Introduction
Major depressive disorder (MDD) is a highly common mental disorder, globally affecting approximately 265 million people of all ages (James et al., Reference James, Abate, Abate, Abay, Abbafati, Abbasi and Murray2018). MDD is often associated with poor health outcomes (Penninx, Milaneschi, Lamers, & Vogelzangs, Reference Penninx, Milaneschi, Lamers and Vogelzangs2013) and non-adherence to medications and treatments (DiMatteo, Lepper, & Croghan, Reference DiMatteo, Lepper and Croghan2000). Further, MDD is often comorbid with medical conditions such as cardiovascular diseases (CD) (Correll et al., Reference Correll, Solmi, Veronese, Bortolato, Rosson, Santonastaso and Stubbs2017; Lett et al., Reference Lett, Blumenthal, Babyak, Sherwoob, Strauman, Robins and Newman2004; Penninx, Reference Penninx2017; Penninx et al., Reference Penninx, Beekman, Honig, Deeg, Schoevers, van Eijk and van Tilburg2001). Numerous studies have shown that both MDD and CD potentially share underlying pathophysiological disturbances such as systemic inflammation, autonomic dysfunction of hypothalamic-pituitary-adrenal (HPA) axis (Angermann & Ertl, Reference Angermann and Ertl2018), and immune system dysregulation (Halaris, Reference Halaris2017).
HR parameters may be used as diagnostic and predictive biomarkers of depression severity. A key indicator of the autonomous nervous system (ANS) function is the heart rate (HR) variability (HRV), consisting of the fluctuations in either the instantaneous HR or the length of heartbeats intervals. Increased variability indicates an improved autonomic nervous system regulation (Berntson et al., Reference Berntson, Thomas Bigger, Eckberg, Grossman, Kaufmann, Malik and Van Der Molen1997). A reduced resting HRV has been related to difficulties in emotion regulation (Williams et al., Reference Williams, Cash, Rankin, Bernardi, Koenig and Thayer2015). Previous studies have also reported that individuals with MDD have a reduced resting mean HRV (Kemp & Quintana, Reference Kemp and Quintana2013; Koenig, Kemp, Beauchaine, Thayer, & Kaess, Reference Koenig, Kemp, Beauchaine, Thayer and Kaess2016; Nabi et al., Reference Nabi, Kivimäki, Empana, Sabia, Britton, Marmot and Singh-Manoux2011). More severe depressive symptoms have been associated with elevated HR (Carney et al., Reference Carney, Steinmeyer, Freedland, Blumenthal, Stein, Steinhoff and Jaffe2008; Carney, Freedland, & Veith, Reference Carney, Freedland and Veith2005; Nabi et al., Reference Nabi, Kivimäki, Empana, Sabia, Britton, Marmot and Singh-Manoux2011) and reduced HRV (Caldwell & Steffen, Reference Caldwell and Steffen2018; Hartmann, Schmidt, Sander, & Hegerl, Reference Hartmann, Schmidt, Sander and Hegerl2019; Kemp et al., Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt2010). Some studies reported that HR differences between individuals with MDD and without MDD might be more evident at night (Carney et al., Reference Carney, Steinmeyer, Freedland, Blumenthal, Stein, Steinhoff and Jaffe2008; Taillard, Lemoine, Boule, Drogue, & Mouret, Reference Taillard, Lemoine, Boule, Drogue and Mouret1993). Furthermore, people with MDD frequently report irregularities in sleep/wakes states (Walker, Walton, DeVries, & Nelson, Reference Walker, Walton, DeVries and Nelson2020), which can also affect HR. However, most of these studies have several significant limitations: (i) either they were conducted in individuals of the general population including those without a diagnosis of MDD (Nabi et al., Reference Nabi, Kivimäki, Empana, Sabia, Britton, Marmot and Singh-Manoux2011; Silva et al., Reference Silva, Aguiar, Reis, Sá, Gonçalves and Carvalho2020); (ii) included only a small study population (Hartmann et al., Reference Hartmann, Schmidt, Sander and Hegerl2019; Li, Hu, Shen, Xu, & Retcliffe, Reference Li, Hu, Shen, Xu and Retcliffe2015; Narziev et al., Reference Narziev, Goh, Toshnazarov, Lee, Chung and Noh2020; Silva et al., Reference Silva, Aguiar, Reis, Sá, Gonçalves and Carvalho2020); or (iii) were conducted under laboratory conditions such as using electrocardiography (ECG) in hospital or research settings (Hartmann et al., Reference Hartmann, Schmidt, Sander and Hegerl2019; Kemp, Quintana, Felmingham, Matthews, & Jelinek, Reference Kemp, Quintana, Felmingham, Matthews and Jelinek2012; Koch, Wilhelm, Salzmann, Rief, & Euteneuer, Reference Koch, Wilhelm, Salzmann, Rief and Euteneuer2019; Koenig et al., Reference Koenig, Kemp, Beauchaine, Thayer and Kaess2016; Nabi et al., Reference Nabi, Kivimäki, Empana, Sabia, Britton, Marmot and Singh-Manoux2011).
The recording of the ECG during daily life and long periods has several limitations. For example, the electrodes of the ECG, can cause skin irritation in long recordings due to its wet compound and adhesive properties, or the gel might dry, resulting in a reduction of the contact between the electrode and the skin negatively affecting the quality of the recording. There are other types of electrodes that are not adhesive but they are highly sensitive to motion artefacts. Morever, Holter devices used for long data acquisitions interfere with the daily life routine, being unfeasible for continuous monitoring (Dias & Cunha, Reference Dias and Cunha2018).
Wrist worn devices that are available today may facilitate the measurement of HR in naturalistic conditions. These technologies have several advantages over previous devices, including being non-invasive, low burden, low cost, and allowing the acquisition and processing in near-real time of a large amount of information. In fact, these technologies are able to provide 24 h of HR monitoring, comfortable design and allowed to worn constantly (Castaneda, Esparza, Ghamari, Soltanpur, & Nazeran, Reference Castaneda, Esparza, Ghamari, Soltanpur and Nazeran2018; Lam, Aratia, Wang, & Tung, Reference Lam, Aratia, Wang and Tung2020; Nelson & Allen, Reference Nelson and Allen2019).
All devices based on the photoplethysmographic (PPG) signal, obtained by illuminating the skin with the light from a light-emitting diode (LED) and then measuring the amount of light reflected to a photodiode to detect blood volume changes in the capillaries above the wrist (Subasi, Reference Subasi2019) from which HR information can be derived. The feasibility of deriving HRV from the PPG signal instead of the ECG signal has been widely investigated. In general, HRV derived from the PPG can be used as a surrogate of HRV derived from the ECG, when pulses can be accurately detected from the PPG, but this is challenging when PPG is recorded at wrist during daily life, mainly due to the sensitivity of PPG to movement artefacts. Only few studies have validated HRV derived from wrist PPG and always in resting conditions (i.e. Hernando, Roca, Sancho, Alesanco, & Bailón, Reference Hernando, Roca, Sancho, Alesanco and Bailón2018). However, many studies have validated the use of HR series provided by these devices to provide mean HR estimates over different periods of time (Fuller et al., Reference Fuller, Colwell, Low, Orychock, Tobin, Simango and Taylor2020; Liu et al., Reference Liu, Han, Puyal, Kontaxis, Sun and Locatelli2022; Nazari, Macdermid, Sinden, Richardson, & Tang, Reference Nazari, Macdermid, Sinden, Richardson and Tang2019; Nelson & Allen, Reference Nelson and Allen2019). Despite the fact that these devices, based on wrist PPG, do not usually allow the study of HRV, they can still be useful to study HR trends and slow dynamics during the day, and might provide an indicator of ANS regulation of HR.
In order to analyse the use of wrist-worn technologies in assessing individuals with a history of recurrent MDD, the Remote Assessment of Disease and Relapse – Central Nervous System (RADAR-CNS) (www.radar-cns.org) project, involving the patients, took the decision to use a commercially available device which is minimally invasive, easy to use and has the sensitivity and precision to generate the desired multimodal information (i.e. HR, activity, sleep) (Owens, Reference Owens2020; Polhemus et al., Reference Polhemus, Novák, Ferrao, Simblett, Radaelli, Locatelli and Hotopf2020; Simblett et al., Reference Simblett, Matcham, Siddi, Bulgari, Barattieri di San Pietro, Hortas López and Wykes2019). In this project, a wrist worn fitness wearable device was used to track the HR dynamics during the whole day, outside the medical environment. This device makes use of the PPG signal from which HR is estimated using a proprietary algorithm and output at different time intervals. HR can vary significantly over 24 h and under different conditions (Shaffer & Ginsberg, Reference Shaffer and Ginsberg2017), so it is essential to take this information into account when analysing and interpreting HR dynamics as a marker of ANS.
This study assessed the relationship of HR parameters during different periods of the day and night and different activity levels with depression severity in a cohort of individuals with a recent history of recurrent MDD. Our first objective was to explore and test the association of HR parameters with the severity of depression. Based on previous literature, we expected that an increase in mean HR and reduced HR variation during the day would be related to an increased depression severity across the follow-up. Furthermore, we expected a similar pattern during the resting periods of the night: an increased mean HR and reduced HR variation associated with higher level of depression. The second objective was to examine whether this relationship can be affected by an individual's characteristics (age, gender body mass index (BMI), smoking and alcohol habit, comorbidity with medical health conditions and antidepressant medication). We also expected that these factors might impact on the association between HR changes and depression severity.
Method
Study design and sample
This study uses data collected from the RADAR-MDD study, as a part of the research RADAR-CNS project. The study was co-developed with service users in our Patient Advisory Board. They were involved in the choice of measures, the timing and issues of engagement and have also been involved in developing the analysis plan and representative (s) are authors of this paper and critically reviewed it.
The RADAR-MDD study explored the use of active and passive remote monitoring technology (RMT), including a wrist-worn Fitbit device to track disease course in people with a recent history of recurrent MDD (with the latest episode within the past 2 years) and follow them up for a 2 years. The active and passive data are collected via the active and passive RMT and then send into the RADAR-base platform (Ranjan et al., Reference Ranjan, Rashid, Stewart, Conde, Begale and Verbeeck2019). For the Fitbit data, they are uploaded to the vendor data warehouse and provided to developers via a Web API. Getting these data into the RADAR system is achieved by implementing a server-side Kafka Source Connector, which continuously queries data from the vendor's Web API and dumps it into Kafka inside the RADAR-base platform; this approach can be used to integrate other Web API/OAuth2 data sources [GitHub. (2019-03-04). RADAR-base/RADAR-REST-fitbit https://github.com/RADAR-base/RADAR-REST-fitbit website].
The full protocol has already been published (Matcham et al., Reference Matcham, Barattieri di San Pietro, Bulgari, de Girolamo, Dobson, Eriksson and Hotopf2019; Matcham et al., Reference Matcham, Leightley, Siddi, Lamers, White and Annas2022). The RADAR-MDD is a multi-centre cohort study involving 623 individuals recruited from three sites: Centro de Investigación Biomédica en Red (CIBER); Barcelona, Vrije Universiteit Medisch Centrum (VUmc), Amsterdam) and the King's College London (KCL). Participants were recruited through primary and secondary mental health care networks (Barcelona and London) and through existing research cohorts (participants from Amsterdam were partially recruited through Hersenonderzoek.nl (https://hersenonderzoek.nl/) and other ways such as advertisements in the https://www.radar-cns.org/participate and mental health charity websites. The study was approved by the ethical committees of participating centres and all participants provided written consent.
Instruments
Depression severity
Depression severity was assessed with the Patient Health Questionnaire 8 items (PHQ-8) (Kroenke et al., Reference Kroenke, Strine, Spitzer, Williams, Berry and Mokdad2009) instrument delivered through an app installed in an Android smartphone (Ranjan et al., Reference Ranjan, Rashid, Stewart, Conde, Begale and Verbeeck2019). Participants were asked by push-notification to complete the PHQ-8 every two weeks. The PHQ-8 score ranges from 0 to 24 (increasing severity). A cut-off score of ⩾10 is the most recommended cut-off point for ‘clinically significant’ depressive symptoms (severe or moderate depression = 1; v. no depression or mild depression = 0), which means that the participant is likely to meet diagnostic criteria for a depressive episode (or moderate and severe depression) in the previous two weeks (Kroenke et al., Reference Kroenke, Strine, Spitzer, Williams, Berry and Mokdad2009). Ratings below 10 are usually defined as an asymptomatic state or sub-threshold (no or mild depression). Internal consistency was calculated with Cronbach's alpha, and it was 0.91.
Heart rate features
HR parameters, such as the mean or the standard deviation, were computed daily from the HR signal provided by Fitbit charge 2 and 3 (Fitbit Inc, San Francisco, CA, USA), which is obtained from the PPG sensor of the device with a a narrowest resolution up to 5 s between samples. This device was previously demonstrate proven to accurately measuring HR (Fuller et al., Reference Fuller, Colwell, Low, Orychock, Tobin, Simango and Taylor2020; Liu et al., Reference Liu, Han, Puyal, Kontaxis, Sun and Locatelli2022; Nazari et al., Reference Nazari, Macdermid, Sinden, Richardson and Tang2019; Nelson & Allen, Reference Nelson and Allen2019). The HR values were not estimated when the Fitbit was not worn. HR was computed during the whole day (24 h) and just at night (from 00:00 to 05:59), as well as just during resting periods and during active periods separately. The HR during nighttime calculated by the Fitbit was strongly associated with resting HR at night (00:00–05:59) (ρ = 0.94, p < 0.0001). However, a previous study (i.e.(Stucky et al., Reference Stucky, Clark, Azza, Karlen, Achermann, Kleim and Landolt2021) proved that the Fitbit underestimated the sleep transition dynamics. For this reason, we selected period from 00:00 to 05:59 as a conservative night time that might work for a large part of the population.
Resting periods were defined when the number of steps and activity level, derived from the accelerometer data were equal to 0.
A total of seven HR features were derived for each day: total mean and standard deviation of HR (mHR/day and stdHR/day), mean and standard deviation of HR during the resting period (resting mHR/day and resting stdHR/day, respectively), mean and standard deviation of HR during resting period at night (resting mHR/night and resting stdHR/night, respectively) and mean HR for the activity periods (activity mHR) (Table 1). We computed the average of the each of the daily HR parameters in the week before the PHQ-8 assessment across the follow-up. An example of the HR parameters for an individual with different depression score during the study was represented in Fig. 1. (Fig. 1). One week mean was considered appropriate to smooth day-to-day variability, especially during weekdays and weekend

Figure 1. An example of 7-day mean HR prior to the PHQ-8 assessment from the same participant at the mild depression level (left) and moderately severe level (right) during the follow-up.
Table 1. Features legend: HR parameters derived from the Fitbit

Sociodemographic, smoking habits and medical health conditions
Information about sociodemographic (age, gender, education years, marital status) and medical history including heaviness of smoking index (yes/no), and current alcohol habit (yes/no) measured trough the questionnaire Alcohol use disorders identification test (Daeppen, Yersin, Landry, Pecoud, & Decrey, Reference Daeppen, Yersin, Landry, Pecoud and Decrey2000) during all the follow-up, self-reported BMI and comorbidity with pre-existing medical health conditions(yes/no), and current antidepressant medications (yes/no) during the study were collected through a the Research Electronic Data Capture (REDCap) package during the enrolment session (Harris et al., Reference Harris, Taylor, Minor, Elliott, Fernandez, O'Neal and Duda2019, Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009).
Data analysis
First, we described the sociodemographic characteristics reporting frequencies and percentages for categorical variables. Categorical comparisons were made with the χ2 test. Median with standard deviation (Std) and interquartile ranges (IQR) were reported for continuous variables. Differences by country in continuous variables were explored with the Kruskal–Wallis test. Spearman correlation (ρ) was calculated between the HR parameters and PHQ-8 to assess the association without considering the clustered structure of repeated observations per individual. We also looked at the association between the PHQ-8 and the number of observations to explore if depression severity was negatively related to the assessment rate (i.e. patients with severe depression might be significantly less likely to complete the assessment because of their symptoms of abulia and apathy). Second, we compared the HR parameters between the observations in individuals with severe or moderate depression = 1; v. no depression or mild depression = 0) to discover HR features explaining the variance of PHQ-8. Cohen's d effect size was calculated for the comparisons: the effect size is considered as small, medium, and large using the 0.2, 0.5 and 0.8 cut-offs. Finally, linear mixed models with PHQ-8 as the outcome were computed, in two steps. In both, random intercepts for the participant and country levels were included as random effects, and data normalisation (z score) of the HR parameters was performed within-participants, so estimates in the mixed models indicate the effect of changes in the HR parameters from the participant-specific mean. In the first step a mixed model was computed separately for each HR feature (mean and standard deviation of HR during the day, resting periods, resting at night and activity periods), having that feature and the baseline of PHQ-8 (first measure of PHQ-8 in the dataset for each participant) as independent variables. In the second step various HR features were included as predictors simultaneously together with the baseline of PHQ-8 to estimate their joint effect. Further, in the second step, sociodemographic and clinical factors were also included as covariates to test their effect in the model, specifically age and BMI as continuous variables and gender, smoking, alcohol and antidepressant consumption, and medical comorbidity as dichotomous variables. All analyses were performed using the R software package ‘lme4’(Bates, Mächler, Bolker, & Walker, Reference Bates, Mächler, Bolker and Walker2015) software R (R Core Team, R Development Core Team, & R Core Team, 2016).
Results
Descriptive analyses
A total of 510 participants (with a total number of HR observations of 6666) in relationship to HR were included in the analyses. The majority of participants were female (n = 386, 76%) and the median age was 50 years old (mean 46.6, std 15.1). About half of the participants were single, separated or widowed (268, 53%). The majority were receiving antidepressant medication during the follow-up (84.3%). The median education years were 15 (Mean: 15.4, std 6.6). The mean BMI was 26 (IQR: 7.6). The sociodemographic information across sites is shown in the online Supplementary materials (Table S1). Only 21.1% (N = 107) reported smoking habits at enrolment. More than half (N = 284, 56%) reported comorbidity with medical conditions (please see details in online Supplementary materials Table S2). The Table 2 displays the descriptives of the variables included in the statistical models.
Table 2. Baseline, clinical and HR features
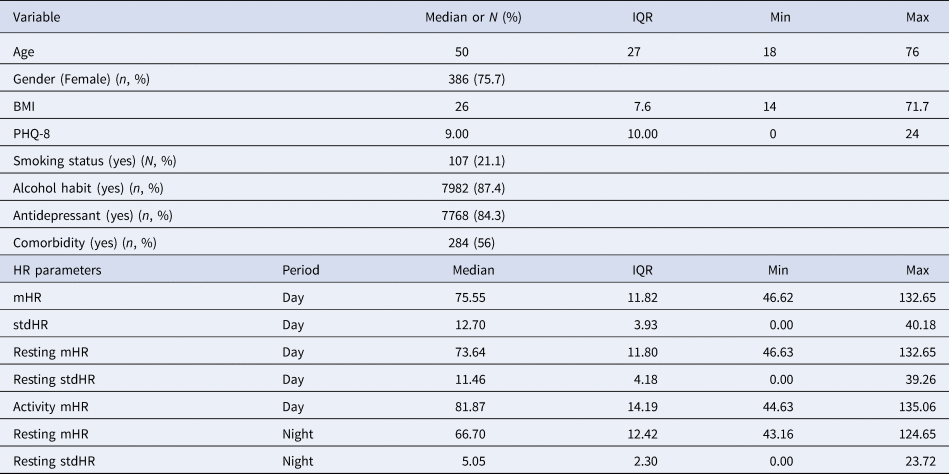
Note: m, mean; IQR, interquartile ranges; std, standard deviation.
Spearman correlation between depression severity and HR features, and comparison between two groups with different current depression severity
Depression severity, as measured with the PHQ-8 was positively related to total mHR/day (ρ = 0.13) and negatively related to the total stdHR/day (ρ = −0.21), (p < 0.001) (Fig. 2). The same pattern of correlations was observed during the resting period: depression severity was positively related to resting mHR/day (ρ = 0.17) and negatively related to resting stdHR/day (ρ = −0.12) (p < 0.001). During the activity period, depression severity was positively related to activity mHR during all day but with a small correlation (ρ = 0.03, p = 0.001). During the night, depression severity was positively related with both measures: resting mHR/night (ρ = 0.21) and resting stdHR/night (ρ = 0.13) (p < 0.001). In fact, participants with no depression or mild depression (PHQ-8 < 10) and moderate or severe depression (PHQ-8 ⩾ 10) severity had different total mHR/day (t = −7.99, Cohen's d = −0.20) and total stdHR/day (t = 16.61, Cohen's d = 0.41) (Fig. 2) (p < 0.001); resting mHR/night (t = −15.30, Cohen's d = −0.39) and resting stdHR/night (t = −7.31, Cohen's d = −0.18) (p < 0.001) (Fig. 3). However, we did not find any significant difference in activity mHR/day (t = −1.67, p = 0.09).
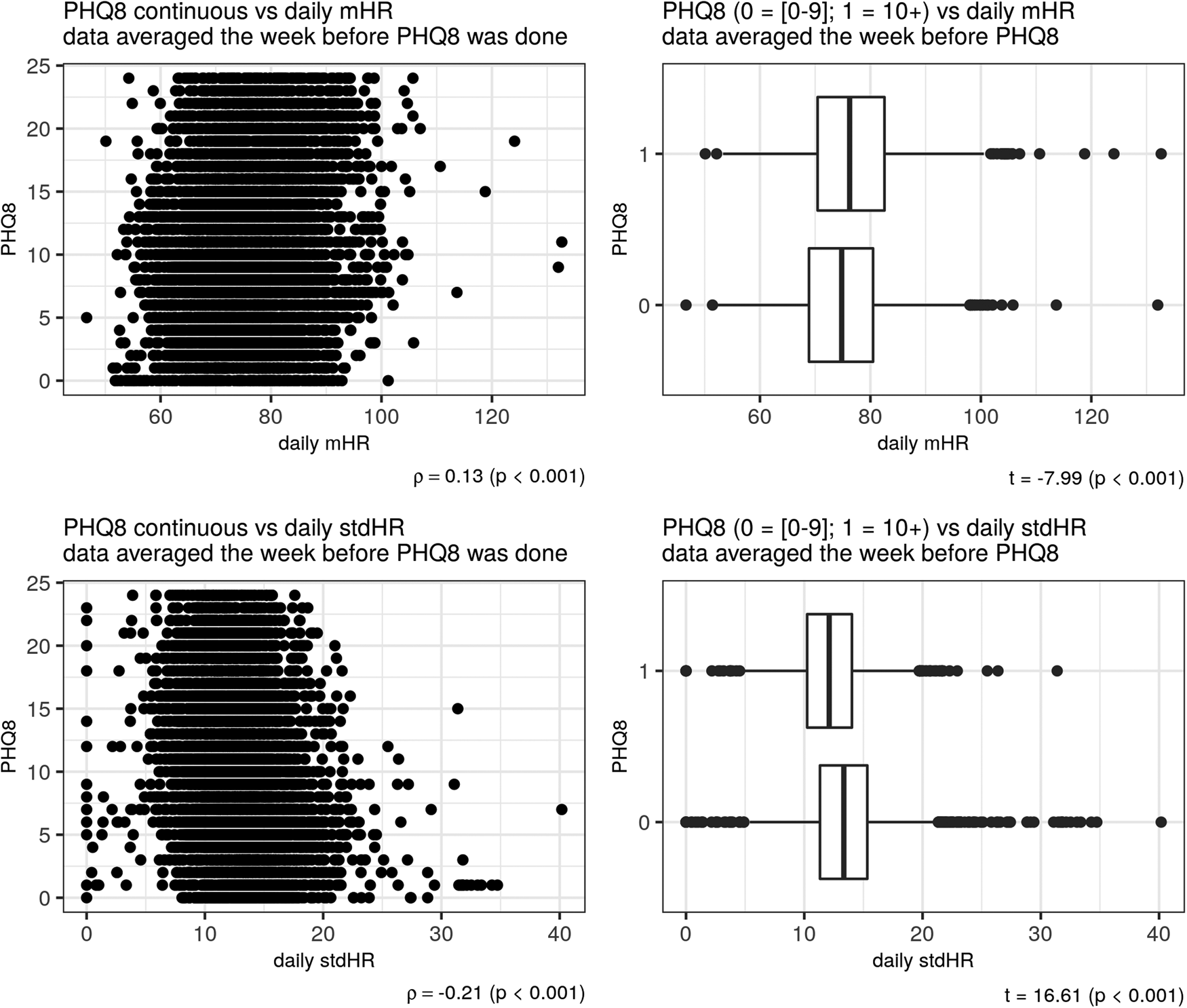
Figure 2. Depression and HR during the day. Scatter plot on the left side showed a correlation between PHQ-8 and mHR (above) and between PHQ-8 and stdHR (below). Boxplots on the right showed a comparison on total mHR (above) and total stdHR (below) between the groups depression v. no depression.
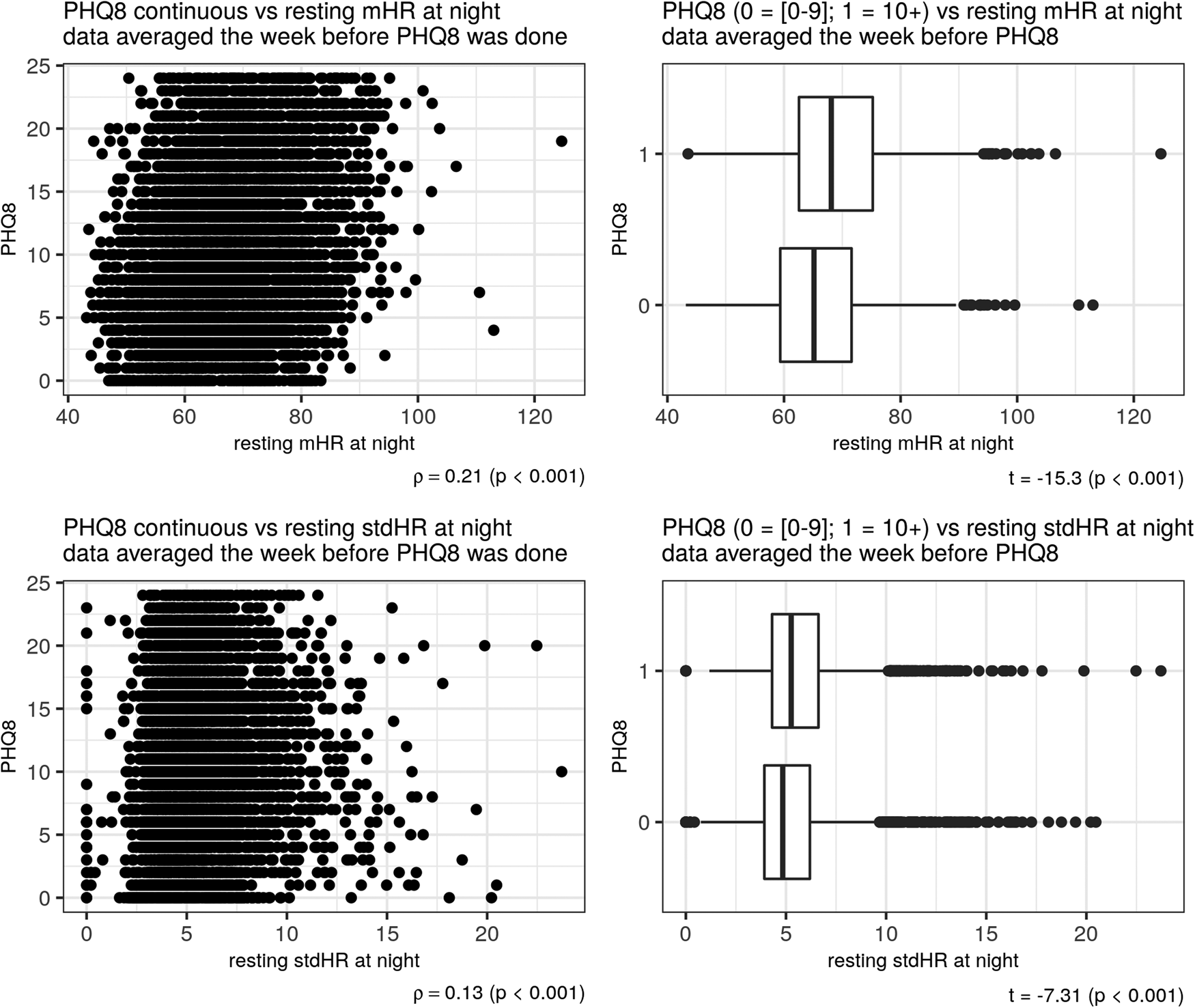
Figure 3. Depression and resting HR at night. Scatter plot on the left side showed a correlation between PHQ-8 and mHR (above) and between PHQ-8 and stdHR (below). Boxplots on the right side showed a comparison on mHR (above) and stdHR (below) between the groups depression v. no depression.
We did not find any significant association between depression severity as assessed with the PHQ-8 and the number of observations (ρ = −0.08, p = 0.07).
Linear multilevel regression analyses for each of the HR parameters
The models included the PHQ-8 rating as dependent variable and each of the HR features and the baseline of PHQ-8 as independent variables. The within-participant coefficient z-scores of the mHR/day (β = −0.18) and stdHR/day (β = −0.34) were negatively associated with depression severity (Table 3). We also observed a similar pattern for resting mHR/day (β = −0.14) and resting stdHR/day (β = −0.32), both were negatively associated with depression severity. While, resting mHR/night was positively associated with depression severity (β = 0.09). On the other hand, no association was found for resting stdHR/night and depression severity.
Table 3. Multilevel analyses for exploring the associations between the HR features and the depressive symptoms severity (PHQ-8)
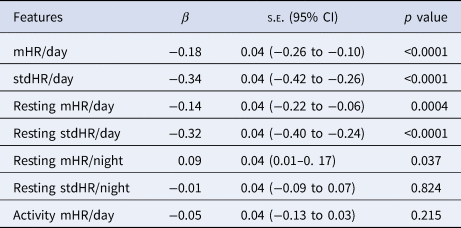
Note: each model includes a HR parameter together with the baseline PHQ-8 as independent variables to predict.
PHQ-8 changes (continuous variable).
Linear multilevel regression analyses for each of the HR parameters adjusting for all parameters and covariates.
The Table 4 shows the results of the multilevel analysis including mean and standard deviation of HR during the day, and resting HR during the night as independent variables (Model 1). Table 4 does shows the same model when adding the sociodemographic and clinical characteristics (adjusted model). The findings are consistent in the two models. Increases in mHR/day (β = −0.23) and stdHR/day (β = − 0.30) were associated with a decrease on PHQ-8, while the increasing resting mHR/night (β = 0.19) was related with an increase on PHQ-8. We then replicated the analyses, replacing the HR during all day with HR at resting state during the day (Table 5). We observed similar patterns for resting HR during the day: depression severity was negatively related to resting mHR/day (β = −0.16) and resting stdHR (β = −0.30), while a positive association was observed with resting mHR/night (β = 0.14, p = 0.04) (Table 5). No significant findings were found for resting stdHR at night. Depression severity was negatively associated with age (β = −0.03, p = 0.01) and positively to medical health condition (β = 1.12, p < 0.0001).
Table 4. Mixed model with HR features during the day (24 h) and night related to depression severity and sociodemographic covariates
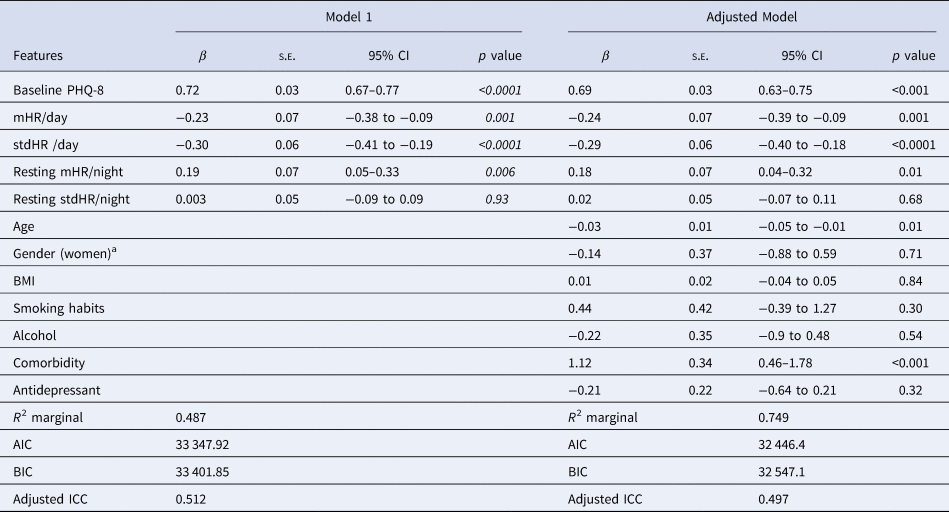
Note: Model 1. Daily measure of HR and HR during nighttime parameters were included together with the baseline PHQ-8 as independent variables in this model. Adjusted model includes all the previous independent variables and covariates.
aThe reference group is men.
Table 5. Linear mixed model with resting HR features during the day (24 h) and night related to depression severity and sociodemographic covariates
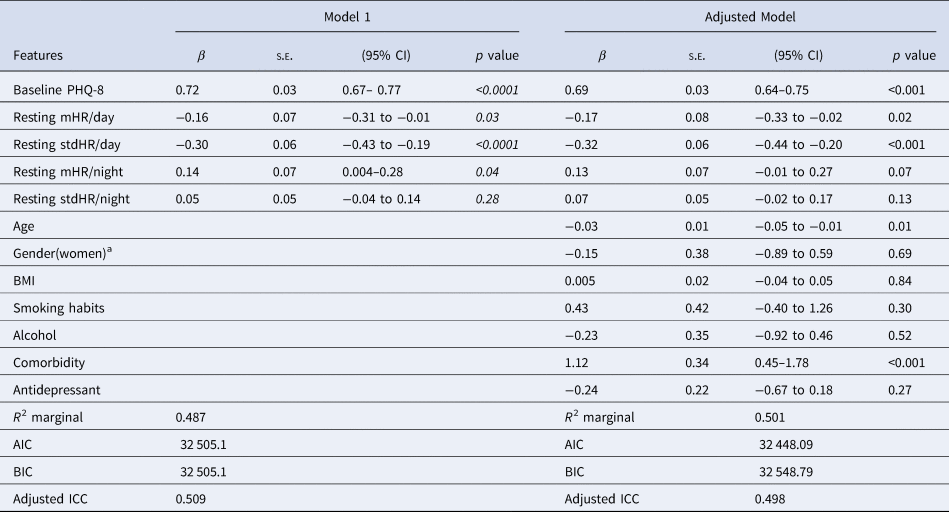
Note. Model 1. Resting HR measures during the day and night were included together with the baseline PHQ-8 as independent variables, Adjusted model: previous parameters were then included in this model with covariates.
a The reference group is men.
We then replicated the previous analyses including mHR during the activity period and the resting mHR/day and resting mHR/night to confirm that the activity mHR does not have an association with depression severity (Table 6). Resting mHR/day and resting mHR/night maintained their association with depression severity, as also the other two covariates did, but activity mHR/day was not associated to depression severity (adjusted model).
Table 6. Linear mixed model with mean HR features during the day (24 h) and night related to depression severity and sociodemographic covariates
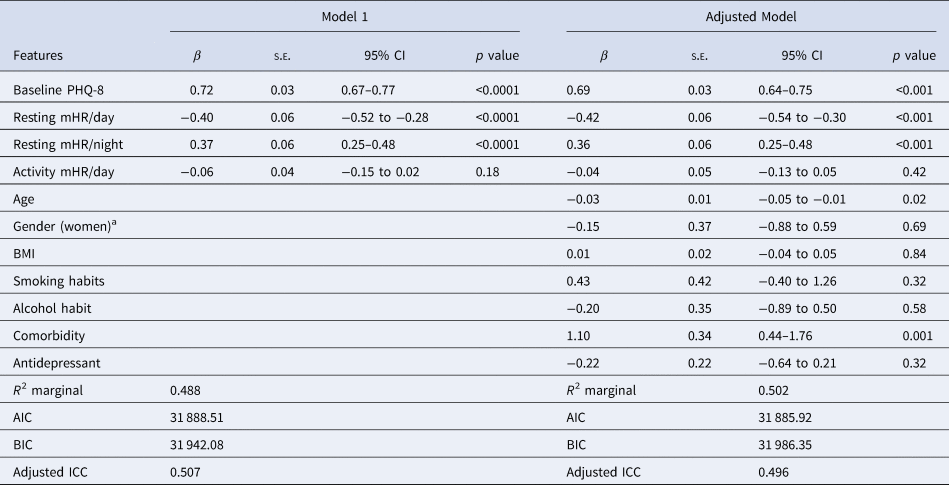
Note. Model 1: Resting and activity HR measures were included together with the baseline PHQ-8 as independent variables, Adjusted model: Previous parameters were then included in this model with covariates.
a The reference group is men.
Discussion
To the best of our knowledge, this is the first study exploring the association between depression severity and HR changes using a wearable device in a sample of people with MDD during a long-term period of monitoring. The two main findings of this study are: first, lower resting HR variation measured with the standard deviation of HR during the day is associated with higher depression severity; and second, resting mean HR at night increases with depression severity. These relationships were maintained when we adjusted for gender, age, smoking and alcohol habits, also pre-existing comorbid medical health conditions, and antidepressant treatment.
These findings are consistent with the study hypotheses. A previous study demonstrated that individuals with more severe depression were less active and did not perform moderate and vigorous activities that increase the HR during the day (Kandola, Lewis, Osborn, Stubbs, & Hayes, Reference Kandola, Lewis, Osborn, Stubbs and Hayes2020). Accordingly, one possible explanation could, therefore, be that individuals with MDD have low physical activity. The relationship was maintained when we also adjusted for mean HR during activity periods.
At odds with the study hypothesis, we also observed decrease in daily mean HR and daily resting HR associated with more severe depression. However, this finding was only present in the regression analysis. Accordingly, this association between mean HR/day and depression severity could have been affected by Simpson's paradox, given that the direction of the correlation between the two parameters changed from positive in the bivariate analyses to negative in the regression analyses when the HR data were within-participants normalised. The negative association was also maintained when the predictors were analysed separately, when they were introduced together and then adjusted with the covariates. Further research may be needed to advance in the relation between HR and depression severity.
One possible explanation of the relationship between HR and depression severity may be that passive behaviours, such as watching TV, listening to music or any other activity that do not include movements, may be more frequent in individuals when more depressed (Hallgren et al., Reference Hallgren, Nguyen, Owen, Stubbs, Vancampfort, Lundin and Lagerros2020). Another explanation might be that a low resting mean HR might be provoked by the effect of the medication; however we did not observe any effect of antidepressant medication when adjusted for it.. Studies conducted both in humans and animals reported that potentially adverse effects of antidepressant interactions could lead to an abnormal decrease in the HR (Ababneh, Ritchie, & Webster, Reference Ababneh, Ritchie and Webster2012; Azizi, Elyasi, & Roodposhti, Reference Azizi, Elyasi and Roodposhti2019; Woroń, Siwek, & Gorostowicz, Reference Woroń, Siwek and Gorostowicz2019). However, a recent meta-analysis found that HR alterations were not fully explained by antidepressant use alone (Brown et al., Reference Brown, Karmakar, Gray, Jindal, Lim and Bryant2018).
During the night, the high resting mean HR was associated with higher depression severity. In the resting state during the night non-activity periods were included, but they can correspond to awake stages where HR could be higher than expected for the night time. It is well-known that people with MDD suffer of sleeping difficulties, especially insomnia, that would be one explanation of the high mean HR. In healthy individuals, resting mean HR decreases significantly during the night due to parasympathetic predominance during sleep (Mancia et al., Reference Mancia, Ferrari, Gregorini, Parati, Pomidossi, Bertinieri and Zanchetti1983). This decrease may be less pronounced in individuals with MDD and related to depression severity. Previous studies have also demonstrated that increasing resting mean HR is a significant predictor of mortality in people with MDD and heart failure (Carney et al., Reference Carney, Freedland, Steinmeyer, Rubin, Stein and Rich2016; Lau et al., Reference Lau, Malik, Foroutan, Buchan, Daza, Sekercioglu and Alba2021; Nabi et al., Reference Nabi, Kivimäki, Empana, Sabia, Britton, Marmot and Singh-Manoux2011). We did not find any association between the resting HR variation during the night and depression severity. Further studies are warranted to explore if the HR variation in relation to depression severity fluctuates according to the different sleep stages.
As we expected, we observed that low HR variation during all day resting periods was related to increased depression severity over time. A low HR variation indicates that the body is under stress from psychological events, or other internal or external stressors. The reduced resting HR variation may depend on a failure of the parasympathetic control via the vagal nerve (Olshansky, Sabbah, Hauptman, & Colucci, Reference Olshansky, Sabbah, Hauptman and Colucci2008) that restores the body from overworking and prior accumulated stress (Kemp & Quintana, Reference Kemp and Quintana2013; Kim, Cheon, Bai, Lee, & Koo, Reference Kim, Cheon, Bai, Lee and Koo2018).
Decreased HR variation, especially during the resting period is associated with increased cardiovascular risk and mortality across different age groups (Brown et al., Reference Brown, Karmakar, Gray, Jindal, Lim and Bryant2018; Koch et al., Reference Koch, Wilhelm, Salzmann, Rief and Euteneuer2019; Koenig et al., Reference Koenig, Kemp, Beauchaine, Thayer and Kaess2016) and it is reported in different medical health conditions (Galinier et al., Reference Galinier, Pathak, Fourcade, Androdias, Curnier, Varnous and Bounhoure2000; Tessier et al., Reference Tessier, Sibon, Poli, Audiffren, Allard and Pfeuty2017). In our group, we observed that depression severity was positively associated with various comorbid medical conditions including cardiovascular disease, metabolic and digestive disease rheumatic disease, pulmonary disease and neurological disorders and other (i.e. psychiatric, as anxiety, eating disorders and other medical conditions. MDD is frequently comorbid with cardiovascular disease (Carney et al., Reference Carney, Freedland and Veith2005; Penninx, Reference Penninx2017), long diseases (Yohannes, Willgoss, Baldwin, & Connolly, Reference Yohannes, Willgoss, Baldwin and Connolly2010), metabolic syndrome (Pan & Hu, Reference Pan and Hu2013; Vancampfort et al., Reference Vancampfort, Correll, Wampers, Sienaert, Mitchell, De Herdt and De Hert2014), and neurological disorder (Raskind, Reference Raskind2008). This comorbidity might impact the prognosis and management of depression and increase risk of mortality.
Moreover, we also observed that younger age was associated with depression severity. Young people may endorse more affective symptoms whereas elderly people may report more cognitive changes and loss of interest (Fiske, Wetherell, & Gatz, Reference Fiske, Wetherell and Gatz2009). Another study found that younger age could have a greater risk of multiple depression episodes (Fergusson & Woodward, Reference Fergusson and Woodward2002). This finding highlights the importance of a prompt and early intervention. Finally, we did not find any association of depression severity with gender. It might be due to the fact that the majority of our sample was composed of women (76%) and both women and men were diagnosed with MDD with recurrent episodes. Neither did we find an association between depression and smoking status, alcohol status and BMI in our group. One explanation could be that only a small subgroup reported smoking habits, and a few reported BMI over the normal range. Previous studies reported an association between depressive symptoms, BMI and smoking habits (Hooker, MacGregor, Funderburk, & Maisto, Reference Hooker, MacGregor, Funderburk and Maisto2014; Strine et al., Reference Strine, Mokdad, Dube, Balluz, Gonzalez, Berry and Kroenke2008; Widome et al., Reference Widome, Linde, Rohde, Ludman, Jeffery and Simon2009).
Strengths and limitations
When considering these results, we should acknowledge the limitation of the device used. As stated previously, the HR data derived from the PPG are highly correlated with the HR data derived from the ECG, both HR and HRV (Gil et al., Reference Gil, Orini, Bailón, Vergara, Mainardi and Laguna2010; Lu et al., Reference Lu, Zhao, Ju, Shin, Lee, Shelley and Chon2008). However, the wrist-worn device used in this study does not provide access to the PPG signal but rather to the HR series derived by proprietary algorithms at different time intervals. This does not allow for analysing HRV properly, as already stated. Wrist-based device HR determination has been shown to have a < 5% error in a range of devices and activities relative to gold-standard and closer to 1% when at rest (Shcherbina et al., Reference Shcherbina, Mattsson, Waggott, Salisbury, Christle, Hastie and Ashley2017). The HR variation can be used as a proxy measure of HR fluctuations (Moser et al., Reference Moser, Lehofer, Sedminek, Lux, Zapotoczky, Kenner and Noordergraaf1994). The HR variation has already been used to explore the variation of HR in other studies (Quer, Gouda, Galarnyk, Topol, & Steinhubl, Reference Quer, Gouda, Galarnyk, Topol and Steinhubl2020; Wang, Lizardo, & Hachen, Reference Wang, Lizardo and Hachen2022). On the other hand, the Fitbit device presents different advantages: comfortable design, it can be used for an extended period and is accessible to a broad population for its low cost. Another strength of this study is the collection of daily mood data which may be superior to retrospective reports to measure depression severity in association with daily HR parameters. Moreover, we used long term longitudinal data from participants. Additional aspects to be considered when analysing the results are that the effect of some medications (i.e. beta-blockers and inhaler treatments) was not analysed; that potential adverse effect on HR might be provoked by other medications; and that various medical conditions have been included in the analysis but were not considered separately. However, the objective was only to explore how co-morbid medical condition might impact the association between HR and depression severity. Finally, depression severity may be exacerbated by anxiety symptoms. Further research should explore the impact of anxiety and sleep, among others, in the association between HR and depression severity.
Conclusion
In this paper, we have demonstrated that HR parameters may be indicators of depression severity and that it is possible to collect them on large numbers of participants and long follow-ups. From a clinical perspective, the current data suggest that reduced daily resting HR variability could represent a correlate of vulnerability to depression severity. Hence, the findings of specific HR biomarkers associated to depression severity fluctuations, providing a valuable tool for the early recognition or post- depressive monitoring of vulnerable individuals. An early warning of potential relapse allows clinicians to take responsive treatment promptly. This represents an opportunity to offer individual trajectories of HR parameters. Moreover, a longitudinal view of HR variations provides great personal health information in real-time and in real-world setting. Further research is needed to translate these results into meaningful clinical recommendations. These findings will be integrated in future analyses with multi-modal data collected within the RADAR-MDD study in order to develop algorithms to predict changes in clinical state.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723001034
Acknowledgements
The RADAR-CNS project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115902. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA, www.imi.europa.eu. This communication reflects the views of the RADAR-CNS consortium and neither IMI nor the European Union and EFPIA are liable for any use that may be made of the information contained herein. The funding body has not been involved in the design of the study, the collection or analysis of data, or the interpretation of data. Participants in the CIBER site came from following four clinical communities in Spain: Parc Sanitari Sant Joan de Déu Network services, Institut Català de la Salut, Institut Pere Mata, and Hospital Clínico San Carlos. Participant recruitment in Amsterdam was partially accomplished through Hersenonderzoek.nl, a Dutch online registry that facilitates participant recruitment for neuroscience studies Hersenonderzoek.nl is funded by ZonMw-Memorabel project no 73305095003, a project in the context of the Dutch Deltaplan Dementie, Gieskes-Strijbis Foundation, the Alzheimer's Society in the Netherlands and Brain Foundation Netherlands. We thank all GLAD Study volunteers for their participation, and gratefully acknowledge the NIHR BioResource, NIHR BioResource centres, NHS Trusts and staff for their contribution. We also acknowledge NIHR BRC, King's College London, South London and Maudsley NHS Trust and King's Health Partners. We thank the National Institute for Health Research, NHS Blood and Transplant, and Health Data Research UK as part of the Digital Innovation Hub Programme. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. This paper represents independent research part funded by the National Institute for Health Research NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. We thank all the members of the RADAR-CNS patient advisory board for their contribution to the device selection procedures, and their invaluable advice throughout the study protocol design. This research was reviewed by a team with experience of mental health problems and their careers who have been specially trained to advise on research proposals and documentation through the Feasibility and Acceptability Support Team for Researchers FAST-R: a free, confidential service in England provided by the National Institute for Health Research Maudsley Biomedical Research Centre via King's College London and South London and Maudsley NHS Foundation Trust. RADAR-MDD will be conducted per the Declaration of Helsinki and Good Clinical Practice, adhering to principles outlined in the NHS Research Governance Framework for Health and Social Care 2nd edition. Ethical approval has been obtained in London from the Camberwell St Giles Research Ethics Committee REC reference: 17/LO/1154, in London from the CEIC Fundacio Sant Joan de Deu CI: PIC-128-17 and in the Netherlands from the Medische Ethische Toetsingscommissie VUms METc VUmc registratienummer: 2018.012 – NL63557.029.17. All authors acknowledged the contribution of the Patient Advisory Board. This work has been dedicated to the memory of Rita Siddi.
Author contributors
SS (Sara Siddi), IG, RB, SK, JMH contributed to the data analysis, figure drawing and manuscript writing. NC, SV contributed with the critical revision of the analysis. AF, YR, RD contributed to the platform design and implementation. EL and EG contributed to the extraction of the features. FM, FL, SS (Sara Siddi), SD, BP, MH, TW, GD, p-M MT contributed to data collection. AF MH and VAN are lead for RADAR-CNS consortium, principal investigator of RADAR-MDD, study funding, design, and oversight of data collection. AF, YR, AR and RJBD contributed to the administrative, technical, and clinical support of the study. All authors have been involved in reviewing the manuscript and have given approval for it to be published. All authors have agreed to be accountable for all aspects of the work, ensuring that questions relating to the accuracy or the integrity of any part of the work are appropriately investigated and resolved.
Competing interests
VAN and VS are employees of Janssen Research and Development LLC. PA is employed by the pharmaceutical company H. Lundbeck A/S. JMH has received economic compensation for participating in advisory boards or giving educational lectures from Eli Lilly & Co, Sanofi, Lundbeck, and Otsuka. No other authors have competing interests to declare
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.












