Coffee contains phenolic compounds called hydroxycinnamates, which principally consist of chlorogenic acids, a family of trans-cinnamic acids conjugated with quinic acid. The main chlorogenic acid in coffee is 5-caffeoylquinic acid(Reference Clifford1). Tea is an infusion of the leaves of the Camellia sinensis plant, and is the most widely consumed beverage in the world after water(Reference Graham2). Some biologically active components of tea include flavonoids, caffeine and fluoride. Both of these caffeine-containing beverages are greatly consumed around the world, and because they contain many different bioactives, including polyphenols (Polyphenols. In the present study, phenolic acid equivalents derived from coffee chlorogenic acids and catechins derived from green tea are the specific compounds described as coffee and green tea polyphenols, respectively), they are studied for their potential health-promoting effects(Reference Natella, Nardini and Giannetti3–Reference Cabrera, Artacho and Gimenez5). Even in Japan, well known for green tea consumption, the contribution of polyphenols in coffee to the Japanese diet is at least equal to that of green tea(Reference Fukushima, Ohie and Yonekawa6). However, the bioavailability (Bioavailability. In the present study, bioavailability is limited to plasma appearance of polyphenols over time) and metabolism of these bioactives remain to be fully investigated, especially for coffee. Indeed, only a few papers have investigated how chlorogenic and phenolic acids present in coffee are absorbed in the plasma and/or excreted in the urine so far(Reference Nardini, Cirillo and Natella7–Reference Renouf, Guy and Marmet11). Bioavailability of the phenolic acids present in coffee is difficult to fully quantify, since it contains many components that cannot be clearly identified (e.g. melanoidin metabolites) due to the lack of proper standards. Because the bioavailability of green tea catechins appears to be more simple, studies on catechin bioavailability seem to provide clearer answers(Reference Lee, Wang and Li12–Reference Stalmach, Troufflard and Serafini21).
In the present study, we investigated the bioavailability of coffee chlorogenic acids expressed as phenolic acid equivalents and the bioavailability of green tea catechins in the same volunteers in a cross-over design. To make the comparison feasible, five major metabolites were studied for each beverage, and plasma appearance of these metabolites was summed and expressed in molar concentrations to facilitate the assessment and comparison of the two different classes of bioactives. We also investigated if there was any correlation between the metabolites. Using bioavailability parameters, such as area under the curve (AUC) and maximum plasma concentration (C max), we also tested the hypothesis that an apparent high absorber of coffee phenolics could also be an apparent high absorber of tea catechins.
Materials and methods
Subjects
Twelve healthy subjects were recruited for the present study. Nine subjects (four males and five females) completed the study. The study was approved by the Ethics Committee of Clinical Research of the University of Lausanne, Switzerland (protocol reference 136/07). Inclusion criteria included age 18–50 years; healthy, average and regular coffee consumption of 1–5 cups/d; and non-smoking.
Study design
The original protocol was a controlled four-treatment cross-over study. Two of the treatments were considered for secondary objectives, and will not be discussed in the present paper. At 1 week before the first treatment, BMI was measured. For 24 h before each treatment until the end of the sampling period, the ingestion of coffee, tea, cola, alcohol, whole-grain cereal (white bread allowed) or any medication was not allowed. Only water could be drunk during the night and in the morning before the treatment. On each study day, the subjects arrived fasted early in the morning at the metabolic unit. Baseline blood was sampled, and then one of the treatments was given to the subjects. Treatments included 4 g of soluble instant coffee dissolved in 400 ml of water (coffee treatment) or 400 ml of 1·25 % green tea infusion (green tea treatment). The composition of these beverages is given in Table 1, showing that the amounts of polyphenols in coffee and tea given to the subjects were very similar. Each subject was given the ‘coffee’ or the ‘green tea’ treatment once at random in a cross-over fashion. The washout period between the treatments was at least 1 week. On each study day, blood was collected at 0·5, 1, 1·5, 2, 3, 4, 5, 6, 8, 10, 11 and 12 h after one of the treatments. A standard lunch and dinner were provided at the metabolic unit. The subjects were encouraged to drink as much as they wanted, and water was available ad libitum. At 24 h after the treatment, a final blood sample was collected to assess return to baseline.
Table 1 Composition of polyphenols present in the coffee and green tea given to the subjects
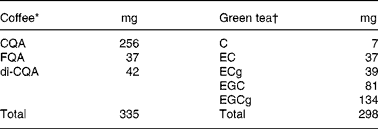
CQA, caffeoylquinic acid; FQA, feruloylquinic acid; di-CQA, di-caffeoylquinic acid; EC, ( − )-epicatechin; ECG, ( − )-epicatechin gallate; EGC, ( − )-epigallocatechin; EGCg, ( − )-epigallocatechin gallate.
* Coffee was prepared using 4 g of soluble coffee dissolved in 400 ml of hot water.
† Green tea was prepared using a 1·25 % infusion stirred for 3 min in 400 ml of hot water.
Plasma extraction after coffee consumption and analyses via LC–electronspray ionisation–MS/MS
The method that was used to extract phenolic acids from plasma samples was performed exactly as described in detail elsewhere, and validated using authentic synthetic standards of phenolic acid aglycones, sulphates and glucuronides spiked into blank plasma(Reference Renouf, Guy and Marmet11, Reference Guy, Renouf and Barron22). The method that was used to quantify coffee phenolics in the plasma has been described in detail elsewhere(Reference Renouf, Guy and Marmet11, Reference Guy, Renouf and Barron22).
Plasma extraction after green tea consumption and analyses via HPLC with electrochemical detection
Extraction of plasma samples after ingestion of green tea was done via a liquid–liquid extraction on ice. In summary, 12 μl of a solution containing 10 % vitamin C and 1 % EDTA (to stabilise), 20 μl of 50 mmol/l K3PO4 (pH 7·4, to achieve the optimal pH for enzymatic activity), 10 μl of internal standard (ethyl gallate, final concentration = 1 μmol/l), 200 μl of plasma and 100 μl of enzymatic mix (500 units β-glucuronidase+10 units sulphatase in 0·1 mol/l sodium acetate, pH 5·5) were mixed in 1·5 ml amber Eppendorf tubes. The tubes were closed, vortexed for 5 s, incubated at 37°C for 30 min (600 rpm) and then immediately put on ice. Extraction was done with 1 ml of ethyl acetate, vortexing was done for 30 s and centrifugation was done for 5 min at 4°C for 15 000 rpm. The supernatant was transferred into a new 2 ml amber Eppendorf vial (on ice), and 10 μl of a solution containing 0·5 % vitamin C and 0·05 % EDTA were added. The lower phase was extracted again with ethyl acetate, vortexed for 30 s and centrifuged for 5 min at 4°C for 15 000 rpm. The supernatant that was obtained was combined with the previous one, and 10 μl of a solution with 0·5 % vitamin C and 0·05 % EDTA were added to stabilise the phase. The latter was dried under N2 in a cold environment. The residue was resuspended in 200 μl of 20 % methanol in water, vortexed for 10 s and centrifuged for 5 min at 4°C for 15 000 rpm. A quantity of 100 μl of the sample was transferred into an amber HPLC vial for injection, and the remainder was kept at − 80°C for back-up analyses.
The method that was used to quantify catechins in the plasma was performed using HPLC coupled with electrochemical detection (Coularray® detector model 5600A fitted with solvent delivery module 582, GuardStat (Euroservice, Genova, Italy)). HPLC analyses were performed on a Macherey-Nagel Nucleosil C18 5 μm 250 × 3 mm column with a Precolumn Macherey-Nagel CC 8/3 Nucleosil 100-5 C18 (Oensingen, Switzerland). The mobile phases consisted of phase A: 75 mmol citric acid and 25 mmol ammonium acetate, pH 2·66, and phase B: 50 % phase A and 50 % acetonitrile. The gradient programme was as follows: 0 min, 75 % A; 0–13 min, 75 % A; 13–22 min, 70 % A; 22–35 min, 50 % A; 35–42 min, 0 % A; and 42–50 min, 97 % A. The flow rate was 0·5 ml/min, and the injection volume was 50 μl. The detection was performed with cell potentials set at − 90, − 10, 70, 150, 230, 310, 400 and 480 mV. To quantify detectable catechins present in the samples, a standard curve was constructed using known spiked samples, and the ratio of the sum of the peak heights of the substance to that of the internal standard was plotted v. the concentration. Similar ratios obtained from unknown plasma samples generated in the present study were then quantified based on this standard curve. For each patient, a standard curve was constructed from his/her baseline plasma spiked with known concentration of catechins to avoid inter-individual variability and plasma matrix effects.
Statistical analyses
Participants were randomised to sequences issued from a 4 × 4 Williams Latin square.
The areas under the available plasma curve of coffee phenolic acids were calculated over 12 h duration using the trapezoidal method.
All the calculated parameters (AUC, C max and time needed to reach maximum plasma concentration (T max)) were skewed to the right, and they needed a log transformation to reach normality. Thus, data are described by the geometric mean and the first and third quartiles.
A linear mixed model was used for the log-transformed data with treatment as the fixed effect and volunteers as the random effect. The estimations of the treatment effects are presented as the ratio of geometric means and their 95 % CI. Correlations between metabolites based on AUC and C max as well as the AUC/C max correlation for each individual metabolite were performed using regression analyses with significance of correlation set at P < 0·05.
Statistical analyses were done with Statistical Analysis Systems software (version 9.1; SAS Institute, Cary, NC, USA). The rejection level in the statistical tests is equal to 5 %.
Results
Plasma pharmacokinetics of coffee
Some data on the bioavailability of coffee obtained from the present study have been already described elsewhere(Reference Renouf, Guy and Marmet11). In summary, we found that caffeic acid (CA), ferulic acid (FA) and isoferulic acid (iFA) equivalents peaked early (T max at approximately 1 h), suggesting absorption in the small intestine. On the other hand, dihydroferulic acid (DHFA) and dihydrocaffeic acid (DHCA) appeared in the plasma later (T max at approximately 8–12 h), suggesting metabolism and absorption in the colon. Green tea metabolites were not detected in the plasma samples of subjects who drank coffee. Calculation of individual AUC was done for each subject and each metabolite with the data available between 0 and 12 h, with the 24 h time point being used only to assess return to baseline. Large inter-individual variability was observed, especially for the colonic metabolites (Fig. 1).

Fig. 1 Mean plasma concentration of caffeic acid (–■–) equivalents, ferulic acid (–♦–) equivalents, isoferulic acid (- -▲- -) equivalents, dihydroferulic acid (– × –) and dihydrocaffeic acid (–△–) in healthy volunteers after ingestion of coffee. Values are geometric means and 95 % CI (n 9).
Plasma pharmacokinetics of green tea
We established a method to measure C, ( − )-epicatechin (EC), ( − )-EC gallate, ( − )-epigallocatechin (EGC) and ( − )-EGC gallate (EGCg) in the plasma after green tea consumption. However, under the in vivo conditions in the present human intervention study, only EC, EGC and EGCg could be detected. Coffee metabolites were not detected in the plasma samples of subjects who drank green tea. These compounds were absorbed very quickly with a C max about 1–2 h after ingestion. Clearance from plasma was also rapid, and was back to baseline 6–8 h after ingestion with a monophasic response (Fig. 2).
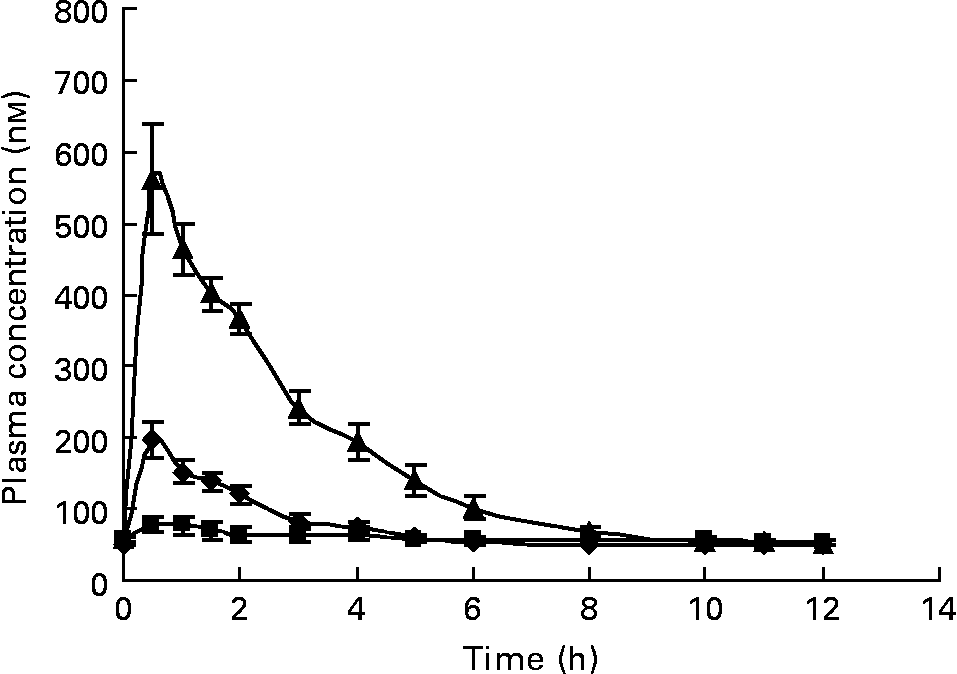
Fig. 2 Mean plasma concentration of ( − )-epicatechin (–♦–), ( − )-epigallocatechin gallate (–■–) and ( − )-epigallocatechin (–▲–) in healthy volunteers after ingestion of green tea. Values are geometric means and 95 % CI (n 9).
Comparison of plasma pharmacokinetics of coffee v. green tea
The comparison was done by summing the individual AUC0–12 h of CA, FA, iFA, DHFA and DHCA for coffee and the individual AUC0–12 h of EC, EGC and EGCg for green tea. The bioavailability of the compounds calculated over the 12 h was significantly higher in coffee than in green tea (mean ratio of coffee:green tea = 1·72; 95 % CI 1·27, 2·34; P = 0·0014) (Fig. 3). This comparison was possible due to the fact that the amounts of coffee and tea polyphenols given to the subjects were similar (Table 1).
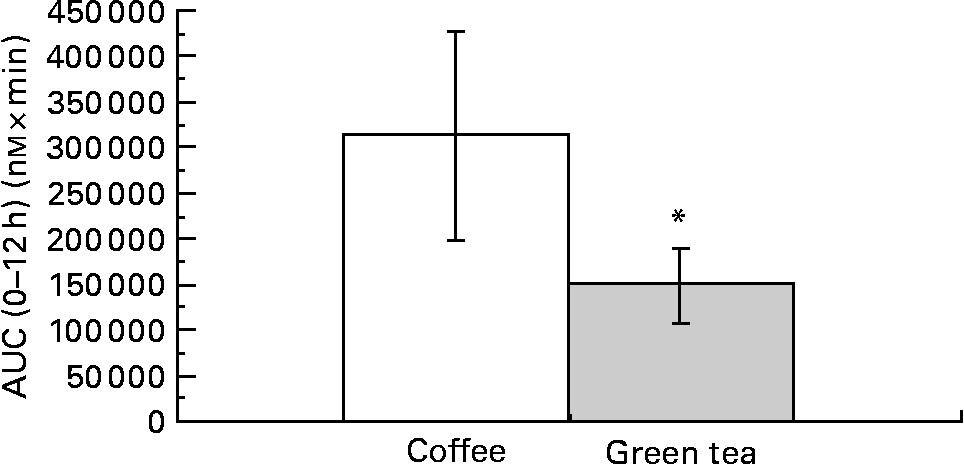
Fig. 3 Comparison of the sum of the area under the curve (AUC) of coffee (□) and green tea (░) metabolites (n 9). * Mean values were significantly different (P = 0·0014).
Correlations between coffee and tea metabolites
Based on AUC, correlations between different hydroxycinnamate metabolites present in coffee were highly significant (Table 2). For tea, only EC and EGC were significantly correlated. When the correlations were based on C max values, only CA/iFA and EC/EGC showed a statistical significance (Table 2). Correlation between AUC and C max was significant for CA, iFA, FA and EGCg (Table 3).
Table 2 Correlation of coffee and tea metabolites based on area under the curve (AUC) and maximum plasma concentration (C max)
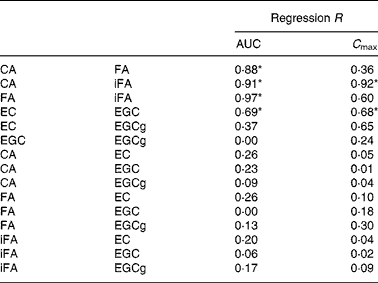
CA, caffeic acid; FA, ferulic acid; iFA, isoferulic acid; EC, ( − )-epicatechin; EGC, ( − )-epigallocatechin; ECg, ( − )-epicatechin gallate.
* Regression significant at P < 0·05.
Table 3 Correlation between area under the curve (AUC) and maximum plasma concentration (C max) for coffee and tea metabolites

CA, caffeic acid; FA, ferulic acid; iFA, isoferulic acid; EC, ( − )-epicatechin; EGC, ( − )-epigallocatechin; EGCg, ( − )-epicatechin gallate.
* Regression significant at P < 0·05.
Correlations could not be accurately established for DHFA and DHCA, as return to baseline was not observed in most of the subjects even 12 h after ingestion. Hence, only truncated AUC was measured for DHCA and DHCA, and C max could not be determined for all subjects.
Discussion
Only a few reports on the bioavailability of coffee bioactives have been published(Reference Nardini, Cirillo and Natella7–Reference Renouf, Guy and Marmet11). Coffee contains a complex profile of different isomers of the chlorogenic acids such as caffeoylquinic acid, feruloylquinic acid and di-caffeoylquinic acid, which upon absorption and metabolism will lead to an even greater number of metabolites (conjugation and cleavage of the ester bond into phenolic acids). Because of this complexity and to make an attempt to compare with green tea, we narrowed the number of metabolites of interest by cleaving all the molecules of interest into simple phenolic acids, which were measured as equivalents of CA, FA, iFA, DHFA and DHCA as reported previously(Reference Renouf, Guy and Marmet11, Reference Guy, Renouf and Barron22). For green tea, several studies have investigated plasma appearance of catechins (EGC, EC and EGCg)(Reference Yang, Chen and Lee13, Reference Chow, Cai and Alberts14, Reference Lee, Maliakal and Chen16, Reference Henning, Niu and Liu20, Reference Stalmach, Troufflard and Serafini21). These data are in agreement with the data reported here on green tea catechins, and show that EGC is the major flavanol appearing in the plasma, and that EGCg is not as well absorbed. In the present study, C max of EGC peaked at approximately 500 nm, while that of EGCg peaked only at approximately 80 nm, which is consistent with the published data. Any variation in AUC, T max and C max observed in all the studies is dependent on the population studied, the dose and source of green tea. The monophasic phenomenon observed in plasma kinetics of green tea catechins with an early T max shows that the small intestine is the key site for catechin absorption, and very few colonic metabolites of green tea catechins have been observed in human subjects in vivo (Reference Lee, Maliakal and Chen16, Reference Mulder, Rietveld and van Amelsvoort23–Reference Del Rio, Calani and Cordero25). We tried to simplify the analyses using full enzymatic hydrolysis in order to make the overall comparison between coffee and tea and between participants. We are aware that the total number of metabolites for both tea and coffee is very high, and that with improved analytical techniques, the accuracy, detection and quantification of these metabolites will improve as has been highlighted recently for both coffee(Reference Stalmach, Mullen and Barron10) and tea(Reference Del Rio, Calani and Cordero25). However, the increased complexity makes the examination of correlations more difficult, since it increases the number of individual compounds.
We further examined the data for differences between the individual volunteers. Based on AUC, we also observed a significant correlation between coffee metabolites. This implies that a subject exhibiting a high AUC for CA would most likely have a high AUC for FA and iFA. Correlation between CA and iFA was expected, as CA is the precursor of iFA in the hepatic methylation pathway. The correlation between CA and FA was less obvious to predict, as FA may come from either methylation of CA or from the cleavage of feruloylquinic acid present in the coffee. Inter-individual differences were observed, but individuals with a high level of CA also showed a correspondingly high level of FA (r 0·88). Similar results were obtained for FA, iFA and CA, and also for EGC and EC. However, no such correlation was observed between the tea catechins and coffee phenolic acids. This implies that the mechanisms of absorption for these two classes of compounds are different, and that a high absorber of catechins is not necessarily a high absorber of phenolic acids. For correlations based on C max, the correlation between EC and EGC was also significant, but it was weaker for coffee metabolites and only the correlation between CA and iFA showed a statistical significance. From the fact that the pathways of catechin metabolism are not as interlinked as those of phenolic acid metabolism, it seems reasonable to predict that correlation coefficients for green tea metabolites would be indeed lower than those for coffee metabolites.
To test the internal consistency for each individual metabolite, we used two parameters, AUC and C max, that were correlated for CA, FA, iFA and EGCg, indicating, as would be expected, that subjects displaying high C max will most likely have large AUC.
In conclusion, because coffee and green tea are two of the most widely consumed beverages, the present study led us to investigate plasma pharmacokinetics of phenolic acid equivalents and catechins after the consumption of instant coffee and green tea, respectively. Our data show that phenolic acids present in coffee are more bioavailable, on a molar basis, than catechins present in green tea by a factor of 1·7. This was shown by comparing the sum of the AUC of coffee metabolites to that of the AUC of green tea. This approach remains conservative, as the AUC of coffee metabolites DHCA and DHFA was truncated, and hence was incomplete at 12 h. In the present study, extrapolation of data between 12 and 24 h for these metabolites was not done to ensure that the data presented were, even though incomplete for DHCA and DHFA, the most reliable to make this coffee–green tea comparison.
Additionally, the present study confirmed published data on the presence of these bioactives in the plasma after ingestion of the beverages, and based on the measurement of the metabolites for 12 h after ingestion, we again emphasise the key role of the colon in the metabolism of coffee phenolic acids. Finally, we observed a greater overall correlation between the absorption capacity of the metabolites present in coffee and that of the corresponding catechins present in green tea.
Acknowledgements
We acknowledge Sylviane Oguey-Araymon, Anny Blondel-Lubrano, Maurice Beaumont, Bernard Decarli, William Sauret, René Fumeaux and Candice Menozzi-Smarrito for their help and support in the present study. M. R., C. C., H. S. and G. W. designed the research; M. R., C. M. and P. G. conducted the research; D. B. provided essential reagents or materials; M. R., P. G., C. M., A.-L. F. and K. L. analysed the data; J. M. performed statistical analyses; M. R., J. M. and G. W. wrote the paper; M. R. had the primary responsibility for final content; F. D. and G. W. helped with the interpretation of data, discussion and consulting. All authors read and approved the final manuscript. The study reported here was supported in full by Nestec SA. All authors are employees of Nestec SA, which fully funded the present study.








