Introduction
Populations of many North American grassland birds are declining (Winter & Faaborg, Reference Winter and Faaborg1999; Winter et al., Reference Winter, Johnson and Faaborg2000; Herkert et al., Reference Herkert, Reinking, Wiedenfeld, Winter, Zimmerman and Jensen2003). Habitat loss and degradation because of encroachment of woody vegetation (Davis, Reference Davis2004), cattle grazing and conversion of grasslands to agriculture are contributing to these declines (Herkert, Reference Herkert1995; Peterjohn & Sauer, Reference Peterjohn and Sauer1999; Vickery et al., Reference Vickery, Tubaro, DaSilva, Peterjohn, Herkert and Cavalcanti1999). The effects of land-cover change on nest survival in grassland habitats are variable depending on bird species, type of grassland and degree of degradation (Fondell & Ball, Reference Fondell and Ball2004; Pavel, Reference Pavel2004; Shochat et al., Reference Shochat, Patten, Morris, Reinking, Wolfe and Sherrod2005a,Reference Shochat, Wolfe, Patten, Reinking and Sherrodb).
The least studied grassland bird communities and populations are those that breed in the desert grassland (sensu Rzedowski, Reference Rzedowski1975; McClaran, Reference McClaran, McClaran and Van Devender1995) of the Chihuahuan Desert ecoregion of northern and central Mexico and the south-eastern United States (reviewed in Askins et al., Reference Askins, Chavez-Ramírez, Dale, Haas, Herkert, Knopf and Vickery2007). Over the last 50 years grassland ecosystems in central Mexico have undergone severe transformations for intensive livestock grazing, which includes shrub encroachment, and from conversion to rain-fed agriculture (agriculture based on summer precipitation only). This process of land-use change has created a landscape of three main land-cover types: natural, grass-dominated grasslands (shortgrass steppes), savannah-type grasslands with a grass and shrub layer, and agricultural lands with strips of native vegetation. Several declining bird species breed in these habitats and there is increasing evidence that land-use and cover changes have diminished available habitat for grassland birds and thus contributed to their decline (Askins et al., Reference Askins, Chavez-Ramírez, Dale, Haas, Herkert, Knopf and Vickery2007).
Studies in similar types of modified grasslands in North America have shown that changes in vegetation structure driven by shrub encroachment (Davis, Reference Davis2004), cattle grazing and agricultural tillaging negatively influence nest site availability (Kantrud & Kologiski, Reference Kantrud and Kologiski1982; Saab et al., Reference Saab, Bock, Rich, Dobkin, Martin and Finch1995) and nest abundance, and increase nest predation rates (Johnson & Temple, Reference Johnson and Temple1990; Riley et al., Reference Riley, Davis, Ortiz and Wisdom1992; Fondell & Ball, Reference Fondell and Ball2004). Therefore, overall reproductive success of birds may differ substantially between habitat types (Rosenstock & Van Riper, Reference Rosenstock and Van Riper2001; Shochat et al., Reference Shochat, Patten, Morris, Reinking, Wolfe and Sherrod2005a,Reference Shochat, Wolfe, Patten, Reinking and Sherrodb). Most of the natural desert grassland in the Chihuahua desert is not protected (Askins et al., Reference Askins, Chavez-Ramírez, Dale, Haas, Herkert, Knopf and Vickery2007) and strategies to preserve natural resources outside reserves are required. Knowledge of how the reproductive ecology of grassland birds varies among habitat types will aid the development of suitable management strategies to protect grassland bird populations in nature reserves, private lands and communal lands (ejidos) throughout northern and central Mexico.
The main objective of our study was to examine nest abundance, survival of nests, number of young fledged per nest attempt, and number of young fledged per successful nest of several bird species in three habitat types in the Mexican desert grassland: shortgrass steppe, savannah-type grasslands and rain-fed agriculture on former shortgrass steppe. As survival of nests may fluctuate substantially and non-linearly with age of the nest and date (Grant et al., Reference Grant, Shaffer, Madden and Pietz2005) we also examined the influence of temporal effects (date and nest age) on reproductive success. Our predictions were that land-use change from shortgrass steppe to heavily grazed savannah-type grasslands and to agriculture would negatively affect nesting success, number of fledglings and nest abundance.
Study area
Our study was conducted in the sub-province of Llanos de Ojuelos, Jalisco, Mexico (Fig. 1) where shortgrass steppe, savannah-type grasslands, and rain-fed agriculture on former shortgrass steppe are dominant at c. 2,100 m altitude. For each habitat type we randomly selected three sites with similar habitat characteristics. The shortgrass steppe sites are grazed by cattle Bos taurus year-round at a stocking rate of 0.125 ha-1. These sites are characterized by the short-statured grass species Bouteloua gracilis, Bouteloua scorpioides, Bouteloua hirsuta, Bouteloua simplex, Aristida divaricata, Microchloa kuntii, Buchloë dactyloides and Muhlenbergia rigida, with isolated sub-shrubs, shrubs and trees. The savannah-type grassland sites are also grazed throughout the year but at higher stocking rates of 0.5 ha-1. These sites are characterized by an open layer of grasses (B. gracilis, B. scorpioides and A. divaricata), and an open layer of shrubs and trees, including Acacia schaffneri, Yucca decipiens, Mimosa biuncifera, Dalea bicolor, Brickellia spinulosa, Opuntia robusta, Opuntia leucothicha, Opuntia imbricata and Opuntia streptacantha. The agricultural sites are usually covered (70%) by annual crops (beans, barley and corn) from June to September. During the rest of the year most crop fields are uncovered or interspersed with grasses, sub-shrubs, shrubs and trees in similar proportions.

Fig. 1 Locations of the nine study sites, with three each of the three habitat types (agriculture, shortgrass steppe and savannah-type grasslands). The shaded rectangle on the inset shows the location of the study area in the sub-province of Llanos de Ojuelos, Jalisco, Mexico. Numbers in parentheses indicate number of nests found at each study site.
For the three habitat types we selected similar-sized and similar-shaped, relatively unfragmented sites (> 340 ha; Fig. 1) to avoid potential confounding effects of different fragmentation patterns at different sites. Because edge effects may extend as far as 500 m (Davis et al., Reference Davis, Brigham, Shaffer and James2006; Grant et al., Reference Grant, Madden, Shaffer, Pietz, Berkey and Kardmas2006) we only searched for nests in areas > 1,000 m from habitat edges.
Methods
During the nesting period in March–August 2004 and 2005 we searched for nests at each site by following adult birds and by dragging a 50-m long rope, held by a person at each end, through the vegetation to flush adult birds from their nests (Ralph & Scott, Reference Ralph and Scott1981). We monitored nests of loggerhead shrike Lanius ludovicianus, savannah sparrow Passerculus sandwichensis, white-winged dove Zenaida asiatica and western meadowlark Sturnella neglecta. The savannah sparrow and western meadowlark are declining across at least 50% of their breeding distribution in the USA and Canada. The loggerhead shrike is declining through most of its breeding distribution in the same region (Sauer et al., Reference Sauer, Hines and Fallon2008) and has been identified as a species of conservation concern (US Fish and Wildlife Service, 2008). We included the white-winged dove as a comparison species as its populations are increasing (Sauer et al., Reference Sauer, Hines and Fallon2008) across most of its breeding range in the USA. Each nest was labelled with a tag, attached to a nearby plant, for easy relocation, and georeferenced with a global positioning system (GPS). We examined each nest every 3–4 days from the day it was found until it failed or fledged young. We used a mirror attached to a pole to monitor nests at heights greater than 2 m above ground (Parker, Reference Parker1972). Indications of successful fledging events were nests with nestlings close to fledging on visit t, and empty nests, fresh droppings and/or activity of juveniles and adults near nests at consecutive visits (t+1, t+2, etc.). Indications of nest failure were shell fragments, pierced eggs and/or destroyed nests (Martin & Geupel, Reference Martin and Geupel1993; Manolis et al., Reference Manolis, Andersen and Cuthbert2000).
For all species we calculated the mean, standard error and range of number of eggs, incubation days and brooding days per nest. To calculate these variables we only used nests whose complete clutch size, incubation and/or brooding periods were known. To estimate clutch size we only used nests that were found during the incubation period and we assumed that the complete clutch size was the largest number of eggs ever recorded in the nest. This assumption is reasonable because we never recorded partial clutch losses. For each species we recorded the number of nests found in each habitat type and the substrate (grass, forb, shrub, cacti and/or tree) on which nests were found. For each species we present clutch size, number of incubation days, brooding days, nests in each habitat type (nest abundance), and nesting substrates.
To analyse nest survival data we used the NLMIXED procedure in SAS (SAS Institute, 2005) for mixed models (Rotella et al., Reference Rotella, Taper, Stephens and Lindberg2007) to fit logistic exposure models (Shaffer, Reference Shaffer2004; Rotella et al., Reference Rotella, Taper, Stephens and Lindberg2007) to the data. We conducted separate analyses for each species. We used Akaike’s Information Criterion corrected for small sample sizes (AICc) and Akaike weights (ωi; Burnham & Anderson, Reference Burnham and Anderson2002) to evaluate support for competing a priori hypotheses related to factors influencing survival of nests in the different habitat types. To calculate AICc for nest survival we used the effective sample size (the sum of the total number of days that all nests were known to have survived plus the number of intervals between visits to nests that ended in failure; Rotella et al., Reference Rotella, Dinsmore and Shaffer2004). Before evaluating the influence of habitat type on nest survival we assessed time effects. The six a priori time-related, nest survival models were: (1) a constant survival model, (2 & 3) linear effects of age (the age of a nest in days) and date (the Julian date at the time in which the nest was visited; e.g. day 1 refers to the date when the first nest became active), (4 & 5) quadratic effects of age and date, and (6) a global model with all parameters from the previous models. Quadratic effects included both linear (x) and quadratic (x2) effects (Grant et al., Reference Grant, Shaffer, Madden and Pietz2005). These polynomial models allowed us to explore potential variation within the nesting cycle related to differences among nesting stages, and variation within the breeding season (Grant et al., Reference Grant, Shaffer, Madden and Pietz2005).
We then conducted an analysis comparing time-specific effects with habitat type effects. We constructed five a priori nest survival models: (1) a constant survival model, (2) the best-supported time effects model, (3) a habitat type effects model, (4) an additive model incorporating time effects and habitat type, and (5) a global model that included time effects, habitat type and the interactions of these two effects. The global model of nest survival was used to assess model fit (Burnham & Anderson, Reference Burnham and Anderson2002) using the Hosmer & Lemeshow (Reference Hosmer and Lemeshow2000) goodness-of-fit test. We then calculated model-averaged weighted parameter estimates and their associated standard errors using Akaike weights (Burnham & Anderson, Reference Burnham and Anderson2002; Shaffer, Reference Shaffer2004). Weighted parameter estimates and standard errors were used to calculate odd-ratios, their standard error, and confidence intervals. We only interpreted variables if the confidence intervals of the odd-ratios did not include 1, as recommended by Hosmer & Lemeshow (Reference Hosmer and Lemeshow2000).
Results
We collected nest survival information for 220 nests: 47 of savannah sparrow, 62 of loggerhead shrike, 75 of white-winged dove and 36 of western meadowlark (Table 1). The number of nests found per site was 19–33 (Fig. 1). White-winged doves were common in all study sites, with the highest nest abundance per site each year in agricultural sites (mean = 9.25 ± SE 1.03) and a lower nest abundance in savannah (mean = 4.0 ± SE 1.05) and grassland sites (mean = 4.0 ± SE 1.16). Although adult western meadowlarks were common in all three habitat types, nests of this species were not found in agricultural sites (Table 1). The number of nests of both loggerhead shrike and savannah sparrow were similar between habitat types (Table 1). Mean numbers of fledglings per nest of savannah sparrow, loggerhead shrike and white-winged dove were fewer in agricultural sites compared to shortgrass steppes and savannah-type grassland but mean fledglings per successful nest varied little between habitat types (Table 2).
Table 1 Number of nests and nesting characteristics of the four studied bird species in the three habitat types at Llanos de Ojuelos, Jalisco, Mexico (Fig. 1).
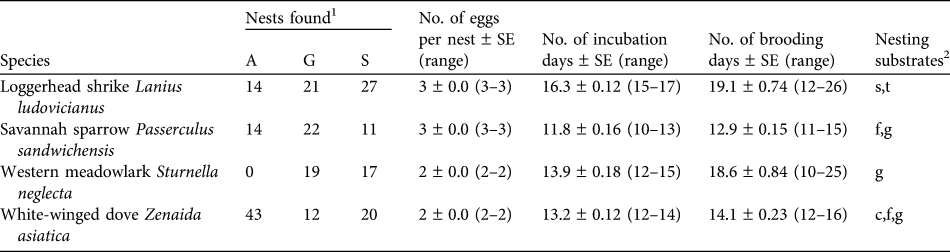
1 A, agricultural lands; G, shortgrass steppe; S, savannah-type grassland
2 c, cacti; f, forbs; g, grass; s, shrubs; t, trees (nesting substrate for ground nests indicates the plant species with which a nest was associated)
Table 2 Mean fledglings per nest and per successful nest of the four studied bird species in the three habitat types at Llanos de Ojuelos (Fig. 1).
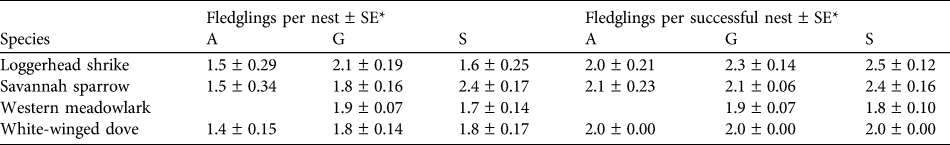
* A, agricultural lands; G, shortgrass steppe; S, savannah-type grassland
No nests were found during either the nest-building or egg-laying stages. For the shortgrass steppe and savannah-type grassland sites the primary cause of nest mortality was predation; trampling by cattle caused the failure of < 1 % of all nests in these habitat types. In agricultural sites ploughing accounted for most nest failure (78%) and predation for the remaining 22%.
Akaike information criterion results indicated that the best models explaining variation in nest success in relation to time-related effects were those including the following independent effects: quadratic date effects for loggerhead shrike and savannah sparrow (loggerhead shrike: ΔAIC = 0, ωi = 0.508; savannah sparrow: ΔAIC = 0, ωi = 0.253), constant survival (no effects) and linear date effects for meadowlark (ΔAIC = 0 and 0.36, ωi = 0.26 and 0.21, respectively), and linear date effects for white-winged dove (ΔAIC = 0, ωi = 0.341). When habitat type effects and time effects were analysed simultaneously, results of the Hosmer & Lemeshow’s (Reference Hosmer and Lemeshow2000) goodness-of-fit tests indicated that model fit was reasonable for all four species (χ2 = 0.999, df = 9, and P > 0.05).
Nest survival of savannah sparrow and loggerhead shrike responded to date, increasing slightly at the beginning and decreasing at the end of the breeding season, although confidence intervals were large. Survival of white-winged dove nests varied among habitat types; it was lower for nests in agricultural sites compared to those in savannah-type grasslands and shortgrass steppe sites, although confidence intervals were large (Fig. 2a). Nest survival of this species also responded to date, increasing as the breeding season progressed, and confidence intervals also increased as the breeding season progressed (Fig. 2b). AIC results (Table 3) and confidence intervals of odd-ratios suggested that nest survival of western meadowlark was unrelated to the independent variables that we evaluated.

Fig. 2 Estimated mean daily nest survival rates of white-winged doves Zenaida asiatica at Llanos de Ojuelos (Fig. 1) during the 2004 and 2005 breeding seasons as a function of (a) habitat type (lower confidence limits, not shown, were 0.23 for agricultural lands, 0.38 for shortgrass steppe, and 0.52 for savannah-type grassland) and (b) date, where 1 = first day of breeding season (lower confidence limit, not shown, ranged from 0.76 on day 9 to 0.20 on day 75). Daily survival rates were estimated from model-averaged coefficients of the best-supported logistic exposure models. Error bars and dotted lines indicate upper 95% confidence limits.
Table 3 Model selection results of a priori models of nest success of the four studied bird species in Llanos de Ojuelos (Fig. 1) during the nesting seasons of 2004 and 2005. Models include the best-supported model (lowest AICc value), candidate models within two ΔAIC units of the best model, and a constant survival model. Number of parameters (k) and AICc weights (ωi) for each model are provided.
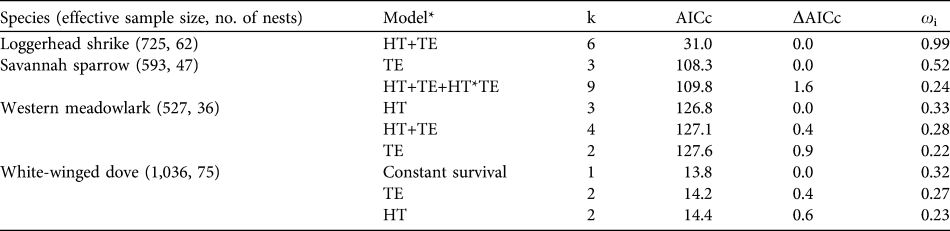
* HT, habitat type; TE, temporal effects
Discussion
We found only limited support for our prediction that nesting success would be lower in human-modified agricultural fields and overgrazed savannah-type grasslands compared to shortgrass steppe. Previous studies have reported low nesting success in shrub-encroached savannah-type grasslands (Rosenstock & Van Riper, Reference Rosenstock and Van Riper2001; Shochat et al., Reference Shochat, Patten, Morris, Reinking, Wolfe and Sherrod2005a,Reference Shochat, Wolfe, Patten, Reinking and Sherrodb). However, other studies have found no, or minor, effects (Bowen & Kruse, Reference Bowen and Kruse1993; Granfors et al., Reference Granfors, Church and Smith1996; Kruse & Bowen, Reference Kruse and Bowen1996; Fondell & Ball, Reference Fondell and Ball2004) of shrub-encroached savannah-type grasslands on nest success and/or nest densities. We did not assess the effects of several variables, including nest concealment, vegetation cover, predators and edges. Variation in these factors could have diluted any potential habitat effects and accounted for the large within-habitat variation that we recorded in white-winged dove nest survival. Therefore we cannot eliminate the possibility that nest survival of grassland-nesting bird species in central Mexico varies between habitats without conducting studies with larger sample sizes, allowing simultaneous examination of several interacting variables. Within-habitat variation of some of these effects should be the focus of future studies.
Date effects were included in at least one of the top nest success models for every bird species analysed. We found some evidence that savannah sparrow and loggerhead shrike nest survival increased at the beginning and decreased at the end of the breeding season, although confidence intervals were large. Nesting success of white-winged dove increased throughout the breeding season. Other studies have reported linear (not quadratic) date effects in grassland-nesting birds (Davis, Reference Davis2005; Grant et al., Reference Grant, Shaffer, Madden and Pietz2005). Patterns of within-season variation in nest success may result from temporal changes in animal community composition, and thus abundances and/or activity patterns of nest predators (reviewed in Grant et al., Reference Grant, Shaffer, Madden and Pietz2005), and/or other prey species (Ackerman, Reference Ackerman2002). Some of these variables could be responsible for the quadratic effects that we observed. Also, in contrast with findings from studies of grass-nesting birds (Davis, Reference Davis2005; Grant et al., Reference Grant, Shaffer, Madden and Pietz2005; Davis et al., Reference Davis, Brigham, Shaffer and James2006), we did not record substantial changes in nest survival with nest age, indicating that other variables that we did not consider (see above) may have had stronger influences in our study area.
The nesting period in our study area was delayed until early June in 2004, compared to 2005 when nesting began in late March. This difference is most likely related to inter-annual differences in precipitation; 2004 was wetter (total annual rainfall 688 mm), and 2005 was drier (284 mm) than the long-term mean annual precipitation (350 mm). Semi-arid ecosystems such as these grasslands are dynamic, in part because of the high spatio-temporal variation in precipitation (Winter et al., Reference Winter, Johnson and Shaffer2005). The first rain event in 2005 occurred on 18 March whereas in 2004 it was in early July. Therefore it is not total annual precipitation but rather the date of the first rain that may trigger the beginning of the breeding season. Although no previous study has recorded similar correlations between nest initiation and distribution of rain events, previous research has documented that at times of severe drought nesting may terminate early, and bird densities, species richness, hatching success, number of young fledged per successful nest and nesting success are low compared to wetter years (George et al., Reference George, Fowler, Knight and McEwan1992). In addition, climate change may induce early laying (reviewed in Crick, Reference Crick2004).
The potential effects of the size and timing of individual precipitation events on the breeding birds of the desert grasslands of central and northern Mexico require further study. Some studies have reported important differences in nest survival between years (Davis, Reference Davis2005; Davis et al., Reference Davis, Brigham, Shaffer and James2006), which could be related to precipitation. However, others have not recorded such differences (Grant et al., Reference Grant, Shaffer, Madden and Pietz2005). Because our study included only 2 years it was difficult to compare nest survival between years and to relate these differences to date of first rainfall. However, given the large disparity in precipitation and nesting times it is likely that precipitation affects nesting success. Long-term studies would be required to investigate this issue.
Based on our results it appears that habitat type influenced nest presence-absence and abundance patterns. Therefore, in spite of the limited support for our hypothesis of lower nesting success in human-modified habitats compared to natural grasslands, conversion to agriculture and savannah-type grasslands may influence demography by modifying the amount of habitat available. The white-winged dove, a species that is widespread in central Mexico, showed apparent nesting preferences for agricultural lands. In contrast, western meadowlarks seemed to avoid agricultural sites for nesting. Hence, extensive conversion of shortgrass steppe to rain-fed agriculture in the desert grasslands of central and northern Mexico may have substantially increased breeding habitat for some bird species at the expense of others and thus led to shifts in the abundance of species. In addition, ploughing contributed substantially to nest mortality and lower number of fledglings per nest in agricultural sites.
A number of management actions need to be implemented to minimize nest failures, reverse habitat decline and enhance conservation of grassland-nesting birds. These actions include implementation of limited-ploughing policies to minimize nest losses, adjusting ploughing times, establishing protected grassland corridors, and reducing land conversion to agriculture on both private and communal lands. To date these recommendations have not been followed despite the increased interest of state and federal government in the conservation of natural resources in Mexico. There are, however, various federal programmes administered by the National Forest Commission that promote conservation of biodiversity through economic incentives. Application of these programmes in combination with initiatives such as the Neotropical Migratory Bird Conservation Act (2010) could promote conservation of grassland-dependent bird populations in central Mexico.
Acknowledgements
We thank the landowners at Llanos de Ojuelos who granted us permission to conduct research on their properties. The Mexican National Council for Science and Technology (CONACYT) provided a full scholarship for the completion of CP-L’s graduate studies. L. Ildrich and C. Cacho helped in the field. Five anonymous reviewers provided helpful comments that improved this article. We also thank F. Thompson III, Javier Salgado and S.K. Robinson for their comments, and J. Rotella and T. Shafer for their advice on statistical analyses.
Biographical sketches
César Posadas-Leal’s main interest is the conservation of the wildlife of the grassland habitats of Central Mexico. Leonardo Chapa-Vargas’s research includes avian ecology, landscape ecology and wildlife ecotoxicology. José Tulio Arredondo-Moreno’s research focuses on management of grassland habitats and the roles of hydrology on the functioning of arid and semi-arid ecosystems of central Mexico. Elisabeth Huber-Sannwald’s research includes climate change, ecosystem services, and functioning of socio-ecological systems in arid, semi-arid and neotropical environments.







