The 2017 outbreak of plague in Madagascar was unprecedented in the African region, resulting in 2417 cases (498 confirmed, 793 probable and 1126 suspected) and 209 deaths by the end of the acute urban pneumonic phase of the outbreak [1, 2]. Plague is endemic in the plateau of Madagascar and approximately 400 cases (mostly, the bubonic form of the disease) are reported annually, most of them from September to April [2, 3]. The 2017 outbreak began on 1 August 2017, occurred primarily in urban, non-endemic areas and was predominantly (77% of cases) of the rapidly fatal, pneumonic form of the disease [2]. A concerted national and international response led by the Ministry of Public Health of Madagascar with support from WHO and other partners was mounted and the outbreak was contained within 3 months (Fig. 1) [1].
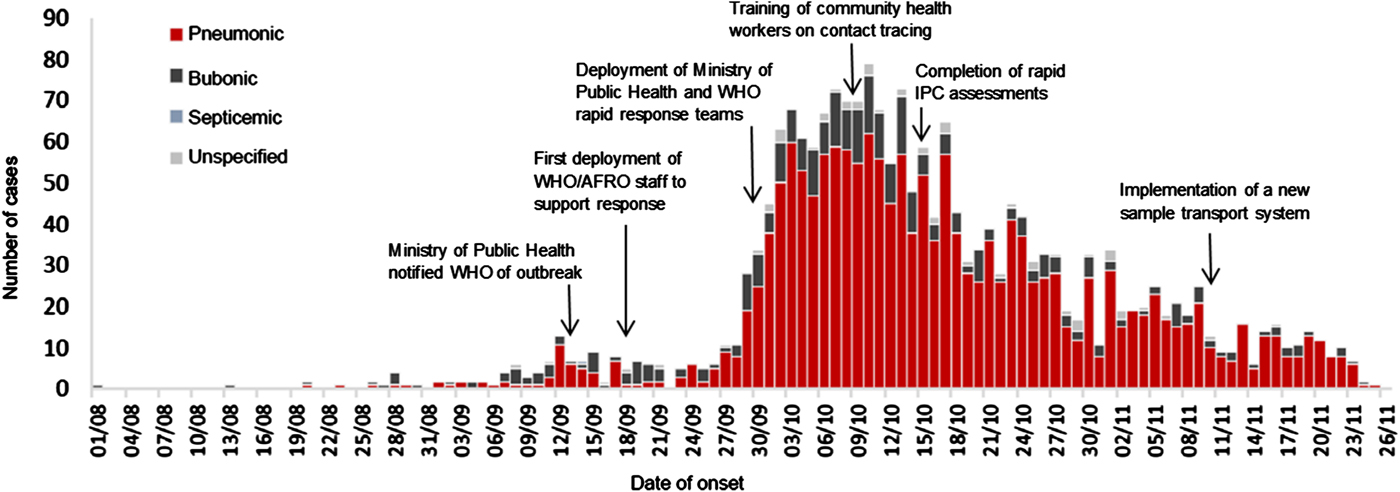
Fig. 1. Epidemic curve of suspected, probable, and confirmed outbreak-associated plague cases in Madagascar and key response actions, 1 August-26 November 2017 (adapted from [2])
The Health Emergencies Programme of the WHO Regional Office for Africa (WHE), together with the WHO Country Office and WHO Headquarters assisted the Ministry of Public Health of Madagascar in the rapid implementation of plague prevention and control measures while collecting and analysing quantitative and qualitative data to inform immediate interventions. WHO recommends 15 strategies for plague prevention and control [2], which reflect WHO guidance on the topic [Reference Dennis4–6]. We supported data collection relevant to eight of those strategies including enhanced surveillance; contact identification, prophylactic antibiotic administration and follow up; laboratory confirmation; infection prevention and control; case management; social mobilisation and community engagement; risk communication; and safe and dignified burials. We document the key findings of the evidence available to date and actions taken as a result. We evaluate the use of evidence to inform response based on the measures of success of operational research: dissemination of results, peer-reviewed publication, changes to policy and practice, and improvements in programme performance and health. Based on this evaluation, we summarise lessons learned for future outbreak response in the WHO African region.
Enhancement and use of surveillance data
Plague surveillance was passive prior to this outbreak, with case reporting to the Ministry of Public Health of Madagascar by healthcare facilities. However, active surveillance was established following detection of a larger than expected number of pneumonic plague cases via field investigations and confirmation of cases by rapid diagnostic testing at Institute Pasteur de Madagascar (IPM); over 4400 community health workers and 340 supervisors were trained to conduct community-based active surveillance, contact tracing and follow up activities across the country. On 1 October 2017, the Ministry of Public Health and WHO deployed rapid response teams to investigate cases detected through surveillance and identify potential exposures and epidemiological links. Case investigations continued throughout the outbreak to inform control measures. The Ministry of Public Health compiled and shared epidemiological surveillance data with IPM and WHO through daily response coordination committee meetings, which included all actors in the response.
Contact identification, prophylactic antibiotic administration and follow up
Contact tracing data were managed by a team hired by the Ministry of Public Health with financial support from WHO and the International Federation of the Red Cross and Red Crescent Societies. Contacts were traced using specially developed Excel tools that facilitated the collection of demographic information for the source case and contacts, type of exposure, last date of contact with the source case and daily follow-up information. Monitoring of contacts was done using VOOZANOO (EpiConcept, Paris, France), a software program implemented during the Ebola outbreak in Guinea in 2014. This facilitated data standardisation across regions. Regional field coordinators entered aggregate contact tracing data into VOOZANOO daily and access was shared with the Ministry of Public Health, making near real-time contact tracing data available for decision making. Field workers followed up contacts to provide post-exposure prophylaxis and treatment with doxycycline or co-trimoxazole. As of 30 November 2017, 7494 contacts were identified and all received prophylaxis and completed the 7-day follow up period.
Laboratory confirmation of cases and relevant challenges
IPM played a critical role in laboratory confirmation, diagnosis and dissemination of information about plague cases during the outbreak. Cases were confirmed by culture, serologic testing, or RDTs, depending on available laboratory capacity. From the start of the outbreak on 1 August to 7 October 2017, samples from endemic and non-endemic areas had different testing protocols, with only samples testing positive by RDT at other laboratories and field sites being sent to the National Plague Control Laboratory (housed within and supervised by IPM) for further testing. Starting on 7 October, following the official declaration of the outbreak, all samples were tested by RDT in the field or by RDT and polymerase chain reacation (PCR) at reference laboratories. Any RDT-positive samples were subsequently cultured for Yersinia pestis at the National Plague Control Laboratory. As of 2 November, to enhance diagnostic capacity, samples were additionally sent to IPM for differential culture. On 3 November, qPCR replaced conventional PCR as a routine testing method, as qPCR was faster and more specific. It was not always feasible to send samples to IPM in a timely manner because of the cost of shipment to the sending facilities. However, IPM confirmed plague infection in the first reported case and provided diagnostic capacity for the Ministry of Public Health throughout the outbreak. IPM shared results regularly with the sending facilities, the Ministry of Public Health, WHO and partners and also provided more than 2000 RDTs to Toamasina, the Centres Hospitaliers d'Antananarivo and the Plague Department of the Ministry of Public Health, thus increasing the regional availability of laboratory testing capacity in the country. The laboratory cultured isolates of Yersinia pestis, testing isolate sensitivity to the antibiotics recommended by the National Plague Control Programme. Thirty-three isolates of Yersinia pestis were identified and all demonstrated sensitivity to the recommended antibiotics, thus informing the current plague treatment protocol in the country.
WHO and partners addressed two key challenges to laboratory confirmation during the course of the outbreak, a delay between sample collection and reporting results and a need for rapid implementation of infection prevention and control (IPC) measures in healthcare facilities. First, to reduce the 5–7-day lag between sample collection and IPM providing results to clinicians, a new sample transport system was implemented on 10 November 2017, enabling twice daily sample collection from plague treatment reference hospitals, with transport to IPM for testing. Staff was trained to ensure samples were always collected with their associated minimum clinical and epidemiological data to ease interpretation and a call line was established for clinicians to contact IPM if documents were missing, thus facilitating identification of suspected case samples. This system reduced the amount of time from sample collection to communication of results to approximately 48 h and will be maintained until the end of plague season. The second challenge to plague surveillance was the absence of an effective feedback mechanism to alert IPM of RDT shortages at facilities. Some facilities, therefore, experienced shortages of RDTs despite having an ample supply in the region. The regional health directorate requested that healthcare facilities send negative RDTs to IPM, which would indicate to IPM when additional RDTs are needed and enable IPM to distribute RDTs in advance of facility shortages.
Infection prevention and control
To facilitate rapid implementation of IPC measures, WHO and partners developed a tool for rapid assessment of IPC in healthcare facilities. IPC experts deployed by WHO used this tool to conduct rapid IPC assessments from 9 to 15 October 2017 in four of five priority healthcare facilities in Antananarivo. These experts continued to assess facility IPC measures throughout the outbreak and supported capacity building of healthcare workers and rapid implementation of IPC measures to avoid healthcare-associated infections among healthcare workers. The main finding of the initial rapid assessments was that IPC measures were not being implemented to standard in most plague triage and treatment centres (PTTCs), with several key deficiencies contributing to the overall inadequacy of IPC. First, healthcare workers had very limited IPC knowledge and training. To address this, Médecins du Monde, Médecins Sans Frontières and other partners provided mentorship and support to PTTC staff in order to increase adherence to IPC standards. WHO IPC experts also conducted short training workshops in areas directly affected by plague (Fianarantsoa, Antsirabe and Tamatave) and trained IPC trainers nationally. However, in spite of training, on-the-job application of IPC knowledge remained poor and the WHO IPC sub-committee recommended the appointment of regional IPC focal points to lead regional management teams in providing additional support to facilities in the implementation of improved IPC measures. As of 1 December 2017, five regional IPC focal points had been selected and recruitment is ongoing in other regions to implement this plan.
A second deficiency identified by the IPC assessments was inadequate supply chain management, resulting in inappropriate re-use and shortages of materials. WHO and partners identified supply needs and implemented an official supply chain management system that included a central storage site managed by the Ministry of Public Health from which supplies were distributed to regions and facilities. The WHO IPC/case management sub-committee recommended a daily inventory of consumables by healthcare workers so that logisticians could monitor stocks and request additional orders when needed. A third key finding of the IPC assessments was the limited number and poor quality of sanitation and facilities for bathing at PTTCs. Response coordination committee partners including Action Contre la Faim, Médecins Sans Frontières and UNICEF established gender-specific sanitation and bathing facilities in PTTCs and other priority facilities. A fourth key finding of the IPC assessments was the inappropriate management of healthcare waste in PTTCs. This was addressed by UNICEF through the construction of waste volume reducers for safe storage and waste incineration, as well as a glass destroyer and facilities for organic waste disposal. Finally, the IPC assessments found that the protocol for patient flow through triage and isolation areas was not clearly defined, leading to suspected cases coming into contact with and potentially infecting others at the hospitals. WHO IPC/case management teams advised healthcare facility managers to clearly demarcate and monitor patient flow with the assistance of a hospital security guard. The standard operating procedures for triage and patient flow were revised based on this experience and are pending validation and finalisation.
Case management
To support the urgent need for case management during the outbreak, WHO and Global Outbreak Alert and Response Network partners deployed international experts in plague case management to evaluate the current plague case management protocol in Madagascar. This evaluation found difficulties in implementation of the protocol, notably the need to provide injectable antibiotics to patients every 3 h post-admission, which carried a significant risk of a missed injection. WHO case management experts decided to revise the treatment protocol to include the administration of levofloxacin; it was piloted in two hospitals and is pending review for feasibility and sustainability.
Social mobilisation, community engagement and risk communication
A risk communication and community engagement technical committee led by the Ministry of Public Health was established to implement plague communication activities during the outbreak. This committee used the pre-existing national plague communication plan to develop regional communication plans to increase community knowledge about the plague and also encouraged community engagement and prompt care seeking by suspected cases. Focus groups of community members and healthcare workers were conducted to assess plague-related knowledge, attitudes and practices in the eight districts initially affected by the outbreak. They noted a generalised fear of plague, misinformation from plague-related rumours and a desire for plague-related information, particularly from community leaders and healthcare workers, along with key messages to be transmitted via posters, TV or radio messaging (Malala Ranarison, written communication). On 10 November 2017, a multi-sectoral communication meeting was held in Toamasina, led by the regional government with participation by WHO and partners, to discuss the need to strengthen community engagement and mobilisation activities and preparation of a polio vaccination campaign integrating plague prevention messages. WHO developed an operational action plan to strengthen community engagement regarding plague prevention during the period from November 2017 to January 2018. A review of risk communication and community engagement and mobilisation activities was held on 30 November and an expanded review of lessons learned was planned.
Safe and dignified burials
In Madagascar, people are traditionally buried in family burial vaults and the corpses are periodically ritually exhumed, a practice known as Famadihana [Reference Andrianaivoarimanana7]. The onset of plague symptoms has been reported during these exhumations, thus the Ministry of Public Health of Madagascar recommends a 7-year period between death and exhumation of a plague case to reduce the possibility of disease [Reference Andrianaivoarimanana7]. There is a national burial protocol for plague cases, dating to 1932, which allows the government to bury plague victims in a mass grave. However, it is controversial and difficult to implement. WHO and UNICEF jointly conducted focus groups to determine the acceptability of changes to increase the safety of burial practices. A key finding was that any change would only be acceptable if definitive proof of plague infection of the deceased could be provided (Malala Ranarison, written communication). However, focus group participants expressed willingness to change certain aspects of traditional burial practices, including shortening or discontinuing wakes, allowing others to prepare the body for burial or families wearing personal protective equipment to prepare the body themselves, using body bags for plague victims and burying adjacent to the family vault instead of inside it. WHO and UNICEF developed a revised safe and dignified burial protocol based on these findings and pre-tested it in Antananarivo and Tamatave; 90% of the population consulted was in favour of the measures proposed in the protocol [2]. WHO engaged the Malagasy Red Cross to take responsibility for the revised safe and dignified burial protocol and submitted the protocol for approval by the government of Madagascar. Training of trainers for the burial teams was conducted to facilitate implementation of the protocol during future plague seasons or outbreaks in the country.
Evaluation of WHO's use of evidence to inform response measures
The success of WHO's use of data and information to inform outbreak response can be determined by assessing progress toward the four goals of operational research [Reference Zachariah8]. In all cases, the findings of data collection activities were communicated back to teams and partners in the field to facilitate evidence-based action, thus meeting the first goal of operational research activities. The response coordination mechanism, directed by a high-level working group chaired by the prime minister and involving health sector response coordination led by the Ministry of Public Health with support by WHO, non-health response coordination led by the National Risk and Disaster Management Office of Madagascar and health sector partners coordination, was critical to facilitating this communication. Future plague outbreak responses would likely benefit from similar high-level interdisciplinary coordination mechanisms to ensure engagement and cooperation of all relevant partners. The second goal, peer-reviewed publication of the research, has not yet been achieved, however, WHE aims to publish their research during the coming months and support colleagues from partner organisations in doing the same. The important qualitative research conducted regarding community mobilisation and engagement, risk communication and safe and dignified burial practices represents an ideal opportunity for peer-reviewed publication; dissemination of this information via the peer-reviewed literature could be used as evidence to advocate for policy change and inform future plague outbreak response. Despite the fact that all papers resulting from this work are in preparation, WHE has already advocated for important changes to practice and policy, the third goal of operational research. The approval and implementation of revised case management and safe and dignified burial protocols represent an important opportunity to improve the future plague prevention and treatment in the country.
The fourth and most important measure of success in operational research is whether implementation of evidence-based actions results in improvements in response and in human health. Anecdotal evidence suggests that WHE's actions increased the speed of laboratory confirmation, IPC capacity of healthcare workers, IPC supply availability, case management and community plague knowledge, but data were not systematically collected to confirm these observations. Data collection to evaluate those aspects of response at this stage could nevertheless be valuable to inform interventions during the remainder of the plague season and during future outbreaks in Madagascar. WHE collected data regarding eight of the 15 strategies for plague control and prevention; although not all of the remaining seven strategies are readily evaluated using data, one strategy, vector control, could have been better informed by data. WHE did not implement vector control activities or collect data to assess their effectiveness during the outbreak due to an absence of available technical expertise. Future plague outbreak responses in the WHO African region would benefit from the early recruitment of staff with that expertise and the integration of vector-related data collection activities into outbreak response. During this outbreak, data collection was managed by a data management team that comprised WHE, Ministry of Public Health and IPM staff, strategy-specific response committees and a qualitative researcher jointly hired by WHO and UNICEF. To implement systematic data collection in the context of future WHE outbreak responses, there is a need for greater institutionalisation of operational research as part of the WHE Programme. The infectious hazards management unit, whose mandate includes knowledge generation to inform control of all hazards, should lead this effort, with participation from other programme areas.
Although WHE was successful in collecting data to inform action during the 2017 plague outbreak in Madagascar and evidence was suggestive that those actions improved the effectiveness of response strategies and human health, increased implementation of operational research activities into outbreak response and publication of this research in the peer-reviewed literature are needed to improve scientific understanding of the effectiveness of outbreak response activities and improve plague outbreak prevention and control measures at the national, regional and global levels.
Acknowledgements
We thank Julienne Anoko, Freddy Banza-Mutoka, Fanny Chereau, Margarita Ghiselli, Konate Issiaga, Gilbert Kayoko, Jean Paul Ngandu Mbanga, Samuel Mesfin, Malala Ranarison, José Rovira-Vilaplana and Annika Wendland for providing their insights and detailed information regarding each aspect of the response to the 2017 Madagascar plague outbreak. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. However, the response and relevant collection of evidence were supported by WHO Contingency Funds for Emergencies, the Norwegian Agency for Development Cooperation, the Italian Ministry of Foreign Affairs, the Korean government, the Bank of Africa Madagascar Foundation, the United Nations Children's Fund (UNICEF), the United Nations Development Programme (UNDP) and the United Nations Population Fund (UNFPA).
Conflict of interest
None.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policies, or views of the World Health Organization.



