Introduction
It is well known that snow rapidly changes its structure when containing liquid water. Since the mechanical property of wet snow depends greatly on the amount of liquid water in snow, the measurement of liquid-water content is important in clarifying the dynamic behaviour of wet snow.
There are various methods which bave been used to measure the liquid-water content of snow: a centrifugal method (Reference Kuroda and Furukawa.Kuroda and Furukawa, 1952; Reference LaChapelleLaChapelle, 1956), a melting calorimetry (Reference YosidaYosida, 1960), a freezing calorimetry (Reference Radok, Stephens and Sutherland.Radok and others, 1961; Reference Jones, Rango and Howell.Jones and others, 1983) and a di-electric method (Reference KuroiwaKuroiwa, 1951; Reference Ambach and Denoth.Ambach and Denoth, 1972). Recently, new methods for making field measurements of liquid-water content, including a solution method (Reference MorrisMorris, 1981), a time-domain reflectometry (Reference Stein and Kane.Stein and Kane, 1983), a dilution method (Reference Davis, Dozier, LaChapelle and Perla.Davis and others, 1985) and an alcohol calorimetry method (Reference FiskFisk, 1986) have been proposed. Comparative measurements of each method and each device have been made to assess the measurement accuracy and operating performance (Reference DenothDenoth and others, 1984; Reference Boyne and Fisk.Boyne and Fisk, 1990).
Recently, in Japan, the melting calorimeter, designed by Reference Akitaya.Akitaya (1978, Reference Akitaya.1985), has been widely used for measuring liquid-water content. This method uses hot water and has the advantage of faster measurement than the freezing calorimeter, because the thermal equilibrium between hot water and the snow sample can be reached quickly. However, the Akitaya-type snow-water content meter has several disadvantages due to its large size, heavy weight and time-consuming operation, which causes a transportation problem and a poor efficiency of measurement in making observations in high mountains. In addition, Reference ColbeckColbeck (1978) has concluded from error analysis that melting calorimetry is inherently inaccurate. Does this imply that melting calorimetry is of no practical use? It may be necessary to reexamine the measurement error for melting calorimetry.
To solve the above problems of the Akitaya-type snow-water content meter, we have designed a portable melting calorimeter (Endo-type snow-water content meter) suitable for use in the mountains. In this paper, the structure, procedure and accuracy of the new device is described to show its practical advantages.
Structure of the Device
The schematic diagram and side view of the Endo-type snow-water content meter are shown in Figures 1 and 2, respectively. It is composed of a container and a lid made of insulating materials (slyrene foam). For durability, the container is put in a metal canister with a wall thickness of 1.5 mm. A small thermistor with a digital display, whose weight and resolution are 60 g and 0.1°C, respectively, is attached to the lid of the container to measure the temperature of water poured into it. The joint between the container and its lid contains a tapered structure, which allows easy installation and removal of the lid, together with airtightness of the container. The weight and volume of the Endo-type snow—water content meter are 250 g and 1.6 × 103 cm3, respectively, showing that they are no more than 10% of those of the Akitaya-type snow-water content meter.

Fig. 1. Schematic diagram of Endo-type snow-water content meter.

Fig. 2. Side-view of Endo-type snow-water content meter.
Measuring Procedures
Measuring procedures of liquid-water content by the Endo-type snow-water content meter are as follows: (1) 60-100 cm3 of hot water (30-40 °C) are put into a small vessel and its mass, M 1, is measured. (2) The vessel is emptied of hot water by pouring into the container of the device and then the mass of the empty vessel, M 2, is measured. (3) After the lid is put on the container, the total mass of the device, including the hot water, M 3, is measured. (4) The temperature of the hot water, T 1, is read on the thermometer while shaking the device. (5) Soon after the snow is taken with a cylindrical sampler which has been stored in the snow adjacent to the sampling site, the lid is taken off for an instant and then the sample (20-30 g) is put into the container. The snow sample is quickly melted by shaking the device, resulting in a rapid drop in temperature of the hot water. When the thermometer provides a stable temperature, T 2, we read it. (6) The total mass of the device, including hot water and snow sample, M 4, is measured.
The liquid-water content, W, is defined as the ratio of the water weight to the total weight of wet snow in per cent, and can be calculated by

where C is the specific heat of water (4.2 × 103 J kg−1 K−1), L is the latent heat of fusion of ice (3.34 × 105 J kg−1). M 1, M 2, M 3 and M 4 are determined with an accuracy of 10−4 kg, and T 1 and T 2 in Celsius with an accuracy of 0.1°c.
Error Analysis
The liquid-water content is a function of six directly measured values and can be described as

The maximum value of expected absolute error in the indirect measurement of liquid-water content,  , is derived by
, is derived by

where δTi and δMj are the errors in the direct measurements of Ti and Mj , respectively.
Equation (3) becomes

where
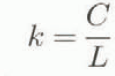
and

For a typical case where δT 1 = δT 2 = 0.1°C, δM 1 = δM 2 = δM3 = δM 4 = 10−4kg, T 2 = 10°c and M 1 = M 2 = 8 × 10−2kg.

Figure 3 shows the maximum absolute error as a function of R for various values of W. This clearly indicates that 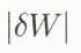 increases when R is large or W is small. If we need an error of
increases when R is large or W is small. If we need an error of 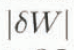 ≤ 2% irrespective of W, it is required to keep R < 3.5. Thus, it can be concluded from the error analysis that the melting calorimeter proposed in this study is practically available, if special attention is given to the mass ratio of the hot-water-to-snow sample.
≤ 2% irrespective of W, it is required to keep R < 3.5. Thus, it can be concluded from the error analysis that the melting calorimeter proposed in this study is practically available, if special attention is given to the mass ratio of the hot-water-to-snow sample.

Fig. 3. Maximum absolute error (![]() ) in the measurement of liquid-water content by the Endo-type snow-water content meter as a function of mass ratio of hot water to snow sample (R).
) in the measurement of liquid-water content by the Endo-type snow-water content meter as a function of mass ratio of hot water to snow sample (R).
Experimental Measurements
Measurement Accuracy
To estimate how accurately the liquid-water content can be determined with the Endo-type snow-water content meter, 49 samples of wet snow having known values of liquid-water content, W 0, were artificially made in a cold room by adding known quantities of water at a temperature of 0°C to known quantities of dry snow at a temperature of 0°C. Measurements of liquid-water content by this device were made in a cold room controlled to 0°C and then measured values, W, were compared with W 0.
In Figure 4, W is plotted against W 0, showing a linear relationship expressed approximately as

Fig. 4. Relation between values of liquid-water content (W0) and those measured by the Endo-type snow-water content meter (W). Solid and broken lines represent W = W0 and W = W0 ± 2%, respectively.
Statistical considerations indicate that the average error and the probable error are 1.07 and 0.903%, respectively. About 86% of W fall in the range within W 0 ± 2%. These imply that the device is capable of measuring liquid-water content with an accuracy of 2% by weight in most cases, which is consistent with the result of error analysis. Furthermore, in spite of the fact that no consideration is given to the effect of heat flow from hot water to the container in Equation (1), the data shown in Figure 4 are evenly distributed above and below the line which represents W = W0 , suggesting that this effect is negligible.
Effect of Heat Loss by Opening the Lid
When the lid is kept open to put a snow sample into the container, heat loss can occur through the open top of the container. To estimate the effect of the time the lid is kept open on the measured liquid-water content, snow samples having a water content of 10% were prepared in the same manner as mentioned above and were measured by the Endo-type snow water content meter with the opened time being changed from 2 s to 100 s. Two series of measurements were made when the temperature of hot water was 34° and 40°C. Changes in liquid-water content due to the opened time are shown in Figure 5. Roughly constant values of 9-12% up to a time of 40 s are followed by a sudden decrease in liquid-water content in the case of a temperature of 34°C, while a sudden decrease begins at a time of only 10 s, when the hot water has a temperature of 40°C. Consequently, we can conclude that the opened time does not have a significant effect on the measured value, if the temperature of hot water does not exceed 40°C, because the opened time is usually as little as about 2 s.

Fig. 5. Changes in liquid-water content measured by the Endo-type snow-water content meter due to the time the lid of the device is kept open. Snow samples with a liquid-water content of 10% were measured, open circles and solid circles represent the measured values when the temperature of the hot water is 34° and 40°C, respectively.
Comparison with Other Devices
Three profiles of liquid-water content were obtained at Nagaoka, Japan on 7 March 1996 using the Endo-type snow-water content meter, the Akitaya-type snow-water content meter and the snow-surface/volume-wetness dielectric device. The last device was developed at the Institute for Experimental Physics, University of Innsbruck, to determine a volumetric liquid-water content which can be transformed into a gravimetric one by use of the snow density. Measuring points are horizontally spaced at 5 cm intervals for each profile. The results are shown in Figure 6, together with the density profile and stratigraphy of the snow cover. The snow cover was entirely metamorphosed into a coarsegrained granular snow with densities of 330-490 kg m−3. Comparisons between three profiles of liquid-water content show an approximate agreement between the three, although the difference in measured values leaches 5% or more at heights of 14,36 and 70 cm. These significant differences, however, are not necessarily attributable to the difference of the measuring device, because the structure and properties of the snow cover become heterogeneous during the melt season.

Fig. 6. Liquid-water content, density profiles and stratigraphy of the snow cover at Nagaoka on 7 March 1996. Grain-shape classification is referred to Reference Akitaya.Colbeck and others (1990).
Using the Endo-type snow—water content meter and the snow-surface/volumc-wetness dielectric device, the liquid-water content was measured at the same depth of the snow-cover to compare measured values by them. Measured points for each device were horizontally spaced about 5 cm apart. A coarse-grained granular snow with densities of 310-520 kg m−3 was used for the measurements. The results, shown in Figure 7, indicate a switching relation between the measured value by the calorimetric method and that by the dielectric method; the former is smaller than the latter at lower water contents and the reverse is true at higher water contents. In other words, the latter has a tendency to fall in a narrower range in comparison with the former. Although the reason for this is not clear, it may be possible that the water-saturation regime and the grain structure in wet snow exert an effect on the determination of liquid-water content by the dielectric method.

Fig. 7. Liquid-water content measured by a dielectric method in comparison with those by Endo-type snow-water content meter.
During the observation shown in Figure 6, we could obtain the time required for each measurement of liquid-water content, which was made by two persons including an operator and a recorder. The mean time necessary for a measurement was calculated for the three types of device (Table 1). As a result, 2 minutes were found to suffice for a measurement by the Endo-type snow-water content meter and the dielectric device, whereas about 4 minutes were needed for a measurement by the Akitaya-type snow-water content meter. The Endo-type snow-water content meter is superior in measurement rate to the freezing calorimeter (Reference Jones, Rango and Howell.Jones and others, 1983), the dilution method (Reference Davis, Dozier, LaChapelle and Perla.Davis and others, 1985) and the alcohol calorimeter (Reference FiskFisk, 1986) by a factor of at least 2.
Table 1. Mean time required for the measurement of liquid-water content for three types of snow-water content meter

Conclusions
A portable melting calorimeter was developed for measuring liquid-water content of snow by weight. Its major advantages lie in its light weight and small size, together with easy fabrication with cheap materials. The time required for a measurement by the device is as little as 2 minutes, which provides a benefit of much speedier measurements than the current device in Japan. Furthermore, the measurement accuracy is within ±2% by weight and is not practically inferior to other snow-water content meters. For these reasons, the new device is suitable for measurements in high mountains where measuring instruments must be transported using human power. On the other hand, it is important to recognize that measured values using this device are sensitive to the natural heterogeneity of water distribution in the snow cover, because rather small snow samples are used for the measurements.
Acknowledgements
The authors wish to express their appreciation to T. Kobayashi and Y. Nohguchi, National Research Institute for Earth Science and Disaster Prevention, and K. Izumi, Niigata University, for their encouragement and for many useful suggestions. The authors are greatly indebted to M. Shimizu, Y. Yamada and T. Ikarashi, National Rest Mich Institute for Earth Science and Disaster Prevention, for providing measuring instruments. The authors are also thankful to K. Nishimura and Y. Ito, Hokkaido University, for their kind cooperation in the field observations.










