Introduction
Introduced species threaten native biodiversity and natural resources (Vitousek et al., Reference Vitousek, Mooney, Lubchenco and Melillo1997; Doherty et al., Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016), are the second most important cause of biodiversity loss, after habitat destruction and fragmentation, and on islands have been the main driver of wildlife population declines and species extinctions (Millennium Ecosystem Assessment, 2005). Island ecosystems are particularly vulnerable to introduced mammals because of loss of anti-predator behaviour in many island species (Blumstein & Daniel, Reference Blumstein and Daniel2005), high rates of endemism (9.5 and 8.1 times higher than on the mainland for vascular plants and vertebrates, respectively; Kier et al., Reference Kier, Kreft, Ming, Jetz, Ibisch and Nowicki2009), low species richness and simple trophic webs (Vitousek et al., Reference Vitousek, Loope and Adsersen1995). High rates of species invasion and extinction of native fauna and flora on oceanic islands have resulted in biotic homogenization (i.e. the gradual replacement of native biota by introduced species; McKinney & Lockwood, Reference McKinney and Lockwood1999).
The introduction of mammals is a major cause of species extinctions on oceanic islands (Biber, Reference Biber2002), and most introduced mammal species became established after European colonization (Vitousek, Reference Vitousek, Wilson and Peter1988). Each new introduction of mammals increases the number of native species lost, and the probability of extinction is higher for oceanic island endemic species than for mainland species (Blackburn et al., Reference Blackburn, Cassey, Duncan, Evans and Gaston2004). The number of bird species that have become extinct following human colonization of oceanic islands is estimated to be in the hundreds to thousands (Blackburn et al., Reference Blackburn, Cassey, Duncan, Evans and Gaston2004), and most extinction events have occurred or are occurring in island ecosystems (Courchamp et al., Reference Courchamp, Chapuis and Pascal2003).
Eradication is only possible or cost-effective at early stages of invasion, before introduced species become abundant and widespread (Nogales et al., Reference Nogales, Martin, Tershy, Donlan, Witch and Puerta2004; Howald et al., Reference Howald, Donlan, Galvan, Russell, Parkes and Samaniego2007). Although prevention, early detection and rapid response are the best management strategies, most oceanic islands lack systems for detecting, responding to and monitoring introduced species. Wildlife managers require reliable information on introduced species to guide, assess and adjust management actions, a process known as adaptive management. Thus, large-scale and long-term monitoring programmes are needed to evaluate the efficacy of eradication operations and confirm the removal of introduced species.
Efficient survey techniques are required to detect and monitor introduced species on islands. Camera trapping is an effective, non-invasive survey technique for monitoring wildlife (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003; Tobler et al., Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008), including introduced small (Rendall et al., Reference Rendall, Sutherland, Cooke and White2014) and arboreal (Gregory et al., Reference Gregory, Rueda, Deichmann, Kolowski and Alonso2014) mammals. Camera traps provide data on multiple species at large spatial and temporal scales, with low impact on wildlife, and at low effort and cost (the relatively high initial cost is offset by the reuse of camera traps in subsequent studies; O'Connell et al., Reference O'Connell, Nichols and Karanth2011). Camera trapping is effective for detecting rare, elusive or cryptic animals (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003; Tobler et al., Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008), as is the case for some introduced species. Camera-trap data can be used to define species distributions (González-Esteban et al., Reference González-Esteban, Villate and Irizar2004) and estimate relative abundance and population trends (e.g. photographic capture rate; Rovero & Marshall, Reference Rovero and Marshall2009).
We evaluated camera trapping as a survey technique for detecting and monitoring introduced small and medium-sized terrestrial mammals on a remote oceanic island. We also report information on birds and reptiles, and we quantify the cost and sampling effort needed to complete the inventory of introduced mammals. Finally, we propose guidelines for designing programmes to monitor introduced species.
Study area
The Azores archipelago is a group of nine volcanic islands located in the North Atlantic Ocean, c. 1,500 km from Europe and 1,900 km from America. The Azores were uninhabited until Portuguese colonization in the 15th century, and since then many species have been introduced to the islands (Frutuoso, Reference Frutuoso1561). The archipelago is a high priority area for seabird conservation. Most native seabird species are categorized as Endangered at the regional scale (Cabral et al., Reference Cabral, Almeida, Almeida, Delliger, Ferrand de Almeida and Oliveira2005). Seabird populations have declined because of habitat loss, human exploitation and predation by introduced mammals, particularly rodents and feral cats (Monteiro et al., Reference Monteiro, Ramos and Furness1996). Some native species have become extinct or are confined to inaccessible cliffs and islets (Monteiro et al., Reference Monteiro, Ramos and Furness1996).
We conducted our study on the 402 km2 Terceira island, which rises up to 1,022 m altitude. Most human settlements are located in the coastal lowlands, where the native laurel forest has been transformed to pastures for cattle grazing. Native vegetation only remains in inaccessible highlands. All terrestrial mammals on Terceira have been introduced since the European colonization (Mathias et al., Reference Mathias, Ramalhinho, Santos-Reis, Petrucci-Fonseca, Libois and Fons1998): the feral cat Felis catus, ferret Mustela furo, weasel Mustela nivalis, rabbit Oryctolagus cuniculus, black rat Rattus rattus, Norway rat Rattus norvegicus, house mouse Mus musculus and hedgehog Erinaceus europaeus.
Methods
Camera-trap survey
We surveyed 72 locations on Terceira (Fig. 1) during October 2013–January 2015. We selected camera-trap locations at random, by creating random points in ArcGIS (Esri, Redlands, USA), but separated by at least 1 km to assure data independence (the home range size of the largest mammal on Terceira, the feral cat, is < 1 km2; Hervías et al., Reference Hervías, Oppel, Medina, Pipa, Díez and Ramos2014). We set up one camera trap (Moultrie 880i, Moultrie Feeders, Alabaster, USA, and Bushnell Trophy Cam, Bushnell Corporation, Overland Parks, USA) in each location, c. 30 cm above the ground and facing outward. Camera traps remained active for 8 days (Rendall et al., Reference Rendall, Sutherland, Cooke and White2014) and recorded the date and time of each photograph. Camera traps were baited with meat or fish, fruit and molasses, and the sensitivity of the infrared sensor was set to high to increase photographic capture rates (O'Connell et al., Reference O'Connell, Nichols and Karanth2011; du Preez et al., Reference du Preez, Loveridge and Macdonald2014).

Fig. 1 Terceira is an island of the Azores archipelago, a group of nine volcanic islands in the North Atlantic Ocean, c. 1,500 km from Europe and c. 1,900 km from America, in the Macaronesia region.
Data analysis
We used EstimateS 9.1 to compute species accumulation curves (Colwell, Reference Colwell2013). Sampling units (i.e. locations) were randomized 1,000 times to remove entry order effects. We estimated the species richness at 95% CI with an incidence-based species richness estimator. Then, we used STATISTICA 10 (StatSoft, 2011) to fit the Clench asymptotic function, a non-linear regression model, to the smoothed species accumulation curve through the quasi-Newton method. The Clench model assumes that the probability of detecting new species increases with time (Soberón & Llorente, Reference Soberón and Llorente1993). We calculated the total number of introduced mammal species from the asymptote of the curve, because species richness (i.e. the cumulative number of species) is a function of sampling effort (i.e. the number of camera-trap locations). Finally, we estimated the minimum sampling effort required to complete the inventory of introduced mammal species.
We defined the photographic capture rate (number of photographs per camera-trap day) as an abundance index, and used PRESENCE 2.2 to build single-season occupancy models (Hines, Reference Hines2006). We used presence/absence data from camera-trap surveys to calculate both the detection probability and estimated occupancy rate. We assumed constant detection probability P(.) for occupancy modelling. We estimated the sampling effort (i.e. number of camera-trap days) needed to determine site-specific absence of each introduced mammal at 95% CI (De Bondi et al., Reference De Bondi, White, Stevens and Cooke2010).
We quantified expenditures in EUR to pay for camera-trap station equipment (camera traps, memory cards, batteries and bait) and for a working day (transport, rent and fuel for a 4 × 4 vehicle and salary of one field technician). Camera-trap equipment cost c. EUR 180 per station and we estimated the cost of a working day to be c. EUR 120. We added 10% to the overall cost to account for the replacement of damaged or stolen equipment. We expressed the survey cost as:
As the number of working days depends on the number of locations and cameras, we transformed this equation into:
Then we calculated the minimum value of this function for each sampling size to create a cost curve.
Results
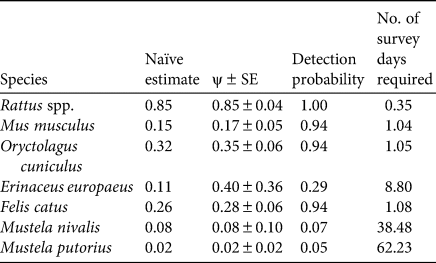
Total sampling effort was 560 camera-trap days, and the mean sampling effort per site was 8.43 ± SD 1.6 days. Samples from seven sites were excluded from the dataset because of camera-trap failures. We then included data from 65 locations in the analysis. Camera traps took a total of 10,558 photographs (10,002 of mammals, 500 of birds and 56 of reptiles). The mean capture rate was 18.85 photographs per camera-trap day. We excluded images of unidentifiable or non-target species (251 photos). Rats were the most common species (8,588 photos) and were present at 86.2% (R. rattus) and 30.8% (R. norvegicus) of sites, whereas mice were less common (77 photos; 15.7% of sites). Cats were the most common carnivore (902 photos; 24.6% of sites). Rabbits were common (310 photos; 32.3% of sites), and hedgehogs were recorded 40 times, at 10.8% of sites. Mustelids were least frequently captured (14 photos) and were present at 9.2% (weasel Mustela nivalis) and 3.1% (ferret Mustela furo) of sites. A total of eight mammal species (Plate 1), 17 bird species and one reptile species were recorded (Table 1). The species accumulation curve approached an asymptote after recording eight mammal species (Fig. 2). Rats were the most widespread species, with an estimated occupancy rate of 85%. The estimated occupancy rate of mustelids was < 10%. The detection probability exceeded 90% for most species, except the hedgehog (29%) and mustelids (7 and 5% for weasel and ferret, respectively). The sampling effort required to determine absence was adequate for all species, except for mustelids (Table 2).
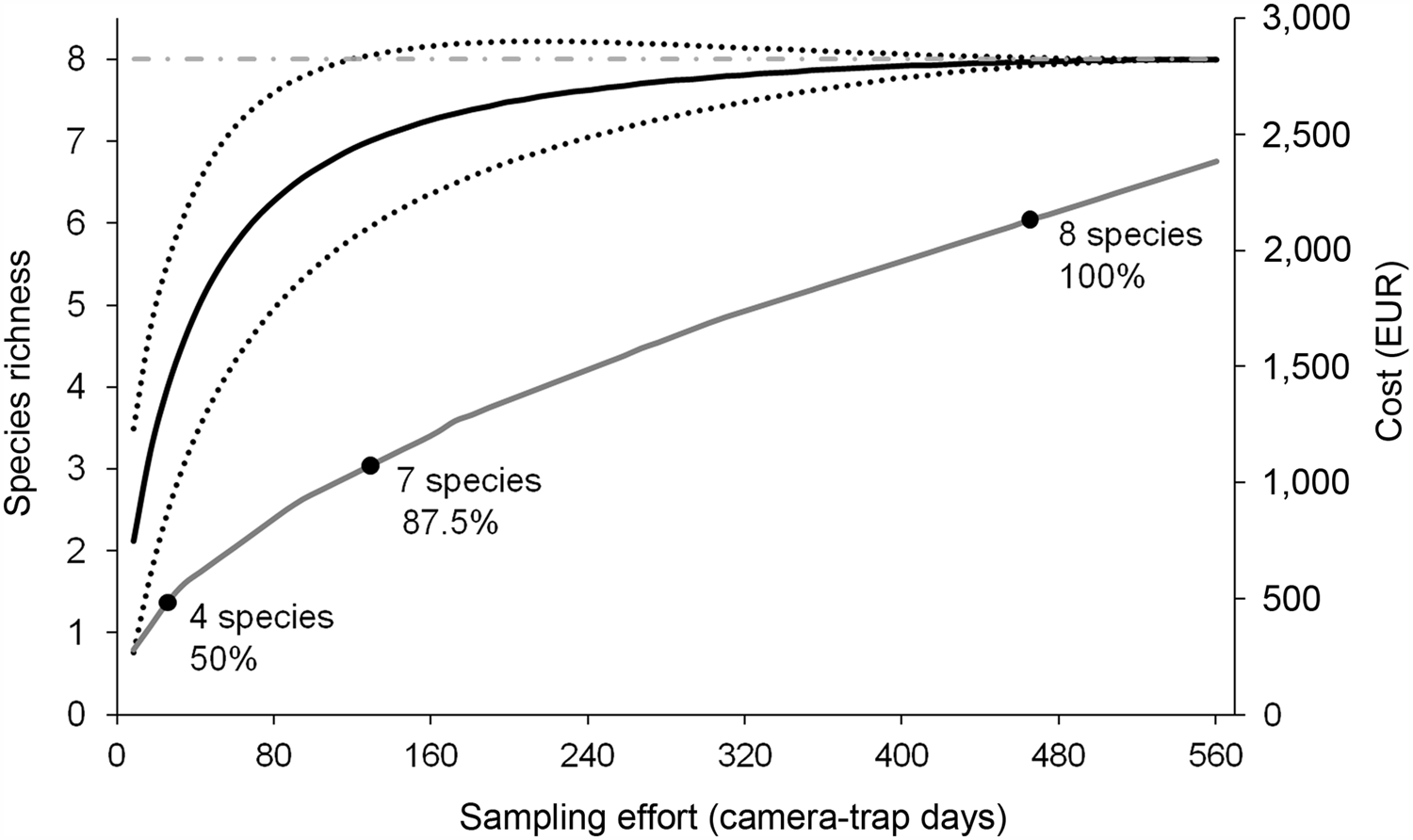
Fig. 2 Relationship between sampling effort, species richness and cost. The black solid line shows the species accumulation curve, which approaches an asymptote (grey dashed-dotted line) as sampling effort increases. The black dotted lines indicate the 95% CI. The grey solid line shows the economic costs of the camera-trapping survey. Black dots indicate the absolute number, and per cent of the total, of introduced mammal species detected during the survey.

Plate 1 Photographic captures of introduced mammals: (a) ferret Mustela furo, (b) weasel Mustela nivalis, (c) hedgehog Erinaceus europaeus, (d) house mouse Mus musculus, (e) European rabbit Oryctolagus cuniculus, (f) domestic cat Felis catus, (g) Norway rat Rattus norvegicus, (h) black rat Rattus rattus.
Table 1 Wildlife inventory through camera trapping in Terceira island (Azores archipelago). Number and percentage of presence sites, and photographic capture rates (photographs/100 camera-trap days) as a relative abundance index.
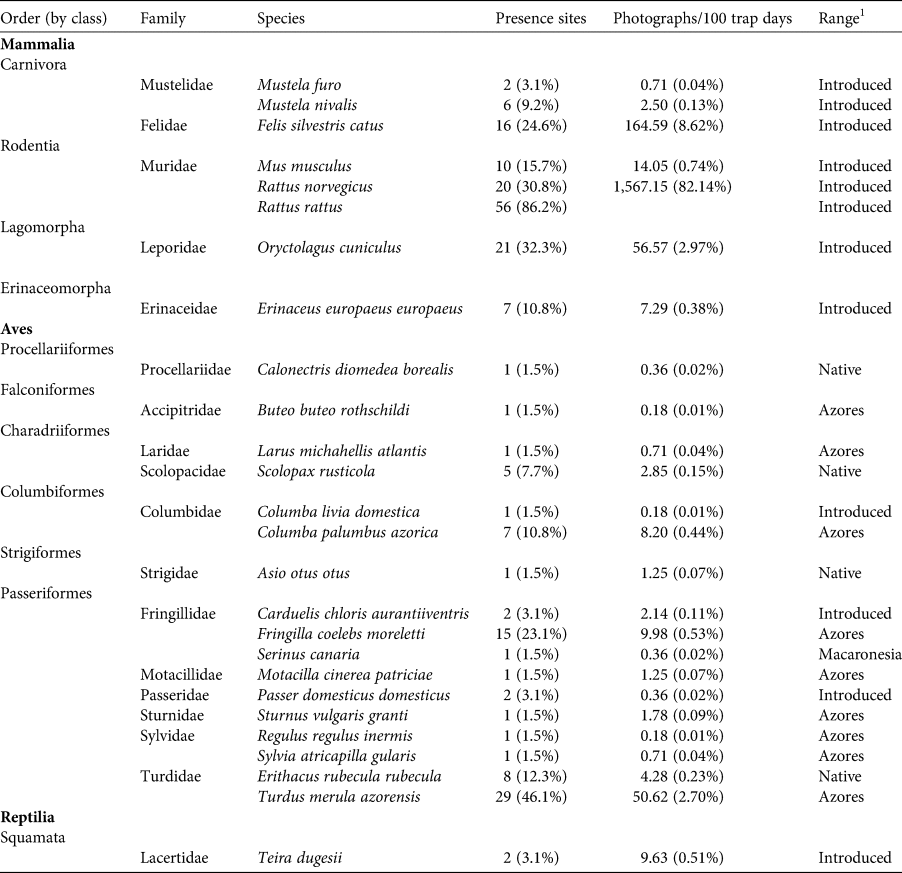
1 Range: Macaronesia, endemic to Macaronesia; Azores, endemic to Azores; Native, species range includes Azores; Introduced, species range does not include Azores.
Table 2 Information at site level of naïve occupancy estimation, estimated occupancy (ψ), cumulated detection probability and number of sampling days required to determine a site-specific absence at 95% CI, assuming constant detection probability P(.).
The sampling effort required to capture the most common introduced species (rodents, cats and rabbits; five species, 62.5% of all introduced mammal species) was 34 camera-trap days, at a cost of c. EUR 600. The sampling effort required to capture also the less common species (the weasel and hedgehog; to a total of seven species, 87.5% of the introduced mammals) was 129 camera-trap days (EUR 1,073), and to capture all eight introduced species, including the rarest (the ferret), was 465 camera-trap days (EUR 2,133; Fig. 2).
Discussion
Camera trapping has become common in wildlife research. It is more efficient than other survey techniques such as live trapping, line transects, track plots or hair snares (Lyra-Jorge et al., Reference Lyra-Jorge, Ciocheti, Pivello and Meirelles2008; De Bondi et al., Reference De Bondi, White, Stevens and Cooke2010; Monterroso et al., Reference Monterroso, Rich, Serronha, Ferreras and Alves2014; Wearn & Glover-Kapfer, Reference Wearn and Glover-Kapfer2019). Our findings confirm that camera trapping is a cost-effective survey technique for detecting and monitoring introduced mammals on oceanic islands. We have recorded all wild mammals known to have been introduced to Terceira island (eight species; Borges et al., Reference Borges, Costa, Cunha, Gabriel, Gonçalves and Martins2010), with a relatively low sampling effort (465 camera-trap days) and at low cost (EUR 2,133).
We used camera-trap data to estimate abundance, detection probability, site occupancy rate and the sampling effort required to determine site-specific absence of introduced species. We detected rats and rabbits with a low sampling effort (< 50 camera-trap days). Rats and rabbits are common (detection probability > 90%; photographic capture rate > 50 photographs/100 camera-trap days) and widespread (estimated occupancy > 30%) species on Terceira, as has been reported earlier (Mathias et al., Reference Mathias, Ramalhinho, Santos-Reis, Petrucci-Fonseca, Libois and Fons1998). However, we needed c. 500 camera-trap days to detect the rarest species, the ferret. Mustelids are rare (detection probability < 10%; photographic capture rate < 5 photographs/100 camera-trap days) and occur in few locations (estimated occupancy < 10%) on Terceira. Mathias et al. (Reference Mathias, Ramalhinho, Santos-Reis, Petrucci-Fonseca, Libois and Fons1998) reported that the ferret is rare in the Azores archipelago, except on Flores island. Camera trapping is thus useful to detect and monitor elusive and rare species.
Recommendations for camera-trap surveys
We propose the following questions to guide the survey design to monitor introduced mammals on oceanic islands: How many camera-trap locations should be sampled (sample size)? How many camera-trap days are needed to sample each location (sampling effort)? When is the best time to carry out the survey (sampling period)? How should camera traps be spaced (camera-trap density)? Where should camera traps be facing (camera-trap setup)? How much will the survey cost (economic cost)?
The number of camera-trap locations depends on the island's size and habitat diversity (Hortal et al., Reference Hortal, Triantis, Meiri, Thébault and Sfenthourakis2009; Whittaker et al., Reference Whittaker, Fernández-Palacios, Matthews, Borregaard and Triantis2017). We recommend separating the sampling locations by at least 1 km, as in previous studies (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003; Tobler et al., Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008; Pettorelli et al., Reference Pettorelli, Lobora, Msuha, Foley and Durant2010; Olson et al., Reference Olson, Marsh, Bovard, Randrianarimanana, Ravaloharimanitra, Ratsimbazafy and King2012; Hedwig et al., Reference Hedwig, Kienast, Bonnet, Curran, Courage and Boesch2018). We detected all eight introduced mammal species on Terceira (402 km2) by sampling 65 locations (i.e. 1 camera trap/6.2 km2). According to Tobler et al. (Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008), camera density and sampling area have a relatively minor effect on the number of species detected, compared to sampling effort.
The sampling effort needed to complete an inventory of terrestrial mammals usually exceeds 1,000 camera-trap days: Azlan (Reference Azlan2006) needed 5,972 camera-trap days to record 61% of large mammals in Malaysian rainforests; Tobler et al. (Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008) needed 1,440 and 2,340 camera-trap days to record 75 and 86% of medium and large mammals in the Amazonian moist forest, respectively; and Nakashima (Reference Nakashima2015) needed 4,165 camera-trap days to record 76% of medium and large mammals in the African lowland rainforest. In contrast, we needed only 465 camera-trap days to detect all introduced mammals on Terceira island. Because species richness generally increases with area, and oceanic islands are usually small and contain fewer species than mainland areas (Kier et al., Reference Kier, Kreft, Ming, Jetz, Ibisch and Nowicki2009), the sampling effort to detect introduced species on oceanic islands is lower than on the mainland (Whittaker et al., Reference Whittaker, Fernández-Palacios, Matthews, Borregaard and Triantis2017).
We recommend deploying camera traps for short periods in a given area and then moving them to enlarge the overall sampling area and to avoid spatial bias, especially if only few camera traps are available. We used five camera traps for 8-day periods, as in other studies (Rendall et al., Reference Rendall, Sutherland, Cooke and White2014). We detected most of the introduced species (Table 2). However, the sampling effort was not sufficient to determine the absence of mustelids (38 and 62 required sampling days for the weasel and ferret, respectively). Overall, the best strategy to detect all introduced mammals on oceanic islands is to sample many locations for short periods (Si et al., Reference Si, Kays and Ding2014), although longer sampling periods are needed to monitor rare species at site level.
The detection probability of species may change because of seasonal effects. Generally it is higher in the breeding season when wildlife activity increases, although some species breed all year (e.g. rodents). The sampling period should thus coincide with high wildlife activity. We also recommend to rotate the camera traps between sites during the year to avoid seasonal bias.
Camera traps are generally used to survey medium-sized and large mammals (Srbek-Araujo & Garcia, Reference Srbek-Araujo and Garcia2005; Tobler et al., Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008). Although there is a positive relationship between body weight and photographic capture rate (Tobler et al., Reference Tobler, Carrillo-Percastegui, Leite-Pitman, Mares and Powell2008), we also used camera traps to detect small mammals, as have previous studies (MCCleery et al., Reference MCCleery, Zweig, Desa, Hunt, Kitchens and Percival2014; Rendall et al., Reference Rendall, Sutherland, Cooke and White2014; Taylor et al., Reference Taylor, Goldingay and Lindsay2014). Species detectability depends on whether cameras face outwards or downwards (Taylor et al., Reference Taylor, Goldingay and Lindsay2014). For example, downward-facing cameras are more effective for detecting small mammals. However, we estimated the same detection probability of rodents as in a previous study (Rendall et al., Reference Rendall, Sutherland, Cooke and White2014), although we could not distinguish between both Rattus species in all images from outward-facing cameras. Capture frequency is higher when camera traps face outwards because the detection area is wider (Taylor et al., Reference Taylor, Goldingay and Lindsay2014). We therefore recommend outward-facing camera traps for monitoring of multiple species across a range of body sizes.
Although camera trapping requires high initial costs, this is offset by the low fieldwork effort (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003). It cost EUR 2,133 to detect and monitor all introduced mammals on Terceira, which decreased by 35% the next year because equipment was reused. Camera trapping is the most cost-effective non-invasive method to monitor wildlife (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003; Lyra-Jorge et al., Reference Lyra-Jorge, Ciocheti, Pivello and Meirelles2008; Monterroso et al., Reference Monterroso, Rich, Serronha, Ferreras and Alves2014), but we recommend complementary techniques (e.g. direct observation and sign surveys) to maximize detection probability, especially for rare species. Camera trapping is the best survey technique for a rapid assessment of introduced wildlife in varying environmental conditions (Silveira et al., Reference Silveira, Jácomo and Diniz-Filho2003). To keep costs low, we recommend purchasing a small number of camera traps and maximizing the number of survey locations by moving the cameras. In conclusion, camera trapping is an efficient survey technique for detecting and monitoring introduced mammal species on oceanic islands.
Acknowledgements
We thank Jose Sarangollo, David Rodilla, María Olivo, Sophie Wallon and Luis Ansias for their assistance during fieldwork. LLL was supported by a grant from the Fundação para a Ciência e Tecnologia—FCT (SFRH/BD/115022/2016).
Author contributions
Both authors contributed equally to all aspects of this research.
Conflicts of interest
None.
Ethical standards
This study abided by the Oryx guidelines on ethical standards.







