Introduction
Scedosporium and Lomentospora species are widely distributed filamentous fungi that emerged as etiologic agents of localized and disseminated diseases in immunocompromised and immunocompetent individuals. Fungal ball and mycetoma are usual clinical manifestations caused by these fungi, resembling a classical biofilm structure (Mello et al., Reference Mello, Bittencourt, Liporagi-Lopes, Aor, Branquinha and Santos2019).
Biofilm is considered a microbial community adhered to a biotic/abiotic surface covered by a complex extrapolymeric substance, which confers resistance to environmental stresses (Flemming et al., Reference Flemming, Wingender, Szewzyk, Steinberg, Rice and Kjelleberg2016). Scedosporium and Lomentospora species form biofilm structures over different substrates, including polystyrene, glass, catheters and lung epithelial cells, which present a typical multidrug-resistance profile (Mello et al., Reference Mello, Aor, Gonçalves, Seabra, Branquinha and Santos2016, Reference Mello, Oliveira, Frasés, Branquinha and Santos2018; Rollin-Pinheiro et al., Reference Rollin-Pinheiro, Meirelles, Vila, Fonseca, Alves, Frasés, Rozental and Barreto-Bergter2017). However, little is known about the environmental conditions that interfere with the biofilm formation in these fungi. In this respect, it is well-established that bacterial/yeast biofilms are influenced by available nutrients, particularly carbohydrates (Jahid et al., Reference Jahid, Lee, Kim and Ha2013; Waldrop et al., Reference Waldrop, McLaren, Calara and McLemore2014; Pereira et al., Reference Pereira, Silva, Ribeiro, Henriques and Azeredo2015).
Objective
The aim of the present study was to evaluate the influence of monosaccharides (glucose and fructose) and disaccharides (sucrose and lactose) on the ability of S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans conidial cells to form biofilm structures over a polystyrene surface containing different nutritional culture media.
Methods
Fungi
Scedosporium apiospermum (RKI07_0416) was provided by Dr. Bodo Wanke (Brazil), S. minutisporum (FMR4072), S. aurantiacum (FMR8630) and L. prolificans (FMR3569) were given by Dr. Josep Guarro (Spain). Fungi were maintained in Sabouraud medium for 7 days. Conidia were obtained as previously described (Mello et al. Reference Mello, Aor, Gonçalves, Seabra, Branquinha and Santos2016).
Biofilm assay
Conidia (106 cells) were placed to interact (72 h/37˚C) with 96-well polystyrene plates containing Sabouraud supplemented with different concentrations (0%, 2%, 5% and 10%) of glucose, fructose, sucrose and lactose. Synthetic cystic fibrosis medium (SCFM) and yeast nitrogen base (YNB) added or not with 2% glucose were also tested. Biofilm parameters (biomass, metabolic activity and extracellular matrix) and scanning electron microscopy (SEM) were evaluated as previously published (Mello et al. Reference Mello, Aor, Gonçalves, Seabra, Branquinha and Santos2016).
Statistics
All experiments were performed in triplicate, in three independent experimental sets. Data were expressed as mean ± standard deviation. Results were evaluated by two-way ANOVA followed by Tukey’s test.
Results
Initially, we analyzed the biofilm formation of Scedosporium/Lomentospora species when cultivated in Sabouraud, a classic culture medium for fungal studies, supplemented or not with different concentrations of monosaccharides (glucose and fructose) and disaccharides (sucrose and lactose). In all fungal species tested, no significant differences were observed regarding the biomass, cellular metabolic activity and production of extracellular matrix comparing the biofilm formed in the absence and presence of soluble carbohydrates, which was also independent of the concentration used (Figs. 1 and 2).
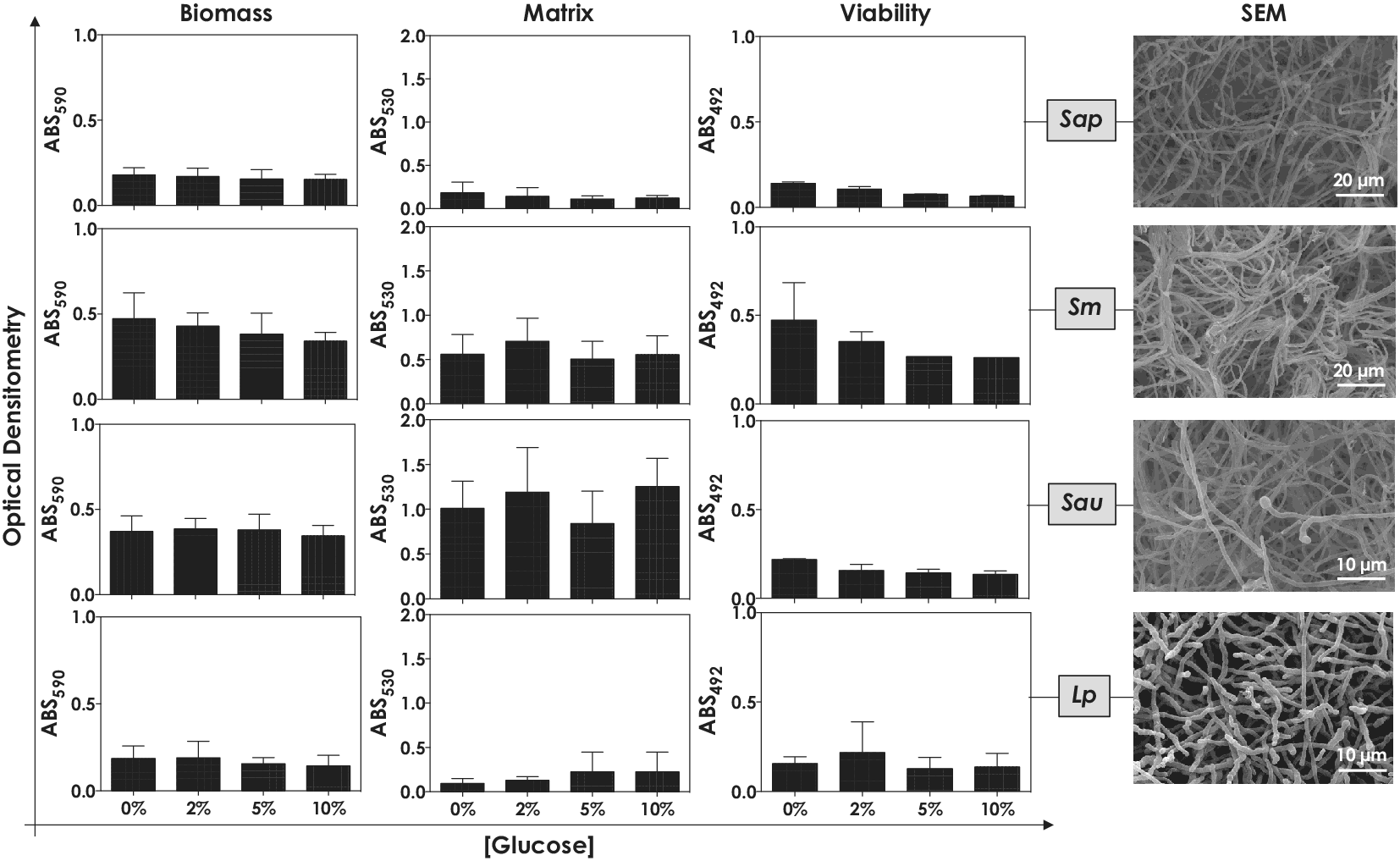
Figure 1. Biofilm formation by S. apiospermum (Sap), S. minutisporum (Sm), S. aurantiacum (Sau) and L. prolificans (Lp) under different glucose concentrations. Conidia (106) were placed to interact with polystyrene for 72 h in Sabouraud containing different glucose concentrations (0%, 2%, 5% and 10%) at 37˚C. Subsequently, in order to assess biofilm formation, the following parameters were spectrophotometrically inspected: biomass was measured in a methanol-fixed biofilm using crystal violet dye (590 nm) as well as extracellular matrix and metabolic activity (viability) were quantified in non-fixed biofilm by safranin incorporation (530 nm) and XTT metabolization (492 nm), respectively. Scanning electron microscopy images of the fungal mature biofilms formed in polystyrene containing Sabouraud supplemented with 2% of glucose were also shown.
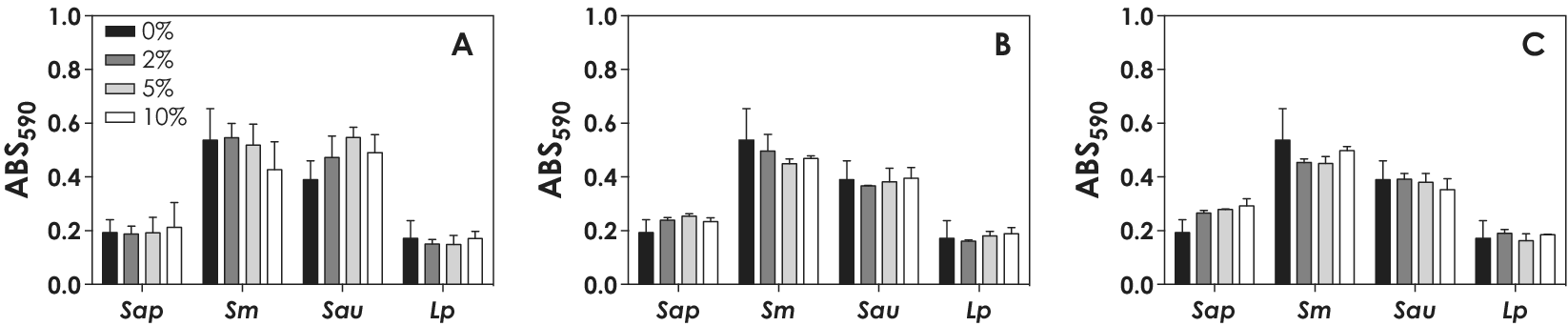
Figure 2. Biofilm formation by S. apiospermum (Sap), S. minutisporum (Sm), S. aurantiacum (Sau) and L. prolificans (Lp) under different saccharide sources. Conidia (106) were placed to interact with polystyrene for 72 h at 37˚C in Sabouraud supplemented with different concentrations (0%, 2%, 5% and 10%) of fructose (A), sucrose (B) and lactose (C). Then, the systems were processed in order to detect the fungal biomass by incorporation of crystal violet in methanol-fixed biofilms at 590 nm.
Posteriorly, two additional culture media were selected based on their chemical composition, SCFM (rich in amino acids, which mimics the cystic fibrosis sputum) and YNB (rich in salts), to evaluate the influence of glucose on fungal biofilm formation. Nevertheless, no differences on biofilm biomasses of S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans were found taking into consideration the media with and without glucose supplementation (Fig. 3).
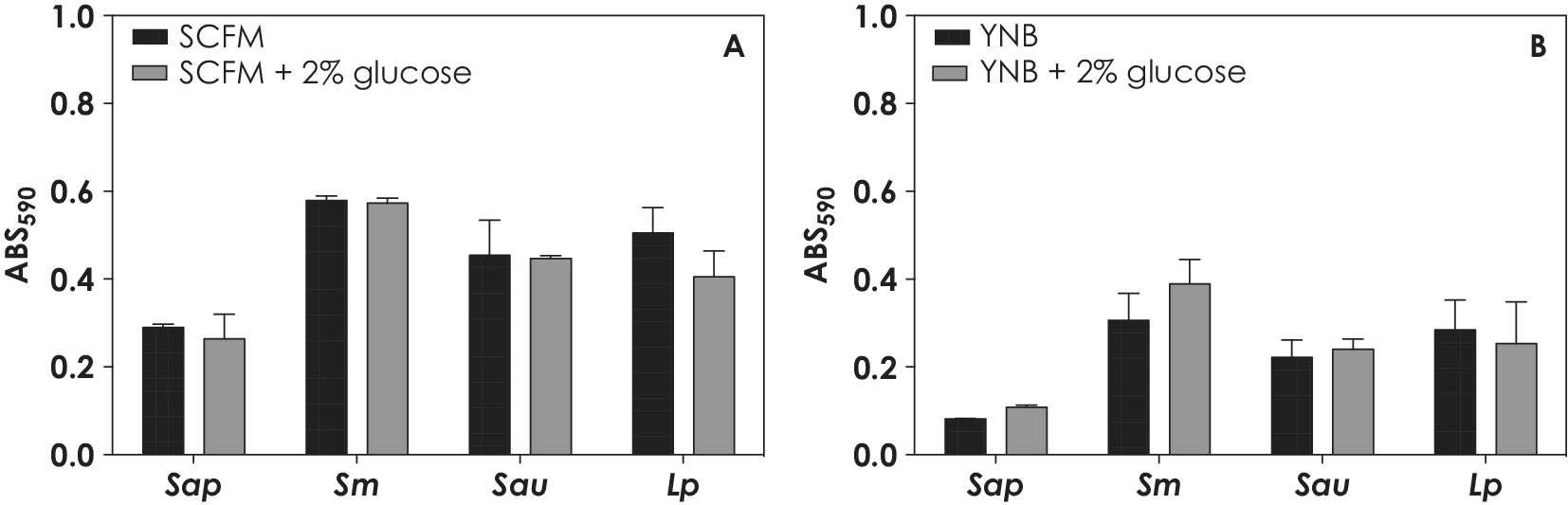
Figure 3. Biofilm formation by S. apiospermum (Sap), S. minutisporum (Sm), S. aurantiacum (Sau) and L. prolificans (Lp) in different culture media. Conidia (106) were placed to interact with polystyrene containing SCFM or YNB media supplemented or not with 2% of glucose for 72 h at 37˚C. Posteriorly, the systems were processed in order to detect the fungal biomass by incorporation of crystal violet in methanol-fixed biofilms at 590 nm.
Discussion
The ability to adapt to different physicochemical conditions is a central vein in the virulence arsenal of pathogens, because it reflects their ability to colonize several natural environments and anatomic sites of human body. Scedosporium/Lomentospora conidia easily adapt and germinate in a wide range of pH, temperature and available nutrients (Mello et al. Reference Mello, Aor, Gonçalves, Seabra, Branquinha and Santos2016). In addition, these fungal species can adapt to distinct types and concentrations of saccharides, forming a similar amount of biofilm under all tested experimental conditions. Similarly, glucose concentration did not affect the Candida albicans biofilm formation (Kolecka et al., Reference Kolecka, Chorvát and Bujdáková2015). Conversely, Candida parapsilosis was able to respond to stress caused by glucose (at 10%) by up-regulating the genes related to biofilm formation, which culminated to a more robust biofilm formation with a higher concentration of carbohydrates and β-(1,3)-glucan in the extracellular matrix composition (Pereira et al., Reference Pereira, Silva, Ribeiro, Henriques and Azeredo2015).
Conclusions
The present findings indicate that soluble carbohydrates (mono- and disaccharides), at different concentrations, were not able to modulate the capacity of S. apiospermum, S. minutisporum, S. aurantiacum and L. prolificans to form biofilm on an inert surface containing distinct culture media (Sabouraud, SCFM or YNB). These data can corroborate the high adaptive capacity of these fungi, reflecting in their metabolic plasticity.
Funding Information
This work was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Finance code 001).
Acknowledgments.
The authors would like to thank Denise Rocha de Souza and Diego de Souza Gonçalves, both supported by FAPERJ scholarships, for their technical assistance.
Disclosure statement
The authors declare that there is no conflict of interest.






Comments
Comments to the Author: In this study, the authors demonstrated that Scedosporium apiospermum, Scedosporium minutisporum, Scedosporium, aurantiacum and Lomentospora prolificans were able to form biofilm, in similar amounts, when conidial cells were incubated in an abiotic substrate containing Sabouraud medium supplemented or not with different concentrations of glucose, fructose, sucrose and lactose. The problem is significant and it can provide a better understanding of the molecular mechanisms involved in resistance to antifungal agents. The paper presents a good discussion and has scientific merit. The article should be accept.