The 1995 National Nutrition Survey in Australia found that only 34 % of men and 21 % of women met the recommended number of daily servings of whole grainsReference Flight and Clifton1. This is despite the advice in national dietary guidelines for adults to increase the consumption of wholegrain cereals2, 3. Population studies have linked the consumption of wholegrain cereal foods with lowered risk of certain cancers (especially of the colon and rectum), CHD and diabetesReference La Vecchia, Chatenoud, Negri and Franceschi4–Reference Truswell6. Increased consumption of wholegrain cereals correlates negatively with obesityReference Koh-Banerjee, Franz, Sampson, Liu, Jacobs, Spiegelman, Willett and Rimm7, itself an important risk factor for greater morbidity and mortality.
Whole grains contain complex carbohydrates – starch and NSP – which can improve health status through a number of mechanisms. NSP resist human small-intestinal digestive enzymes completely, contributing to their faecal bulking and laxative propertiesReference Marlett, McBurney and Slavin8. NSP are the principal components of dietary fibre and fibre-rich foods and are well established in the promotion of normal colonic function and prevention of functional diseaseReference Marlett, McBurney and Slavin8, Reference Aldoori, Giovannucci, Rockett, Sampson, Rimm and Willett9. A large multi-centre population study (European Prospective Investigation into Cancer and Nutrition; EPIC) has also shown that dietary fibre (measured using country-specific methods) is protective against colorectal cancerReference Bingham, Day and Luben10.
Plant polysaccharides improve large-bowel health by mechanisms other than increasing stool mass. A fraction of NSP is fermented by the large-bowel microflora, providing the bacteria with energy for growth and maintenance and yielding SCFAReference Cummings and Macfarlane11. One of the major acids, butyrate, is thought to be especially important for bowel health and an increasing body of experimental evidence suggests that it acts to promote a normal phenotype in colonocytes and, hence, lower the risk of colorectal cancerReference Brouns, Kettlitz and Arrigoni12, Reference Topping and Clifton13. Early studies (for example, those of BurkittReference Burkitt14) on low-risk African populations who consumed diets high in unrefined foods led to the conclusion that greater dietary fibre was responsible for the protection. However, it is becoming apparent that at least for some low-risk groups (including the Africans), their intakes of fibre are not especially highReference Topping and Clifton13, Reference Segal15. Studies in southern Africa have shown that one of those low-risk groups consumes diets high in starch, including resistant starch (RS)Reference Segal15. RS is that fraction of ingested starch which enters the large bowel where it is fermented by the microflora, leading to greater SCFA productionReference Brouns, Kettlitz and Arrigoni12, Reference Topping and Clifton13. RS fermentation seems to favour butyrate productionReference Weaver, Krause, Miller and Wolin16. This may explain the apparent protection against colorectal cancer conferred by dietary starchReference Cassidy, Bingham and Cummings17. Studies with ileostomists and normal volunteers have confirmed high levels of colonic and faecal SCFA (including butyrate) in Africans consuming traditional foods putatively high in RS but low in fibreReference Segal15.
Support for beneficial effects of butyrate on colonocyte integrity has come from a range of studies. A human trial has shown that higher faecal butyrate was associated with lower colonocyte proliferationReference van Munster, Tangerman and Nagengast18. Butyrate supplied directly to the colon has been shown to oppose azoxymethane-induced large-bowel cancer in ratsReference Caderni, Luceri, Lancioni, Tessitore and Dolara19. More recently, animal studies have shown diet-induced colonocyte genetic damage was inversely related to large-bowel butyrate contentReference Toden, Bird, Topping and Conlon20. The optimal effects of complex carbohydrates on large-bowel function appear to be obtained through combining RS and NSPReference Muir, Yeow, Keogh, Pizzey, Bird, Sharpe, O'Dea and Macrae21. NSP and RS consumption is relatively low in many countriesReference Baghurst, Baghurst and Record22, Reference Bingham23. RS occurs for a variety of reasons including cooking, the presence of dietary fibre and the relative proportions of amylose and amylopectin in starchReference Brown, McNaught and Moloney24. Amylose gelatinises slowly on cooking and retrogrades more quickly on cooling compared with amylopectinReference Colonna and Mercier25. The amylose content of most cereal food starches is ≤ 30 % and raising its contribution through selective cereal breeding has been used to supply RS via processed foodsReference Brown, McNaught and Moloney24. Recently, a novel variety of a waxy, hull-less barley (Hordeum vulgare var. Himalaya 292; Himalaya 292) with a single genetic change in the pathways of starch synthesis has been identified and grown in quantityReference Morell, Kosar-Hashemi, Cmiel, Samuel, Chandler, Rahman, Buleon, Batey and Li26. This change is located in the gene encoding starch synthase IIa (SSIIa; EC 2.4.1.21) and results in a shrunken grain containing less total starch but a greater proportion of amylose and a much higher content of total and soluble NSP (including β-glucan). Animal feeding studies have shown that this new variety contains more RS than other wholegrain cereals and has the potential to improve indices of large-bowel healthReference Bird, Flory, Davies, Usher and Topping27, Reference Bird, Jackson, King, Davies, Usher and Topping28. Himalaya 292 has been used to make human foods and the aim of the present study was to determine, in free-living human volunteers, whether foods made from this barley have greater capacity to improve those indices than current wholegrain foods at equivalent levels of intake.
Subjects and methods
Subjects
Prospective volunteers were sought by local advertisement and screened using a dietary and health questionnaire and blood tests. Exclusion criteria were a history of diabetes, gastrointestinal, renal, hepatic and cardiovascular disease, an intolerance to cereal-based foods, fasting plasma glucose concentrations >6·1 mmol/l and medications or supplements likely to affect experimental endpoints.
Twenty-four subjects, eleven men and thirteen women, were enrolled in the study. Three of these withdrew before the study commenced while three dropped out during the first intervention phase, which was the Himalaya 292 phase for these three volunteers, due to either personal reasons, an inability to eat the required amount, or abdominal discomfort attributed to the bulkiness of the study foods. Baseline characteristics of the ten men and eight women who completed the study were: age 55·9 (se 2·0; range 31–66) years; weight 79·5 (se 3·6; range 57–109) kg; BMI 27·2 (se 1·2; range 21–38) kg/m2.
The study was explained fully to the subjects, both verbally and in writing, and each gave their written, informed consent before participating. The study was approved by the Human Ethics Committee of the Commonwealth Scientific and Industrial Research Organisation (CSIRO).
Study foods
Himalaya 292 grain was developed and supplied by CSIRO Plant Industry (Black Mountain, ACT, Australia). The grain was first heat stabilised with steam under pressure by Food Science Australia (North Ryde, NSW, Australia). The jacket temperature in the cooker was 100–120°C, with steam delivered at 110 kPa for 12 min, followed by vacuum at − 80 kPa for 20 min. Study foods were breakfast cereal, sweet muffins, bread and savoury cracker biscuits which were formulated and manufactured under contract by the Bread Research Institute (BRI; North Ryde, NSW, Australia). The stabilised barley grain was milled by the BRI to specifications appropriate for each of the foods. White (refined) and wholemeal wheat flours of specifications appropriate for each food application were obtained by the BRI from commercial sources.
Recipes for bread, sweet muffins and savoury cracker biscuits were developed to enable the maximum possible inclusion of Himalaya 292 consistent with a product that would be acceptable to study participants. The levels of inclusion of Himalaya 292 (as wholemeal flour) in the final products were: bread, 20 %; crackers, 37 %; muffins, 21 %. For corresponding wholemeal wheat products, wholemeal wheat flour replaced Himalaya 292 flour and for refined cereal products white wheat flour replaced Himalaya 292 flour. All other ingredients were identical for the Himalaya 292, wholemeal wheat and refined wheat products. Breakfast cereal flakes comprised either 100 % wholegrain Himalaya 292 or 100 % wholegrain wheat. Because it was not possible to make a refined wheat-flake product, a commercial puffed rice breakfast cereal (Coles Farmland, Gepps Cross, SA, Australia) was used in the refined cereal diet. The nutrient composition of Himalaya 292 has been published previouslyReference Bird, Flory, Davies, Usher and Topping27, Reference Topping, Morell, King, Li, Bird and Noakes29. The composition of the study foods is presented in Table 1.
Table 1 Nutrient composition of the cereal-based treatment foods consumed by volunteers* (Mean values of duplicate determinations)

* Volunteers consumed the following servings of each food on a daily basis for each intervention phase: 60 g breakfast cereal, two slices of bread (76 g), six crackers (19–24 g) and one muffin (85–95 g).
† Determined by the Prosky procedure.
‡ On an ‘as-is’ basis.
The foods were produced in three batches at approximately monthly intervals and shipped to CSIRO Adelaide (Australia). Bread and muffins were transported frozen and stored at − 20°C at CSIRO until required. Flakes and crackers were shipped and stored at room temperature. Puffed rice breakfast cereal was purchased at a local supermarket and stored at room temperature.
Study design and diets
Subjects were allocated randomly to one of three dietary treatments according to a cross-over study design with each intervention phase lasting 4 weeks. There was no washout period between phases. The habitual diet of each subject was modified to incorporate Himalaya 292, wholemeal wheat or refined cereal-based treatment foods. On each day of the intervention periods, volunteers were asked to consume a combination of bread, breakfast cereal, muffins and crackers that would supply in total 103 g of the test cereal. The aim was for each volunteer to consume 60 g cereal flakes (or puffed rice for the refined cereal diet), two slices of bread, one muffin and six savoury crackers each day. Volunteers were not told the identity of the test cereal in the foods provided to them. Subjects were seen by a research dietitian at the beginning of each intervention for advice on a low-fibre intake background diet ( < 15 g/d) and to assist in the accommodation of the test foods in their diet without changing total energy intake. They were also seen after 2 weeks to ensure compliance with the test foods and background diet. In a few cases it was necessary to reduce the designated serving size of a test food in order to satisfy individual preference but in most instances the loss in intake could be offset by a proportional increase in consumption of another test food from the same treatment. All other foods eaten during the study were provided by the volunteer and they were instructed to avoid consuming foods, or supplements, that were high in fibre or contained probiotics (for example, yoghurts), and to record study food consumption on a checklist. Dietary intake data are summarised in Table 2.
Table 2 Estimated daily energy and nutrient intakes for the three dietary interventions† (Mean values with their standard errors for seventeen observations)
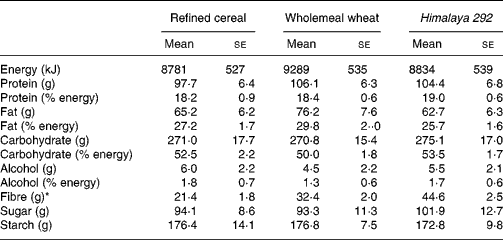
* Estimated total dietary fibre intake was significantly higher in the wholemeal wheat group compared with the refined cereal group (F ratio 7·931, P = 0·001; repeated-measures ANOVA) (P = 0·013; Bonferroni post hoc test), and in the Himalaya 292 group compared with the refined wheat group (P = 0·001; Bonferroni post hoc test) and the wholemeal wheat group (P = 0·001; Bonferroni post hoc test). There were no statistically significant differences between the three groups using repeated-measures ANOVA for energy, protein, fat, carbohydrate, sugar or starch.
† Results were based on self-reported dietary intakes.
Study protocol and sample collections
The volunteers visited the Clinical Research Unit during week 0 and at the end of weeks 2, 4, 6, 8, 10 and 12. To monitor intake of individual foods, volunteers were instructed to maintain 3 d weighed food records in the period before the study commenced (baseline, week 0) and then at the end of each 4-week intervention period. At each clinic visit, volunteers returned any uneaten test foods, which were weighed in order to assess compliance, and collected their allocation of test foods for the next 2 weeks. The volunteers were also weighed and the background diet adjusted accordingly, in consultation with a study dietitian, if significant weight change had occurred. At all visits a study dietitian counselled volunteers to motivate them and encourage compliance.
Complete 48 h faecal collections were made by all participants at the end of each intervention period (weeks 4, 8 and 12). A plastic bag was placed over the toilet bowl to collect each stool. Air was expelled from the plastic bag, which was then sealed, labelled and frozen ( − 20°C) and taken to the Clinical Research Unit to await laboratory analyses. Separate bags were used for each bowel motion. Defecation frequency was calculated according to the number of bags used per collection period. A spot urine sample was obtained from each volunteer during their clinic visit and immediately frozen ( − 80°C) to await analysis. Samples of each cereal test food were obtained during the second intervention period and analysed for macronutrients and total dietary fibre (TDF).
Assessment of nutrient intake and compliance
Volunteers were provided information on recording the amount and type of foods eaten daily over a 3 d period twice during each intervention in addition to completing a daily checklist of the study food items. Training for completing food intake records was provided by study dietitians and food records were analysed with the participants when they visited the clinic. Daily energy and macronutrient intakes were calculated by using Diet 4 Nutritional Calculation software (Xyris Software, Highgate Hill, Qld, Australia) based on Australian food composition tables and compositional data on the test products.
Compliance with the study protocol was calculated by measuring differences in the amount of test foods issued and returned for each period and compatibility with that reported in the test food checklists. Refined cereal intake was 99 g with 96 % compliance, wholemeal wheat intake was 97 g with 94 % compliance and intake of Himalaya 292 was 96 g with 94 % compliance.
Sample processing and analyses
Biochemical, nutrient and microbiological analyses were performed in duplicate using standard published procedures. Samples of each cereal test food were obtained during the second intervention period and freeze dried to constant weight, ground to pass through a 0·5 mm sieve using a cyclonic mill (Cyclotec 1093; Tecator, Höganäs, Sweden) and proximate and other analyses then performed on this material. Moisture was calculated as loss in mass resulting from lyophilisation. The gravimetric method of Prosky et al. Reference Prosky, Asp, Furda, DeVries, Schweizer and Harland30 was used to determine TDF. Total, and soluble and insoluble neutral NSP (NNSP) were measured by the gas chromatographic procedure of Theander et al. Reference Theander, Aman, Westerlund, Andersson and Pettersson31 subject to a slight modification which involved a 2 h hydrolysis with 1 m-sulfuric acid followed by centrifugation for insoluble NNSP, and hydrolysis using 2 m-trifluoroacetic acid for soluble NNSP. Total starch was analysed according to the enzymic method of McCleary et al. Reference McCleary, Solah and Gibson32 using a commercial assay kit (K-TSTA; Megazyme International Ireland Ltd, Bray, Republic of Ireland). Fat content was determined gravimetrically after enzymic digestion of samples with clarase followed by homogenisation and extraction with chloroform–methanolReference Daugherty and Lento33 as outlined by the Association of the Official Analytical Chemists (AOAC official method 983·23). Simple sugars were extracted with aqueous methanol (80 ml/100 ml; AOAC method 982·14) and then quantified by HPLC using a polyamine-bonded polymeric gel column, acetonitrile–water (75:25, v/v) as the mobile phase and a refractive index detector. Total N was analysed by the Dumas combustion methodReference Kirsten, Ternud and Hesselius34 using an automated N analyser (model 1500; Carlo Erba, Milan, Italy). The protein content of food samples (g/100 g) was estimated by multiplying N by 6·25.
Urine samples were thawed at room temperature and samples assayed for creatinine by the Jaffe colorimetric method using a commercial kit (Metra™ Creatinine Assay Kit no. 8009; Quidel Corp., San Diego, CA, USA). Phenols were determined by an HPLC procedure based on the methods of Murray & AdamsReference Murray and Adams35 and Yoshikawa et al. Reference Yoshikawa, Taguchi, Arashidani and Kodama36. Briefly, samples containing internal standard (4-ethyl-phenol, 0·3 mmol/l; Sigma Aldrich, Castle Hill, NSW, Australia) were acidified with 2 m-HCl and boiled for 30 min before distilling under vacuum. Distillates were analysed for phenol and p-cresol using a reverse-phase Chromopack HPLC Microsorb column (250 × 4·6 mm; Varian, Melbourne, Victoria, Australia). The mobile phase was acetonitrile–water (30:70, v/v) (pH 3·2), the flow rate was 1 ml/min, and the injection volume was 20 μl. The column oven temperature was set at 28°C. Phenol and p-cresol were detected at a wavelength of 275 nm with a UV/Vis-detector (LC1200; GBC, Adelaide, SA, Australia).
Stools from each volunteer were thawed at room temperature, pooled within collection period, mixed thoroughly, the composite weight recorded, and then duplicate samples analysed as follows. For determination of DM, samples weighing approximately 3 g were freeze dried to constant weight. For SCFA determination, samples (1 g) were diluted three-fold with internal standard (1·68 mm-heptanoic acid; Sigma Aldrich), centrifuged (3000 g for 15 min at 4°C) and the pH of the resultant supernatant fraction measured by inserting an appropriate glass probe. A sample (150 μl) of supernatant fraction was then acidified with 30 μl 0·16 m-orthophosphoric acid and distilled under vacuum. Individual SCFA in the distillates were separated and quantified by capillary GC (5890 series II; Hewlett Packard, North Ryde, NSW, Australia) as described previouslyReference Bird, Flory, Davies, Usher and Topping27. The GC was equipped with a flame ionisation detector, split-less injector and a Zebron ZB-FFAP 30 m × 0·53 μm capillary column with 0·1 μm film thickness (Phenomenex, Lane Cove, NSW, Australia). Injector and detector temperatures were both 210°C, and the column temperature program was 120°C held for 0·5 min and then raised at 30°C/min to reach a final column temperature of 190°C. The carrier gas used was He (head pressure 50 kPa) and an injection volume of 0·2 μl was used.
Total SCFA concentration was calculated as the sum of acetic, propionic, butyric, isobutyric, caproic, isovaleric and valeric acid concentrations. Faecal SCFA excretion (mmol/2 d) was calculated as: SCFA concentration (mmol/l) × wet faecal weight (g/2 d) × faecal moisture content (g/100 g) × 10–5.
Ammonia in faeces was measured using the indophenol blue procedureReference Chaney and Marvach37. Faecal specimens (0·5 g) were mixed with 9 volumes of distilled water and the slurry centrifuged at 2000 g for 10 min. A sample (100 μl) of supernatant fraction was diluted 1:10 with water and 2 ml of an aqueous phenol (0·1 mol/l) plus sodium nitroprusside (0·17 mmol/l) solution added followed immediately by 2 ml of alkaline sodium hypochlorite (5·4 mmol/l). The samples were vortexed before being heated for 10 min at 60°C in a shaking water-bath and then quickly cooled to room temperature. The optical density (625 nm) of the blue-coloured endproduct (indophenol) that formed was measured by colorimetry. Ammonia concentration was determined from a standard curve based on appropriate reference solutions.
Faecal phenols were determined using the same reverse-phase HPLC procedure as outlined for urine samples. Each faecal sample was diluted 3-fold with 4-ethyl-phenol (4·1 mmol/l) as internal standard and the slurry centrifuged at 3000 g for 15 min at 4°C. A 150 μl sample of supernatant fraction was acidified with 30 μl 0·16 m-o-phosphoric acid and distilled under vacuum at low temperature. Phenol and p-cresol were isolated and separated by the same HPLC method referred to earlier except that an injection volume of 30 μl was used.
Bacteria were enumerated using conventional selective plating methods. Subsamples of homogenised faeces were mixed with 90 ml of pre-reduced buffered peptone water (20 g/l buffered peptone (Oxoid CM509; Oxoid Australia Pty Ltd, West Heidelberg, Victoria, Australia), 0·5 % cysteine HCl and 0·1 % Tween 80) and the resultant suspension thoroughly homogenised followed by 10-fold serial dilutions. Samples (100 μl) of appropriate dilutions were inoculated in duplicate directly onto plates containing either Bifidus bloodReference Pachenari, Conway and Playne38, Rogosa (Oxoid CM627), Columbia blood (Oxoid CM 331) or chromogenic Escherichia coli–coliform (Oxoid CM956) medium for the selective enumeration of bifidobacteria, lactobacilli, total anaerobes, and E. coli, coliforms and total aerobes, respectively. Bifidus blood, Columbia Blood and Rogosa plates were incubated at 37°C under anaerobic conditions (Anaerogen Compact system; Oxoid AN010C) for between 3 and 7 d depending on the culture. Chromogenic plates were incubated at 37°C under aerobic conditions for 24 h. Colonies characteristic of each bacterial group were visually counted and the concentration calculated as log10 colony-forming units/g wet weight.
Calculations and statistical analysis
Data were analysed using SAS software (release 8.02; SAS Institute Inc., Cary, NC, USA). Eighteen volunteers completed the study but one subject did not undertake complete faecal collection on two occasions. Data for this non-compliant individual, and for the three volunteers who failed to finish the study, were excluded from the final statistical analysis. The results are presented as mean values with their standard errors for seventeen observations, except where otherwise indicated.
Effects of the dietary treatments were determined by ANOVA with the following terms included in the model: subject, period, carryover and treatment. Carryover and period were subsequently removed from the model as they were not found to be significant (P>0·05). Fisher's least significant difference test was applied to assess pair-wise comparisons between individual treatments. Logarithmic transformation of bacteriological data was performed before statistical analysis and the results expressed as log10 colony-forming units/g wet weight. Repeated-measures ANOVA was used to examine estimated nutrient intake data. A P value < 0·05 was considered statistically significant.
Results
Body weights of the volunteers remained steady throughout the study and were similar at the end of each of the three dietary interventions (refined cereal, 80·1 kg; wholemeal wheat, 80·5 kg; Himalaya 292, 79·3 kg; P>0·05) and were not different from baseline. Mean intake of test cereals estimated from diet records was 96, 97 and 99 g for the Himalaya 292, wholemeal wheat and refined cereal diets, respectively, which also indicated good compliance. The study foods were well accepted and their consumption was not associated with any serious adverse effects. There were no significant differences in energy or macronutrient intake between the different dietary interventions (Table 2). TDF intake was different for each of the diets (all comparisons P < 0·001). Himalaya 292 and whole-wheat diets supplied about an extra 23 and 11 g dietary fibre/d, respectively, over the 21 g/d (approximately) consumed during the low-fibre period.
Faecal output was greater when Himalaya 292 or the whole-wheat diets were consumed than the refined cereal diet (P < 0·05; Table 3). Faecal bulking was about 8 % larger for Himalaya 292 than wholemeal wheat but the difference was not statistically significant. Frequency of defecation was not different between treatments. Faeces were drier on the refined cereal diet than the wholemeal diets (P = 0·04, Himalaya 292 and wholemeal wheat data combined) but the effects of the individual high-fibre diets were not significant (Table 3). Faecal pH was lower with Himalaya 292 than either the wholemeal wheat or refined cereal diets (P < 0·05).
Table 3 Effect of dietary treatments on faecal weight, water content and pH, and defecation frequency (Mean values with their standard errors for seventeen observations)
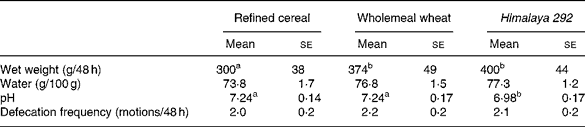
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
Faecal concentrations of total and major individual SCFA were similar for the refined cereal and wholemeal wheat treatments (Table 4). The faecal concentrations of acetate and propionate for these two diets were not significantly different from Himalaya 292. However, faecal butyrate was significantly higher on the Himalaya 292 diet than with the other two treatments, with concentrations 27 and 42 % greater than wholemeal wheat and control (refined) diets, respectively. Faecal total SCFA, acetate and propionate excretion was similar for the two wholegrain cereals and significantly greater than when the control diet was consumed. However, the combination of higher concentrations and greater stool bulk in subjects consuming Himalaya 292 led to a 41–91 % increase in faecal excretion of butyrate relative to the wholemeal wheat and refined cereal diets (Table 4).
Table 4 Effect of dietary treatments on faecal excretion of short-chain fatty acids expressed as concentration (mmol/l) and amount (mmol/48 h) (Mean values with their standard errors for seventeen observations)
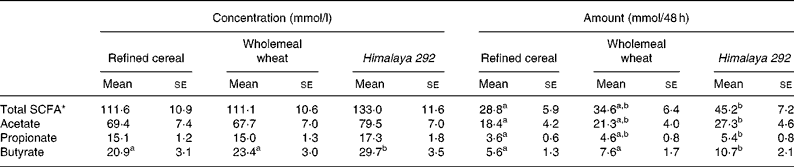
a,b Mean values within a row for concentration or amount with unlike superscript letters are significantly different (P < 0·05).
* Sum of acetic, propionic, butyric, isobutyric, caproic, isovaleric and valeric acids.
The dietary interventions had no significant effect on faecal ammonia or phenol concentrations (data not shown). However, p-cresol concentrations were significantly lower with Himalaya 292 (426 (se 71) nmol/g) and whole wheat (363 (se 65) nmol/g) than with the control diet (641 (se 115) nmol/g) (P < 0·05). Diet also had no effect on urine creatinine concentration, and phenol and p-cresol content relative to creatinine (data not shown). Urine p-cresol concentration was greater on the wholemeal wheat diet compared with refined wheat (7·4 (se 1·6) and 4·2 (se 0·6) μg/ml; P < 0·05) whereas Himalaya 292 (5·7 (se 0·9) μg/ml) was not significantly different from these treatments.
Neither the faecal numbers per g of stool nor the daily excretion of aerobic, bifidobacteria, lactobacilli or coliform bacteria differed between treatments (data not shown). However, the excretion of anaerobes was significantly (P < 0·05) higher during the consumption of Himalaya 292 foods compared with the refined foods with mean values of 11·49 (se 0·13) and 11·84 (se 0·14) log10 colony-forming units/d, respectively. Excretion did not differ from the other treatments during the whole-wheat period with a mean value of 11·73 (se 0·08) log10 colony-forming units/d.
Discussion
The aim of the present study was to compare the effects of foods made from the novel barley variety (Himalaya 292) and from wheat on indices of bowel health. Both sets of foods were made from whole grains while a further comparison was with refined wheat products. As expected from the analytical data, TDF intakes were significantly higher during the wholegrain arms of the trial with mean values of 45 and 32 g/person per d for the Himalaya 292 and whole-wheat foods, respectively, compared with 21 g/person per d for the refined cereal products at equivalent levels of foods consumption. Compared with the refined cereal foods, consumption of Himalaya 292 foods resulted in 33 % higher faecal weight, a lowering of faecal pH and higher faecal concentration and excretion of butyrate.
In comparison with wholegrain wheat, foods made from Himalaya 292 contained approximately double the level of TDF and about 80–90 % more NSP. The difference between the TDF and NSP values in the Himalaya 292 foods can be ascribed to a contribution by RS. Previous experiments with rats have shown that Himalaya 292 consumption leads to raised large-bowel starch (i.e. RS) by 100–200 % at equivalent levels of dietary fibreReference Bird, Flory, Davies, Usher and Topping27. Studies with human volunteers support these findings with lower glycaemic index values for Himalaya 292 foods relative to a standard barley or wheatReference Topping, Morell, King, Li, Bird and Noakes29, Reference Keogh, Lau, Noakes, Bowen and Clifton39. While RS and glycaemic index are not synonymous, the lower values for the latter are suggestive of slower small-intestinal starch digestion and absorption in humans.
The randomised cross-over design is a strong one and the dietary records (plus the food returns) show good compliance and similar intakes of macronutrients during all three periods. It has been reported that food consumption was higher in the short term following meals containing Himalaya 292 compared with whole wheatReference Keogh, Lau, Noakes, Bowen and Clifton39. This did not occur in the present study and body weight showed no change in any experimental period. Rates of withdrawal from the study were low (n 3) and those that did so withdrew in the first study phase due to personal reasons (n 1), an inability to eat the required amount (n 1) or abdominal discomfort attributed to the study foods (n 1). The only significant difference was in the greater TDF intake with the barley (45 g/person per d) and wheat whole grain (32 g/person per d) foods over the refined wheat (20 g/person per d). Even considering these high fibre intakes, there were only a few reports overall of gastrointestinal upsets (bloating, distension, flatulence, etc) and they were unrelated to dietary treatment. Fibre consumption during the refined wheat period was high by international standards but is consistent with earlier findings in another nutritional study with AustraliansReference McIntosh, Noakes, Royle and Foster40, in population surveys of AustraliansReference Baghurst, Baghurst and Record22 and a large study of older AustraliansReference Barclay, Brand-Miller and Mitchell41. Intakes of this order have been reported to promote laxation in youngReference Haack, Chesters, Vollendorf, Story and Marlett42 and elderlyReference Baghurst, Hope and Down43 subjects but higher intakes do not appear to increase defecation frequencyReference Haack, Chesters, Vollendorf, Story and Marlett42. The present data replicate the latter finding. The high stool weights (300 g/48 h) recorded during the refined wheat period are consistent with dietary fibre intake. They are of the order which has been suggested as a minimum value for protection against colon cancerReference Cummings, Bingham, Heaton and Eastwood44 and are associated with improvements in a variety of putative markers of bowel healthReference Birkett, Jones, de Silva, Young and Muir45. Both high-wholegrain periods showed increased stool output. Faecal bulking is in proportion to dietary fibre intake with wheat bran as a possible standard in this regardReference Topping46 with an increase of about 5 g stool/g fibre (as NSP) consumedReference Cummings and Spiller47. The increment of 5·1 g stool/g fibre (measured as NSP) with the whole-wheat diet compared with 2·9 g stool/g NSP for barley foods accords with this suggestion. The increase in stool weight in response to the barley products is similar to that reported for oats and maize, about 3·4 g faeces for each additional g dietary fibre. The difference between the two dietary treatments may reflect the fermentabilities of the major fibre components of whole wheat and the new barley. The latter is higher in soluble fibres which, in general, seem to be relatively more fermentable than insoluble fibres, making them less effective faecal-bulking agentsReference Cummings and Spiller47. In the present study, 76 % of the total NSP in the whole-wheat foods was insoluble compared with 29 % in the barley foods. Nevertheless, stool mass was 20 % higher than anticipated when the Himalaya 292 foods were consumed (100 g/48 h compared with an estimated 80 g/48 h). These data support the suggestion that other dietary, non-NSP, components (for example, RS) can contribute to faecal bulkingReference Birkett, Jones, de Silva, Young and Muir45. It is possible also that differences in the water-holding capacity of the unfermented residues may also play a roleReference McBurney48. However, faecal water content did not differ between treatments, suggesting that this was not a contributor. Finally, it is possible that some of the difference was due to greater bacterial excretion, although we have no measure of the total biomass.
As noted, previous animal studies have shown more large-bowel starch, greater digesta mass and higher SCFA at equivalent fibre intakes for Himalaya 292 than comparable grains. The present study extends these findings with greater SCFA excretion and lower faecal pH following consumption of foods containing Himalaya 292 Reference Bird, Flory, Davies, Usher and Topping27, Reference Bird, Jackson, King, Davies, Usher and Topping28. The present experiment was conducted with equivalent servings of foods (rather than matched intakes of dietary fibre), as this conforms to human consumption patterns. It should be noted also that we used consumer foods prepared by industrial processes involving heat. We acknowledge also that it is not possible to specify that the effects were due to RS alone. Based on the animal studies with Himalaya 292, it was expected that faecal SCFA would be higher during both the whole-wheat and (particularly) the barley periods. However, the impact of whole wheat on SCFA concentrations and excretion was not significant relative to the refined wheat foods. Only Himalaya 292 foods raised the excretion of butyrate significantly. This could be a reflection of the difference in faecal bulk. Greater stool mass is associated with more rapid transitReference Burkitt, Walker and Painter49, Reference Read, Miles, Fisher, Holgate, Kime, Mitchell, Reeve, Roche and Walker50 which is a determinant of faecal SCFA through diminished colonic absorptionReference Topping and Clifton13. However, transit alone may not account for the higher faecal butyrate levels during the Himalaya 292 period. Evidence for greater large-bowel fermentation during consumption of barley foods came from the faecal pH values which were significantly lower than in the other two periods. Similar data were obtained in rats and pigsReference Bird, Flory, Davies, Usher and Topping27, Reference Bird, Jackson, King, Davies, Usher and Topping28. Lowering of pH comes through direct acidification by increased SCFA concentrations and, also, through the fixing of NH4+, as greater bacterial massReference Weber51. As there were no differences in faecal ammonia, it appears that the higher SCFA (through greater fermentation) were responsible. Lowered pH is thought to promote bowel health through altering the absorption of potentially toxic metabolites and preventing overgrowth by potential pathogenic bacteriaReference Topping and Clifton13.
Greater polysaccharide fermentation can lower the levels of bacterial protein degradation products such as phenols and cresolsReference Birkett, Muir, Phillips, Jones and O'Dea52. These compounds have been linked to a greater risk of cancers, including those of the large bowelReference Bingham23. p-Cresol (but not phenols) was lowered in both faeces and urine during both wholegrain periods, indicating lower exposure to these agents.
Previous studies have shown that RS can function as a prebiotic, stimulating the growth of lactic acid bacteria in the bowelReference Topping, Fukushima and Bird53. In the present study no differences were observed in faecal bifidobacteria and lactobacilli numbers. This is consistent with our finding of a lack of prebiotic action of Himalaya 292 in pigs although numbers of anaerobes and aerobes were increasedReference Bird, Jackson, King, Davies, Usher and Topping28. These data provide further evidence that not all RS stimulate proliferation of probiotic bacteriaReference Topping, Fukushima and Bird53. However, it must be recognised that the culture-based techniques that were used to enumerate faecal bacteria in our studies on the novel barley may not have had the necessary sensitivity to detect subtle changes in bacterial populations. The observation that faecal anaerobes were more numerous when volunteers were on the barley diet is consistent with greater supply of fermentable substrate.
Prototype foods made from the new barley were a rich source of fibre and their texture was acceptable to volunteers who had no apparent difficulty adapting to the wholegrain dietary regimen. The use of Himalaya 292 offers an opportunity to expand and diversify the range of food products available to consumers, thereby making it easier for them both to increase their intake of fibre and to meet dietary recommendations for increased consumption of whole grains.
Acknowledgements
The present study was supported financially by CSIRO and ACVL Ltd. The authors thank Michael Mular and Sylvia Usher for compositional analysis of food ingredients, Debbie Davies and Corinna Flory for sample processing and biochemical and bacterial analyses, and Anne McGuffin, Kathryn Bastiaans and Julia Weaver for recruitment and management of volunteers. Russell Heywood and Zhongyi Li at CSIRO Plant Industry managed the growth, harvesting and milling of grain samples and staff at Food Science Australia (North Ryde, NSW, Australia) stabilised the grains with heat. Ken Quail and staff at BRI Ltd (North Ryde, NSW, Australia) are acknowledged for developing and manufacturing the cereal-based foods used in the human study.
M. K. M. and D. L. T. are named inventors of a disclosure describing the barley cultivar (PCT/WO02/37 955/A1 Barley plant with high amylose starch). They have no financial interest in this invention, which was made as part of their normal duties as employees of CSIRO.






