Cadmium is a metal found in nature in low concentrations(1). However, high levels of the metal can be found in the environment due to the burning of fossil fuels, the manufacture of batteries and the production of pigments and stabilisers(Reference Orisakwe, Asomugha and Afonne2). The consequent contamination of soil and water results in the exposure of plants and animals to the metal and its bioaccumulation(Reference Ryan, Scanlon and MacIntosh3, Reference Martorell, Perelló and Martí-Cid4). Moreover, tobacco smoke is another major source of human exposure to cadmium(Reference Järup and Akesson5).
In the male reproductive system, cadmium is known to impair reproductive physiology and decrease sperm quality(Reference Blanco, Moyano and Vivo6, Reference Oliveira, Spanò and Santos7). In addition, in vitro studies have shown that cadmium can stimulate Sertoli cell apoptosis, leading to the disruption of the blood–testis barrier(Reference Kusakabe, Nakajima and Nakazato8, Reference Chung and Cheng9). Endocrine disruption is another consequence of cadmium exposure, caused by Leydig cell apoptosis and decreased serum levels of testosterone, as demonstrated in animal studies(Reference Villanueva, Vigueras and Hernández10, Reference Messaoudi, Banni and Saïd11). Clinical trials have shown an inverse correlation between high serum levels of cadmium and semen quality, including sperm DNA damage, sperm count, motility and morphology(Reference Xu, Shen and Zhu12–Reference Balabanič, Rupnik and Klemenčič15).
Oxidative stress is related to cadmium-induced damage, increasing the formation of free radicals and decreasing the activity of antioxidant enzymes(Reference El-Missiry and Shalaby16). This event promotes the oxidation of cell structures, consisting mainly of PUFA which are present in high amounts in mammalian sperm, and facilitates DNA fragmentation and mitochondrial and plasma membrane peroxidation damage(Reference Saradha and Mathur17, Reference Vernet, Aitken and Drevet18). This results in a decrease in the motility and viability of the gametes(Reference Saradha and Mathur17, Reference Vernet, Aitken and Drevet18).
Grape and its derivatives are rich in polyphenols, including flavonoids (flavanols, flavonols and anthocyanins) and non-flavonoids (hydroxycinnamic acid derivatives, benzoic acid, hydrolysable tannins and stilbenes)(Reference Xu, Simon and Welch19, Reference Waterhouse20). The antioxidant activities of polyphenols include the modulation of endogenous antioxidant system, the scavenging of free radicals, the inhibition of lipid peroxidation and the formation of hydroperoxides(Reference Rodrigo, Miranda and Vergara21). In addition, flavonoids have demonstrated anti-inflammation and anti-carcinogenic activities, as well as metal-chelating properties(Reference Rodrigo, Miranda and Vergara21–Reference Castañeda-Ovando, Pacheco-Hernandez and Paez-Hernandez23).
Grape juice concentrate (GJC) is an alternative natural food colourant obtained by the nanofiltration of the juice from red grapes (Vitis labrusca, mostly of the Concord variety), with subsequent concentration to 68° Brix, by evaporation. This concentrated product provides five times more polyphenols than grape juice and, for this reason, might exert physiological effects. Aguiar et al. (Reference Aguiar, Gollücke and de Moraes24) have previously studied the phenolic amount and composition of this grape product and reported its capacity to decrease oxidative DNA damage induced by H2O2 in peripheral blood cells. The authors have suggested that further investigations should be conducted in vivo to verify the possible physiological effects. Thus, the objective of the present study was to examine the effects of GJC consumption, in two dosages, on long-term cadmium-induced reproductive damage in male rats.
Materials and methods
Animals
All experimental protocols involving animals conformed to the procedures described in the Guiding Principles for the Use of Laboratory Animals. The study was approved by the Animal Committee of the Federal University of Sao Paulo, UNIFESP, SP, Brazil.
Male adult Wistar rats (90 d old) weighing approximately 350 g were obtained from Centro de Desenvolvimento de Modelos Experimentais, Federal University of Sao Paulo, SP, Brazil. They were maintained under controlled conditions of temperature (24 ± 2°C) and light–dark periods of 12 h, with free access to water and a commercial diet (Nuvital®). All the rats were acclimatised for 2 weeks before the experiment.
Experimental design
A total of fifty-four rats were randomly divided into six groups. CdCl2 (Nuclear®) was administered in a single dose of 1·2 mg/kg body weight (BW) intraperitoneally, according to the method described by Predes et al. (Reference Predes, Diamante and Dolder25), and GJC was given orally daily. The non-treated rats received a single dose of saline solution intraperitoneally and/or daily water by oral administration. The first group (Cd) was treated only with cadmium (CdCl2) injection (n 10). The second group (CdGJ1) received the CdCl2 injection and 1·18 g/kg BW of GJC (n 10). The third group (CdGJ2) received the CdCl2 injection and 2·36 g/kg BW of GJC (n 10). The fourth group (GJ1) was treated only with 1·18 g/kg BW of GJC (n 7). The fifth group (GJ2) was treated only with 2·36 g/kg BW of GJC (n 7). The sixth group (CTRL) did not receive any treatment (n 10). The rats were euthanised by decapitation after 56 d, chosen considering the period necessary to complete a spermatogenic cycle(Reference Russell, Etin and Hikim26). BW was recorded four times per week.
Grape juice concentrate intake
The rats were given 1·18 or 2·36 g/kg BW per d of GJC (G8000™; Golden Sucos) by oral administration. The samples were tested for their polyphenol content, and the doses were calculated to be equivalent to four or eight glasses (200 ml each) of natural grape juice and adjusted to the faster metabolism of the rats, according to the method proposed by Aguiar et al. (Reference Aguiar, Gollücke and de Moraes24). The dosage was adjusted every day according to the weight of the rats.
Plasma testosterone levels
Immediately after decapitation, blood samples were collected in EDTA tubes and centrifuged at 5000 rpm for 15 min at 4°C. Plasma samples were kept at − 80°C before analysis. Plasma testosterone levels were determined by chemiluminescence immunoassay, using acridine ester as the chemiluminescent marker (ADVIA Centaur® XP Immunoassay System; Bayer Corporation). The CV for testosterone was 7·7 %.
Tissue collection and preparation
After decapitation, the left testis and epididymis, ventral and dorsolateral prostate and seminal vesicle (with the coagulating gland) were immediately removed and fixed in ALFAC solution (80 % ethanol, formaldehyde and glacial acetic acid, 8·5:1·0:0·5, by vol.) for 24 h and then weighed. Relative weight was used for a comparative analysis (absolute weight/total BW × 100). Paraplast-embedded testes were sectioned at 3–5 μm thickness and stained with haematoxylin and eosin.
Histopathological analysis
Inflammatory characteristics of the testis were analysed by the semi-quantitative method adapted from Zhang et al. (Reference Zhang, Xu and Liu27), establishing the following scores: 0, no inflammatory characteristic; 1, vascular congestion/necrosis; 2, mononuclear cell infiltrate; 3, tissue degeneration. For the evaluation of spermatogenesis, fifty horizontally sectioned seminiferous tubules per animal were scored from 10 (complete spermatogenesis and organised seminiferous epithelium) to 1 (no cells in a tubular section/tubular sclerosis) using Johnsen's score as described by Lee et al. (Reference Lee, Sul and Oh28).
Histomorphometrical analysis
The percentage of interstitial and tubular areas of the testis was determined by measuring the area occupied by the seminiferous tubules and interstitium in fifteen fields per animal(Reference Predes, Diamante and Dolder25). For each animal, thirty round tubules were selected randomly to measure the tubular diameter(Reference Predes, Diamante and Dolder25). This analysis was performed using AxioVision 4.8 software (Carl Zeiss) linked to a microscope (Carl Zeiss) at 200 × magnification.
Sperm parameters
Daily sperm production
A major portion of the right testis was stored at − 20°C and used to determine the daily sperm production (DSP). After tissue homogenisation in a standard solution (0·9 % NaCl2, 0·05 % Triton X-100 and 0.01 % Thimerosal) and dilution in the same solution in 1:10 proportion, the spermatids were counted in four Neubauer haemocytometer chambers to obtain the average count. The calculation was performed according to the method described by Robb et al. (Reference Robb, Amann and Killian29), corrected by testis mass used, and the data are expressed as the number of sperm ( × 106) per testis per d.
Sperm morphology
To analyse the morphology of sperm stored in the epididymis, a small incision was made on the right epididymal cauda (previously frozen at − 20°C) that was immersed in a PBS solution for diffusion for 15 min. Drops of the sperm suspension were put on microscope slides and exactly 200 spermatozoa per animal were analysed under a light microscope at 400 × magnification, and classified as normal or abnormal, according to the method described by Seed et al. (Reference Seed, Chapin and Clegg30). The abnormalities included head alterations (isolated, amorphous, absent or reduced hook) and tail alterations (isolated, coiled, broken or bent). The results are expressed as a percentage of normal sperm.
Testicular antioxidants
A small piece of the right testis, stored at − 80°C, was used for the evaluation of antioxidant markers. The enzymatic activity of superoxide dismutase (SOD) and catalase (CAT) and the tissue levels of glutathione (GSH) were analysed.
Superoxide dismutase
The enzymatic activity of testicular SOD was measured through the microplate method proposed by Ewing & Janero(Reference Ewing and Janero31), in a spectrophotometer at 560 nm, for 3 min at 25°C, using SoftMax software (Bioanalytical Company). Mitochondrial SOD (Mn-SOD) was quantified by the same method, adding potassium cyanide to block cytoplasmic SOD (Cu,Zn-SOD). The activity of cytoplasmic SOD was established by the difference between total SOD and mitochondrial SOD. The obtained values were corrected by tissue protein and are expressed in U SOD/mg protein.
Catalase
The enzymatic activity of testicular CAT was obtained according to the method described by Adamo et al. (Reference Adamo, Llesuy and Pasquini32) by the kinetic of H2O2 degradation in a spectrophotometer (Hitachi-200; Hitachi) at 230 nm, for 3 min at 30°C, using UV Solutions software (Hitachi High Technologies America). The obtained values were corrected by tissue protein and are expressed in U CAT/mg protein.
Glutathione
The tissue levels of GSH were measured based on the method proposed by Tietze(Reference Tietze33). An acid extract of the testicular tissue was obtained using perchloric acid and analysed in a spectrophotometer (Hitachi U-2010; Hitachi) at 412 nm and 25°C, using UV Solutions software (Hitachi High Technologies America). Data are expressed as μmol GSH/g tissue.
Statistical analysis
Considering that the present study is a part of a larger experimental design, including an additional (sub-chronic) period of treatment, statistical procedures were performed using the ANOVA model for three fixed factors (cadmium, grape juice and time) and multiple-comparisons method of Bonferroni. For semi-quantitative histopathological analyses, simultaneous non-parametric comparisons were established. The significance level was set at P≤ 0·05.
The DSP was chosen for planning the sample size. The minimal differences were established according to the researchers' expectation about cadmium and GJC effects. The DSP was expected to be normal (approximately 30 × 106 sperm/d per testis) in the CTRL group, being reduced to approximately 8–10 × 106 in the Cd group, possibly being elevated by the first GJC dose (to approximately 14 × 106) and further elevated by the second dose (approximately 20–22 × 106) without returning to the normal values. By the presence of resveratrol, the GJC was expected to increase the normal DSP in healthy rats (to approximately 37–40 × 106). On the basis of these assumptions and fixing the significance level at 5 %, the power of the tests using different sample sizes was calculated, and this resulted in the following value: 0·906 (90·6 %). Such a value was only 1 % lower when compared with the value obtained with all groups containing ten rats (91·6 %).
Results
Body mass gain
The Cd group showed less body mass gain than the CTRL group (P =0·009). No statistically significant differences were observed after GJC consumption. These results are given in Table 1.
Table 1 Values of body mass gain and plasma levels of testosterone (Mean values and standard deviations)
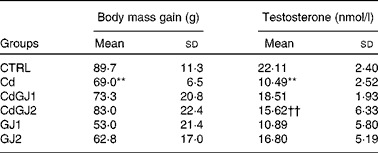
CTRL, control; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate.
**Mean value was significantly different from that of the CTRL group (P≤ 0·01).
††Mean value was significantly different from that of the Cd group (P≤ 0·01).
Plasma testosterone levels
Plasma testosterone decreased in the Cd group (P =0·001), as described in Table 1. Whereas dosage I of GJC (1·18 g/kg BW) showed no effect, the intake at dosage II (2·36 g/kg BW) was able to recuperate the hormonal levels after cadmium exposure (P =0·002). GJC consumption did not significantly change this parameter in the non-exposed rats.
Reproductive organ weight
Cadmium exposure decreased the relative weight of the testis (P =0·001), epididymis (P =0·001), ventral prostate (P =0·008) and seminal vesicle (P =0·002) in the Cd group (Table 2). Dosage I of the GJC showed no effect on the weight of any organ, while dosage II successfully recovered the relative weight of the testis (P =0·003), epididymis (P =0·013) and ventral prostate (P =0·052). In the GJ1 group (non-exposed), decreased seminal vesicle weight (P =0·002) was observed. Interestingly, the effect was not observed in rats of the dosage II group.
Table 2 Relative weight (%) of the testis, epididymis, prostate (ventral and dorsolateral) and seminal vesicle (Mean values and standard deviations)
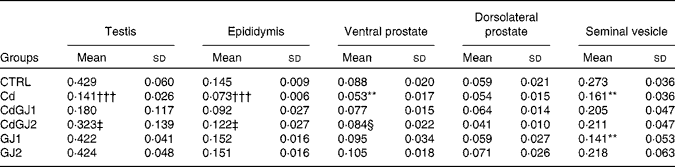
CTRL, control; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate.
**Mean value was significantly different from that of the CTRL group (P≤ 0·01).
†††Mean value was significantly different from that of the CTRL group (P≤ 0·001).
‡ Mean value was significantly different from that of the Cd group (P≤ 0·01).
§ Mean value was significantly different from that of the Cd group (P≤ 0·05).
Histopathological analysis
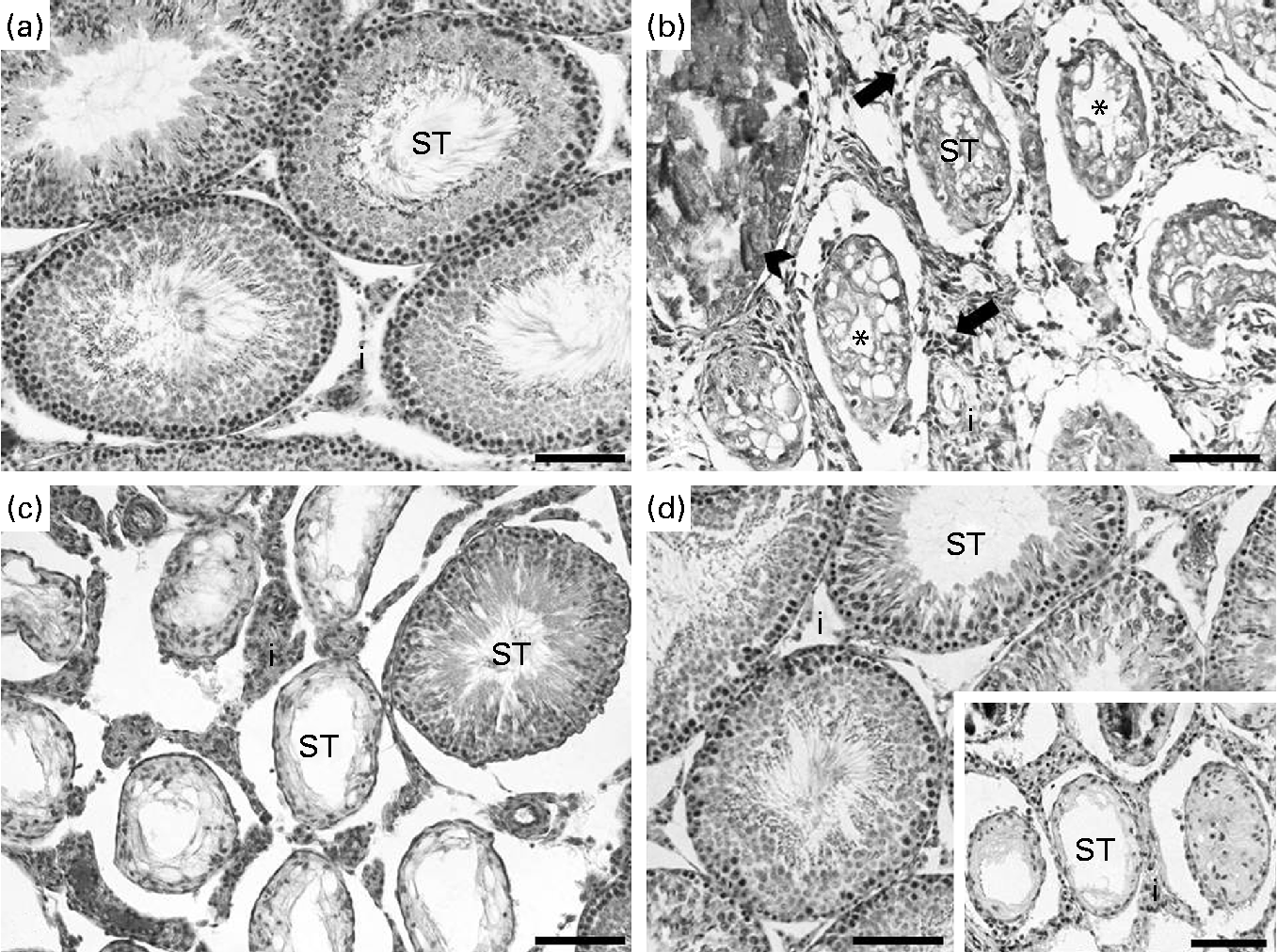
In the analysis of the inflammatory process, the Cd and CdGJ1 groups showed statistically significant histopathological changes when compared with the CTRL group (P =0·001 and P =0·048, respectively). Nevertheless, there were significant differences between the Cd v. CdGJ1 (P =0·002) and the Cd v. CdGJ2 (P =0·001) groups. GJC consumption did not change testicular histopathology in the healthy rats (Table 3). The spermatogenic process was disturbed by cadmium exposure, according to Johnsen's score, in the Cd (P =0·001), CdGJ1 (P =0·001) and CdGJ2 (P =0·001) groups. The main findings were the presence of inflammatory processes, tubular atrophy and tissue necrosis, with variable degrees among the groups. In spite of less pronounced damage in the CdGJ2 group, a statistically based analysis showed that the GJC was not able to attenuate cadmium-induced changes or modify spermatogenesis in the non-exposed groups (Table 4). The results of the histopathological analysis are illustrated in Fig. 1.
Table 3 Total number of rats in all groups according to the degree of histopathological changes in the testicular tissue
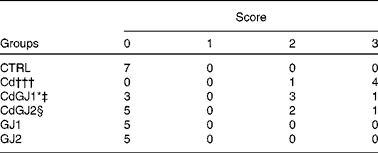
CTRL, control; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate.
*Values were significantly different from those of the CTRL group (P≤ 0·05).
†††Values were significantly different from those of the CTRL group (P≤ 0·001).
‡ Values were significantly different from those of the Cd group (P≤ 0·01).
§ Values were significantly different from those of the Cd group (P≤ 0·001).
Table 4 Johnsen's score and histomorphometrical analysis (Mean values and standard deviations)
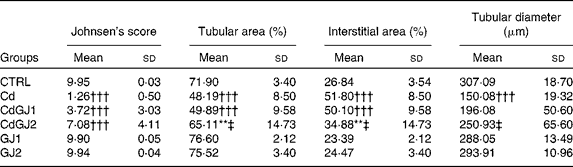
CTRL, control; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate.
**Mean value was significantly different from that of the CTRL group (P≤ 0·01).
†††Mean value was significantly different from that of the CTRL group (P≤ 0·001).
‡ Mean value was significantly different from that of the Cd group (P≤ 0·01).

Fig. 1 Histopathological analysis of the testis. Control rats presented normal testis tissue organisation with intact seminiferous tubules (ST) (a), while in the cadmium-treated group, tissue disorganisation with inflammatory infiltrate (arrows) in the interstitium (i), tissue necrosis (arrowhead) and general tubular atrophy (*) were found (b). In spite of the presence of few intact ST, the CdGJ1 group presented the same degree of damage as that observed in the group exposed only to cadmium (c). Qualitatively, less pronounced damage was observed in the group receiving the second grape juice concentrate dosage (CdGJ2) (d), in spite of extensive areas of some rats showing intense damage (d, onset) (scale bar 100 μm). The GJ1 and GJ2 groups (not shown) presented the same histological architecture as that observed in the control group. CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate.
Histomorphometrical analysis
In the histomorphometrical analysis, the Cd, CdGJ1 and CdGJ2 groups showed a decrease in the tubular area (P =0·001, P =0·001 and P =0·009, respectively) and an increase in the interstitial area (P =0·001, P =0·001 and P =0·008, respectively). Dosage II of the GJC attenuated tubular area (P =0·016) and interstitial area (P =0·015) alterations. Tubular diameter was decreased only in the Cd group (P =0·001). Although the GJC at dosage I did not promote histomorphometrical improvements, at dosage II, the values of tubular diameter were normalised (P =0·001). No changes were observed in the histomorphometrical parameters of the non-exposed groups (CTRL, GJ1 and GJ2; Table 4).
Sperm parameters
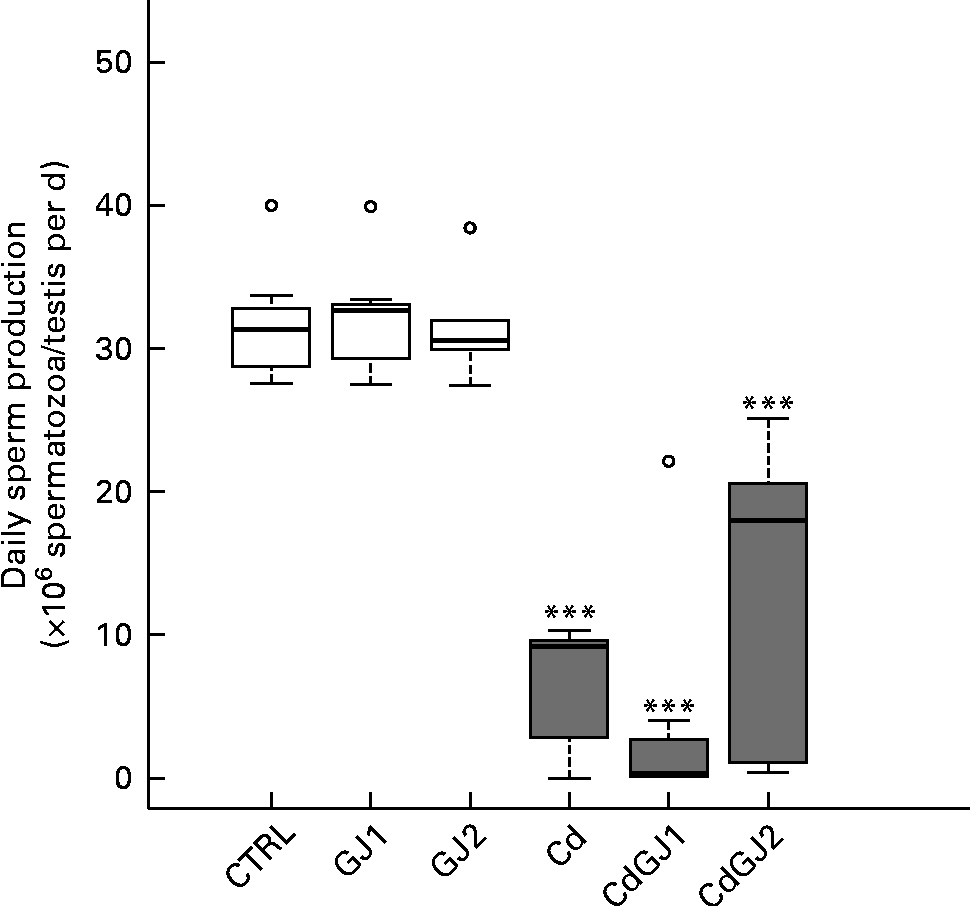
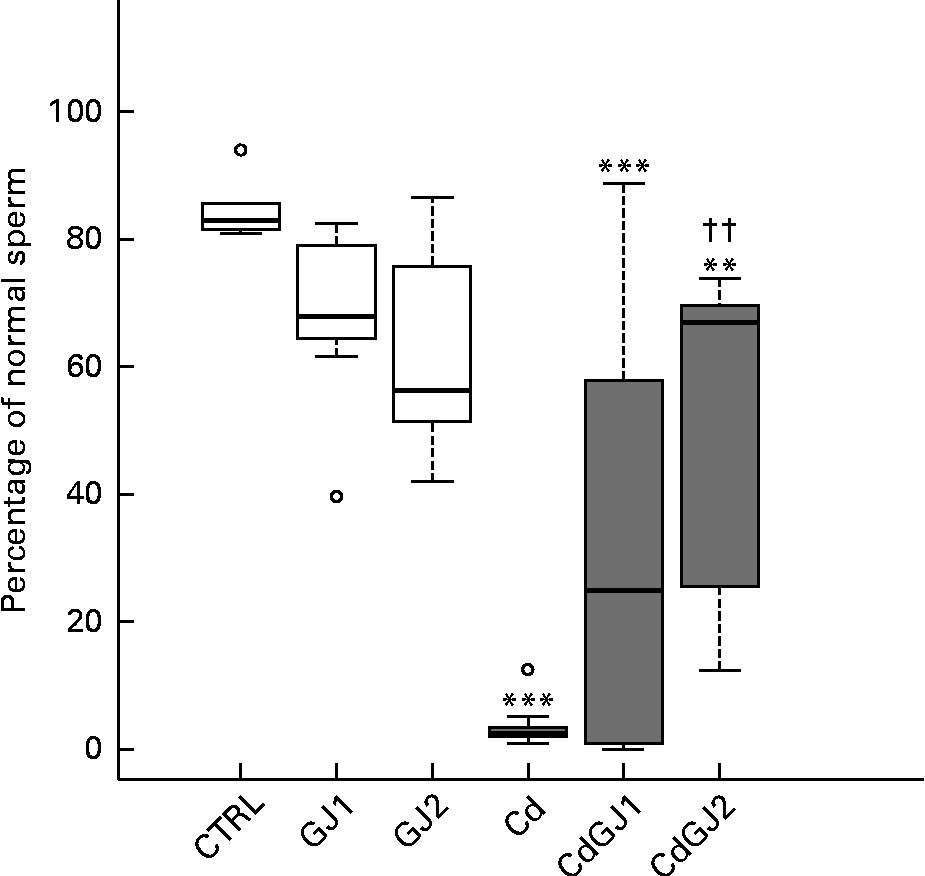
DSP was decreased by cadmium exposure in the Cd (P =0·001), CdGJ1 (P =0·001) and CdGJ2 (P =0·001) groups. The GJC was not able to attenuate the cadmium-induced effect. The parameters were not changed in the non-exposed groups at any dosage (Fig. 2). Moreover, CdCl2 was able to decrease the percentage of normal sperm in the Cd (P =0·001), CdGJ1 (P =0·001) and CdGJ2 (P =0·006) groups. In this case, GJC intake at dosage II improved the sperm morphology of the exposed rats (P =0·003). GJC consumption did not change sperm morphology in the non-exposed groups (Fig. 3).

Fig. 2 Daily sperm production. CTRL, control; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate. *** Values were significantly different compared with that of the CTRL group (P≤ 0·001). □, No cadmium; ![]() , cadmium, ○, outliers.
, cadmium, ○, outliers.

Fig. 3 Percentage of normal sperm. CTRL, control; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate. Values were significantly different compared with that of the CTRL group: **P≤ 0·01, *** P≤ 0·001. †† Values was significantly different compared with that of the Cd group (P≤ 0·01). □, No cadmium; ![]() , cadmium, ○, outliers.
, cadmium, ○, outliers.
Testicular antioxidants
The enzymatic activity of total SOD in the Cd group was increased (P =0·001), as well as that of Mn-SOD (P =0·001) and Cu,Zn-SOD (P =0·006). A recovery was not observed in the group treated with the GJC at dosage I. At dosage II, however, a decrease in total SOD (P =0·001) and Mn-SOD (P =0·007) activities was observed. Additionally, CAT activity was not changed in any group, in spite of the significant differences between the CdGJ1 and GJ1 groups (P =0·001). The tissue levels of GSH decreased in the Cd (P =0·004) and CdGJ1 (P =0·01) groups. Dosage II of the GJC effectively recovered the GSH levels after cadmium exposure (P =0·03). The GJ1 and GJ2 groups did not show statistically significant differences when compared with the CTRL group. Detailed results are given in Table 5.
Table 5 Enzymatic activity of catalase (CAT) and superoxide dismutase (SOD) and testicular levels of glutathione (GSH) (Mean values and standard deviations)
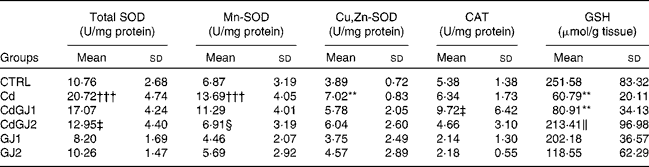
CTRL, control; Cd, cadmium injection; CdGJ1, CdCl2 injection+1·18 g/kg BW of grape juice concentrate; CdGJ2, CdCl2 injection+2·36 g/kg BW of grape juice concentrate; GJ1, 1·18 g/kg BW of grape juice concentrate; GJ2, 2·36 g/kg BW of grape juice concentrate.
**Mean value was significantly different from that of the CTRL group (P≤ 0·01).
†††Mean value was significantly different from that of the CTRL group (P≤ 0·001).
‡ Mean value was significantly different from that of the Cd group (P≤ 0·001).
§ Mean value was significantly different from that of the Cd group (P≤ 0·01).
∥ Mean value was significantly different from that of the Cd group (P≤ 0·05).
Discussion
It is well established that cadmium accounts for several biological changes, mainly leading to oxidative stress and inflammation(Reference Siu, Mruk and Porto34). Conversely, GJC has demonstrated anti-inflammatory, pro-apoptotic and cell-cycle regulation properties(Reference Chahar, Sharma and Dobhal35, Reference Kelly36). The major phenolic compounds in GJC have been assessed earlier by Aguiar et al. (Reference Aguiar, Gollücke and de Moraes24) by a qualitative analysis. The authors have observed the relevant presence of flavonoids (quercetin, kaempferol, peonidin-glucoside, malvidin-glucoside, petunidin-3-O-acetylglucoside, peonidin-3-p-coumaroylglucoside, malvidin-3-O-p-coumaroylglucoside and dimethoxyflavylium) and non-flavonoids (fertaric acid, caffeic acid derivatives and resveratrol).
From a general perspective, the metal induced an overall toxicity evidenced by reduced body mass gain, in agreement with previous studies(Reference El-Demerdash, Yousef and Kedwany37–Reference Santos, Graça and Zeni39). Although the mechanism is not clear, Rencuzogullari & Erdogan(Reference Rencuzogullari and Erdogan40) suggested that free radical production may be involved in cadmium-induced weight changes. GJC consumption did not change body mass gain in any condition, in agreement with previous clinical studies(Reference Hashemi, Kelishadi and Hashemipour41, Reference Hollis, Houchins and Blumberg42).
Severe cadmium-induced damage was observed in the male reproductive system, including testicular and epididymal atrophy as well as prostate and seminal vesicle weight reduction. The effects of cadmium on testis and epididymis weight are well known(Reference El-Demerdash, Yousef and Kedwany37–Reference Santos, Graça and Zeni39). However, data on other organs are controversial. Although some authors have observed no change in seminal vesicle and prostate weight(Reference Predes, Diamante and Dolder25, Reference Wade, Foster and Younglai43), others have found a decrease(Reference Monsefi, Alaee and Moradshahi44, Reference Amara, Abdelmelek and Garrel45). Harmful effects of cadmium on reproductive organs may be related to low testosterone levels after cadmium exposure, as evidenced in the present study. This result was probably caused by DNA damage-induced Leydig cell apoptosis as a consequence of oxidative stress, also observed in the present study(Reference Messaoudi, Banni and Saïd11, Reference Monsefi, Alaee and Moradshahi44, Reference Pandya, Pillai and Nampoothiri46). The present results suggest that the protective effects of GJC consumption on reproductive organs are related to the maintenance of plasma levels of testosterone, observed after the administration of dosage II in cadmium-exposed rats. It has been described that resveratrol and other polyphenols are able to increase testosterone levels and avoid Leydig cell apoptosis(Reference Juan, González-Pons and Munuera47, Reference Yu, Pu and Chen48). In the non-exposed rats, changes in seminal vesicle after the consumption of dosage I were observed. Given that similar results were not found in the literature, these findings require further investigations.
Spermatogenesis disruption may be attributed to the high prevalence of Sertoli cell-only seminiferous tubules, as a consequence of cadmium exposure, in agreement with previous studies(Reference Yari, Asadi and Bahadoran49, Reference Al-Azemi, Omu and Kehinde50). Furthermore, CdCl2 caused histomorphometrical changes, similar to the findings reported by Predes et al. (Reference Predes, Diamante and Dolder25), and intense inflammatory processes, including interstitial mononuclear cell infiltrate, vascular congestion, necrosis and tissue degeneration. These effects were generally due to the stimulation of pro-inflammatory cytokines(Reference Al-Azemi, Omu and Kehinde50). Although GJC consumption did not improve spermatogenesis changes, both dosages I and II of the GJC attenuated the cadmium-induced inflammatory profile and dosage II attenuated the histomorphometrical changes. It is known that grape polyphenols modulate endothelial function and cytokine expression, which may be related to the beneficial effects observed(Reference Bu, Mi and Zeng51–Reference Speciale, Canali and Chirafisi56).
Morphological changes are directly related to physiological dysfunction(Reference Sinha Hikim, Bartke and Russell57), and spermatogenesis disruption may have led to reduced DSP, as has been also demonstrated in earlier studies(Reference Oliveira, Spanò and Santos7, Reference Monsefi, Alaee and Moradshahi44, Reference Saïd, Banni and Kerkeni58). In a recent review, Wong & Cheng(Reference Wong and Cheng59) have reported that cadmium-induced oxidative stress seems to be the main cause for the decline observed in sperm count. Oxidative stress has been stated to occur after cadmium exposure and may cause DNA damage and lipid peroxidation of sperm, contributing to sperm morphology changes as found in the present investigation(Reference Aitken and Curry60, Reference Zini, Phillips and Courchesne61). In spite of the inability of the GJC to completely restore the testicular physiology, a dose-dependent attenuation of cadmium-induced changes in sperm morphology was observed.
An imbalance between oxidant and antioxidant agents, triggered by cadmium, was shown in the present study, characterised by decreasing GSH levels, increasing SOD activity and the maintenance of CAT activity. Low levels of GSH in the testicular tissue, after cadmium exposure, have been well described(Reference Koyuturk, Yanardag and Bolkent62–Reference Ola-Mudathir, Suru and Fafunso64) and mainly occur because the thiol group binds covalently with metals(Reference Martelli, Rousselet and Dycke65, Reference Cuypers, Plusquin and Remans66). After GJC consumption, the tissue levels of GSH were normalised by dosage II. In general, flavonoids are able to modulate both γ-glutamylcysteine synthetase and glutamate cysteine ligase involved in the synthesis of GSH(Reference Moskaug, Carlsen and Myhrstad67–Reference Cheng, Wu and Ho69).
SOD is especially abundant in the mitochondria due to the release of reactive molecules by the respiratory chain(Reference Cuypers, Plusquin and Remans66). Cadmium targets the mitochondria, changing its membrane permeability and accumulating semi-ubiquinone molecules, which can transfer electrons to molecular oxygen(Reference Cuypers, Plusquin and Remans66, Reference Iranzo70). This mechanism can be involved in the overproduction of superoxide anions in the testicular tissue, which explains the increase in SOD activity, especially in the mitochondrial fraction, found in the present study. A decrease in SOD levels was found after GJC consumption at dosage II, mainly in the mitochondrial fraction. This event may be explained by the property of grape bioactive compounds to reduce reactive oxygen species production in the mitochondria and to scavenge superoxide anions, preserving enzymatic activity(Reference Pervaiz and Holme71).
In contrast, CAT activity was not altered, which was unexpected, as CAT activity is directly related to SOD activity(Reference Day72). These findings suggest that a possible increment in CAT activity was inhibited by cadmium, probably due to the metal's capacity to bind sites for other metals, such as Fe, which catalyses the reactions of CAT(Reference Vlasits, Jakopitsch and Bernroitner73, Reference Moulis74). In this sense, a statistically significant difference was observed between the CdGJ1 and GJ1 groups, showing not only the potentiality of cadmium to increase CAT activity but also the capacity of GJC to revert cadmium-induced inhibition, in agreement with the Eybl et al. (Reference Eybl, Kotyzova and Koutensky75) data. No changes were found in the CdGJ2 group as expected, since SOD activity maintained the levels that were similar to those observed in the CTRL group.
Although alterations in enzymatic antioxidant activities indicate oxidative stress, some studies have demonstrated a reduction in testicular SOD and/or CAT activity after cadmium exposure(Reference Pandya, Pillai and Nampoothiri46, Reference Saïd, Banni and Kerkeni58, Reference Ola-Mudathir, Suru and Fafunso64, Reference Fouad, Qureshi and Al-Sultan76, Reference Konar, Kara and Yilmaz77). Disparity between the present results and those reported in the literature may be related to different administration routes and CdCl2 concentrations, generally higher in a single dose or in a high-frequency exposure. In agreement with our hypothesis, Wang et al. (Reference Wang, Xu and Lei78) demonstrated that while low cadmium concentrations can maintain or increase CAT, SOD and GSH peroxidase activities, high dosages inhibit these enzymes in the testicular tissue. Similarly, Kojima et al. (Reference Kojima, Ishihara and Hirukawa79) reported an increase in testicular CAT activity and the maintenance of SOD activity 30 min after a 5 mg/kg BW CdCl2 injection. This may indicate that the increase/maintenance of antioxidant enzymatic activity is an event that take place before its inhibition, as has been widely described in the literature.
In conclusion, the present results show that regular dietary doses of GJC were able to attenuate long-term cadmium-induced reproductive damage in a dose-dependent manner, through various possible mechanisms, including modulation of the antioxidant system.
Acknowledgements
The present study was supported by FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo) (grant no. 2011/22811-5). V. C. P. received fellowships from FAPESP (process no. 2011/03873-0) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior ). The authors also thank Fabrícia de Souza Predes for valuable discussion during the work; Hanna Karen Moreira Antunes for providing the testosterone dosage; Leonardo Parreira Silva Nascimento and José Simões for helping with the animal oral administration; Wederley Alexandre Januário for euthanasia; Dr W. G. Kempinas for teaching O. A. sperm counting method; and Mary Anne Heidi Dolder for providing the CdCl2. The authors' contributions are as follows: V. C. P. was the main executor, being responsible for all parts of the paper and executing all the laboratory procedures, as well as the writing of the manuscript; A. P. B. G. was responsible for providing the GJC and conducting all the discussions concerning the composition and properties of this compound, and also contributed by critically reading the manuscript; D. A. R., together with V. C. P., conducted the histopathological analysis of the testis and critically read the manuscript; L. L. assisted V. C. P. in performing the analysis of the antioxidant markers, and also contributed by critically reading the manuscript; V. D. allowed V. C. P. to use her laboratory facilities to conduct the analysis of antioxidant markers, and also contributed to the discussions concerning the results of the analysis and by critically reading the manuscript; O. A. was V. C. P.'s advisor in the Master's course, being the coordinator of the laboratory where most of the procedures were performed and the coordinator of the Male Reproductive Biology group. All authors contributed significantly to the study development and manuscript preparation. The authors have no conflict of interest.