal elongation. The balance between MMP activity and MMP inhibition provides coordinated growth and development of the mammary gland. In contrast, MMP degradation may occur without consequent action of the inhibitors. On the other hand, high inhibitory activity is reflected by low MMP activity. We examined MMP2 because of its action on collagen degradation in the mammary gland during the period before puberty. TIMP2 was measured because it degrades MMP2. With dietary soya and α-LA, we observed the highest number of TEB in addition to the most longitudinal growth in the wild type. However, the effects of the treatments on the TEB differed in the mutant strain. The MMP2:TIMP2 ratio was highest for α-LA, supporting the elongation; however, there were fewer TEB compared with the soya- and oestradiol-treated groups. The use of total DNA accounted not only for the action in the epithelial cells but also for the cells associated with the stroma. In parallel, the group fed α-LA displayed a similar pattern of growth that also included secondary and tertiary ductal growth. This pattern suggested that changes in the ECM were affected to allow both longitudinal and collateral ductal growth. The highest ratio observed between MMP2 and TIMP2 in the groups fed α-LA and soya would suggest that the balance was in favour of degradation to provide a pathway for growth and elongation to occur. The results reflect those of others who found that ductal elongation resulted in changes in the balance of degradation and inhibition(Reference Weaver and Gilbert22, Reference Woodward, Xie and Fendrick27). The longitudinal and collateral ductal growth suggests an interaction of the epithelial cells and the ECM. Comparison of the MMP2:TIMP2 ratios supports this result.
A relationship between the ECM and EGF has been established(Reference Revillion, Bonneterre and Peyrat24). The protein composition of the ECM regulates the response to EGF related to gene expression(Reference Jin and MacDonald25). In addition, EGF modulates the adhesion of tumour cells to ECM proteins(Reference Woodward, Xie and Fendrick27). EGF is important in early development, relating epithelial to mesenchymal transition. Interestingly, despite the action of α-LA on increasing serum concentrations of EGF, α-LA did not alter the concentrations of oestradiol in the serum, suggesting that the action of α-LA on the mammary gland was not through oestrogen directly. This result may have more to do with the negative feedback mechanism by exogenous oestrogen at the level of the hypothalamus than any influence of EGF. To our knowledge, there are no studies linking an interaction between α-LA and EGF. There are several considerations of the effects of α-LA as it relates to mammary gland development and EGF. The first consideration is that a specific digested fragment of whey milk protein is similar to EGF and is recognised by the EGF receptor. This possibility is plausible, as it is known that fragments of milk proteins including α-LA are biologically active(Reference Wiseman, Sternlicht and Lund28, Reference Grant, Hunt and Xia29). The second, and possibly the more feasible explanation, is that α-LA or its digested fragments may also serve to facilitate the synthesis and/or the activity of EGF in the mammary tissue or in other tissues to increase the serum concentration, which in turn may mediate oestrogenic response in the stroma and epithelia of the mammary gland. If this were the case, it would support the findings of others(Reference Woodward, Xie and Fendrick27).
Conclusion
The premise that soya has protective effects on mammary cancer development is based on its inclusion in the diet of Asian women before puberty. Similar inverse correlations of milk consumption and tumour incidence have been demonstrated for human diets. The caveat is to recognise that lifestyle, genetics, age, stage of development of the mammary gland and the total diet are all factors in the environmental milieu promoting tumour development. We have demonstrated that the whey milk protein α-LA can affect the morphology of the mammary gland. Whereas soya may work directly on the epithelial cells via stem cells or through the proteins of an intracellular pathway, α-LA appears to modulate the activity of ECM proteins in newly weaned mice after only 1 week of dietary supplement. α-LA may possibly promote the activity of EGF; however, this suggestion is inconclusive based on our data. If this protein allows the gland to grow and differentiate before the critical period of tumour initiation, then including milk products in the diet at an early age may have implications in the intervention or prolonging the latency of mammary tumour development in later life.
Acknowledgements
The authors wish to thank William Swallow and Joy Smith, Department of Statistics, North Carolina State University. The authors would also like to thank the many undergraduate students who assisted. The present study was funded by the North Carolina Institute of Nutrition, North Carolina Nutrition Association, North Carolina Dairy Foundation and North Carolina Agricultural and Research Station. None of the authors has conflicts of interests. B. A.-M. contributed to the experimental design, experimental procedures, data interpretation and manuscript writing. J. J. L. contributed to the experimental design, experimental procedures and data interpretation. C. A. M. contributed to the experimental procedures, data analysis, data interpretation and manuscript writing.











