Introduction
Type 1 diabetes mellitus (T1DM) is caused by damage to beta cells in the pancreatic islets and often develops from a preclinical phase of islet autoimmunity [Reference Skrodeniene1]. T1DM represents the primary form of diabetes in children <10-years-old. It can cause serious short-term consequences, such as hypoglycaemia and diabetic ketoacidosis, as well as a series of macrovascular or microvascular complications in later life [Reference Daneman2]. It is estimated that 10-year-old children with diabetes would lose approximately 19 life-years compared with non-diabetic children [Reference Narayan3]. The aetiology of T1DM is considered to be a combination interplay of genetic and environmental factors. The incidence of T1DM has been increasing at the rate of 2–5% a year worldwide, especially in children <5-years-old and genetic factors alone could not account for this proportion [Reference Daneman2]. Therefore, environmental factors initiating this process should be explored for early intervention. Several studies have reported that the process of T1DM may originate from intrauterine factors such as maternal infection. Antibodies produced after infection could be transmitted to newborns through the placenta [Reference Zinkernagel4, Reference Hermann5]. On the other hand, the fetal immune system could also drive the T cells to respond to infection. Pathogen-specific T cells can be found in cord blood samples from children whose mothers had infections during pregnancy [Reference Goldman and Marchant6]. Studies on whether maternal infection could trigger the initial destruction to fetal beta cells have recently increased. However, the findings of these studies are not consistent. Therefore, we conducted a systematic review and meta-analysis to investigate the association between maternal infection during pregnancy and the risk of childhood T1DM.
Methods
This study is reported following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and was registered at the International Prospective Register of Systematic Reviews (number CRD42018091459).
Literature search
The systematic search was conducted using PubMed, Embase, the Cochrane Library and Web of Science. In each database, the studies were limited to those published before June 2018. The search strategy had no restrictions on language. The following medical subject heading terms and free-text terms were used to search for articles: ‘gestational’, ‘mother’, ‘pregnancy’, ‘prenatal’, ‘maternal’ and ‘child’, or ‘offspring’; and ‘infection’, ‘risk factors’ and ‘Diabetes Mellitus, Type 1’, ‘Insulin-Dependent Diabetes Mellitus’, ‘type 1 diabetes mellitus’, ‘islet autoimmunity’, or ‘Autoimmune Diabetes’. In addition, eligible studies were added by searching reference lists in the included articles.
Study selection
Study selection was independently conducted by two reviewers (YY and YT). Disagreements were settled by discussions with another author (TZ). Irrelevant studies were excluded after screening titles and abstracts. Furthermore, an additional screening was conducted by reviewing the full texts. Inclusion criteria were: (1) cohort or case-control studies; (2) studies evaluating the association between childhood T1DM and maternal gestational infection; and (3) studies in which odds ratios (ORs), risk ratios, hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) (or the total number of participants and number of cases) were reported. Exclusion criteria included studies that were reviews, case reports, animal studies, abstracts and letters. Many studies were conducted in the same centers. When two or more studies appeared to have overlapping data, only the data with the larger sample size or those recently published were included.
Data extraction
For included studies, data extraction was independently carried out by two reviewers (YY and YT). Information about the first author, publication years, study design, source of participants, number of patients, ways to define the exposure and source of information, risk estimates with associated 95% CIs were collected. Disagreements were settled by discussions with another author (TZ).
Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the included studies according to the recommendation from Cochrane collaboration [Reference Wells7]. NOS contains eight items with a maximum score of 9. The quality of studies was divided into three categories: low quality with a 0–3 score, moderate quality with 4–6 score and high quality with a 7–9 score. Quality assessment was independently conducted by two reviewers (YY and YT) Disagreements were settled through discussions with the third reviewer (TZ).
Statistical analysis
ORs were used to determine the association between maternal gestational infection and the risk of T1DM in the offspring among studies. HRs were considered as relative risks (RRs); RRs were transformed into ORs based on the formula RR = OR/[(1−P 0) + (P 0 × OR)], where P 0 represents the incidence of T1DM in the group with mothers without infections during pregnancy. Heterogeneity was explored using the I 2 statistic and Q statistic among studies. Statistical significance of the meta-analysis was set at P < 0.05. Because there is significant heterogeneity, we only presented the results from a random-effects model. Subgroup analyses were performed based on study design, study quality, pathogens, methods of determining maternal infection (serological tests or mothers’ recall or medical records) and stage of T1DM (islet autoimmunity or developed T1DM). Furthermore, a sensitivity analysis was used to assess the robustness of the associations. A funnel plot, as well as Begg's and Egger's tests, were performed to test for publication bias. All analyses were conducted using Stata version 12.0 (StataCorp, College Station, TX).
Results
Results of literature search
Overall, 594 publications were identified. After 72 duplicates were removed, 522 articles were screened and the full texts of 71 articles were reviewed. Two case reports were excluded [Reference Otonkoski8, Reference Lönnrot9]. one letter and two abstracts were excluded according to our exclusion criteria [Reference Dahlquist, Boman and Juto10–Reference Ali12]. One study that tested islet autoantibodies in cord blood was excluded because the islet autoantibodies came from their mother [Reference Ludvigsson and Wahlberg13]. Six studies were excluded because their participants were overlapped and had a small sample size [Reference Lönnrot14–Reference Hiltunen19] compared with the four studies [Reference Blom20–Reference Viskari23]. Finally, 18 studies were included in our study [Reference Blom20–Reference Wahlberg37] (Fig. 1).
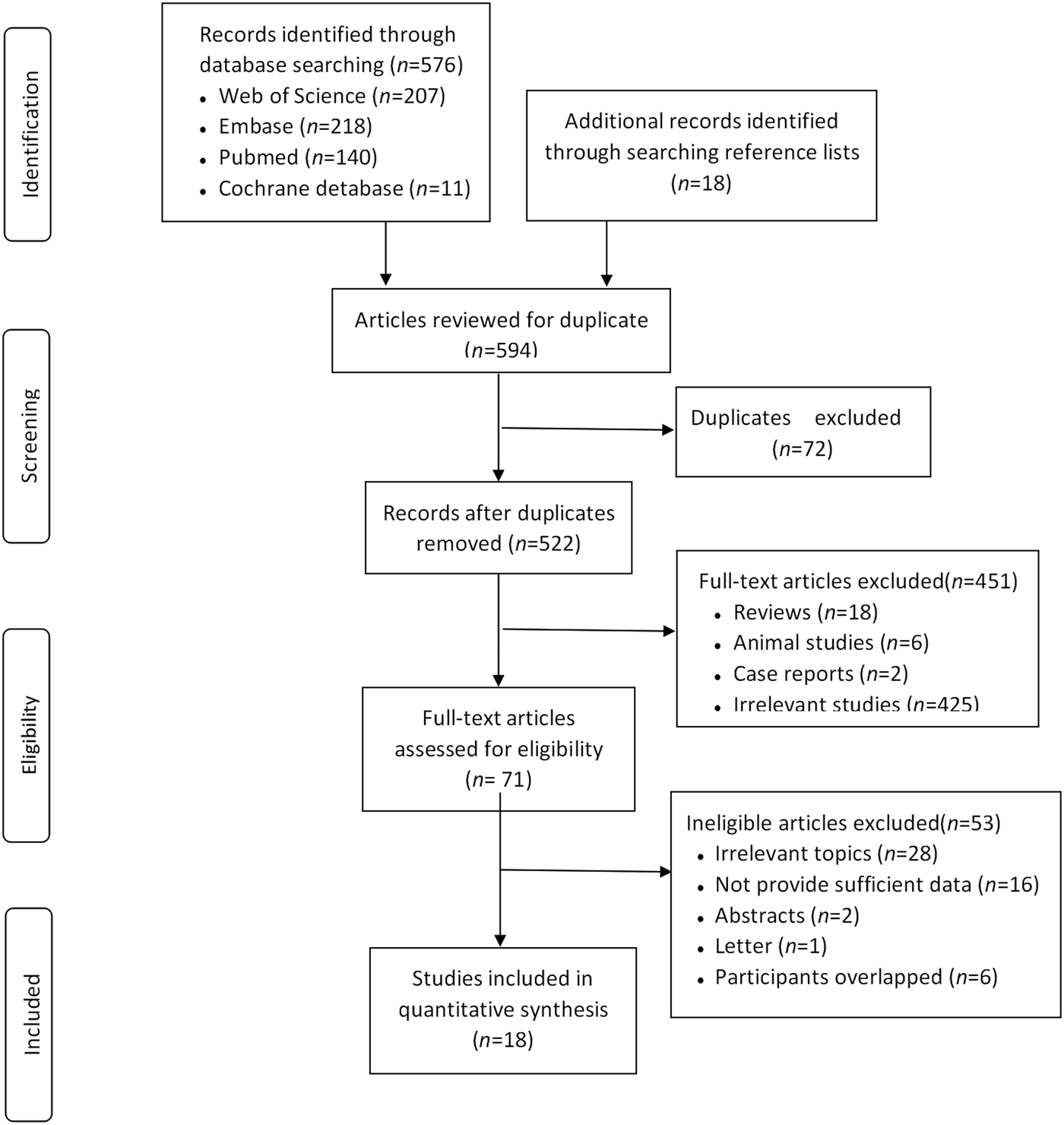
Fig. 1. Flow diagram of the study selection process.
Study characteristics
Ten case-control studies [Reference Blom20, Reference Dahlquist21, Reference Viskari23, Reference Awadalla24, Reference Majeed and Mea28–31, Reference Šipetić34, Reference Visalli36], seven nest case-control studies [Reference Viskari22, Reference Füchtenbusch25, Reference Lee26, Reference Sadeharju32, Reference Salminen33, Reference Stene35, Reference Wahlberg37] and one cohort [Reference Lynch27] study were identified in this meta-analysis, comprising a total of 4304 cases and 25 846 participants. Six studies [Reference Füchtenbusch25, Reference Lynch27, Reference Sadeharju32, Reference Salminen33, Reference Stene35, Reference Wahlberg37] examined islet autoimmunity as the outcome, while another 12 studies [Reference Blom20–Reference Awadalla24, Reference Füchtenbusch25, Reference Majeed and Mea28–31, Reference Šipetić34, Reference Visalli36] examined type 1 diabetes. Nine studies confirmed maternal infection based on mothers’ recall through questionnaires or interviews regarding gestational infection at any stage [Reference Blom20, Reference Awadalla24, Reference Lynch27–Reference Marshall29, Reference Šipetić34–Reference Wahlberg37]. Three studies carefully assessed medical records to investigate whether the mothers had any infectious diseases during pregnancy [Reference Lee26, Reference McKinney30, 31]. Additional six studies ascertained maternal infection using serological tests to detect viral IgM, IgG, antibodies, or viral RNA at the end of the first trimester or/and at delivery [Reference Dahlquist21–Reference Viskari23, Reference Füchtenbusch25, Reference Sadeharju32, Reference Salminen33]; all these six studies tested for enterovirus or subtypes of enterovirus. Study characteristics are presented in Table 1. NOS demonstrated that the overall methodological quality was good with scores ranging from 6 to 8 in all studies (Supplementary Table S1).
Table 1. Characteristics of studies investigating maternal infection during pregnancy and childhood T1DM or islet autoimmunity

Maternal infection and the risk of T1DM in the exposed offspring
Maternal infection during pregnancy increased the risk of childhood T1DM with a pooled adjusted OR of 1.32 (95% CI 1.08–1.62) from all 18 studies, indicating a statistically significant positive association. Statistically significant heterogeneity was observed between the included studies (I 2 = 70.1%; P < 0.001) (Fig. 2).
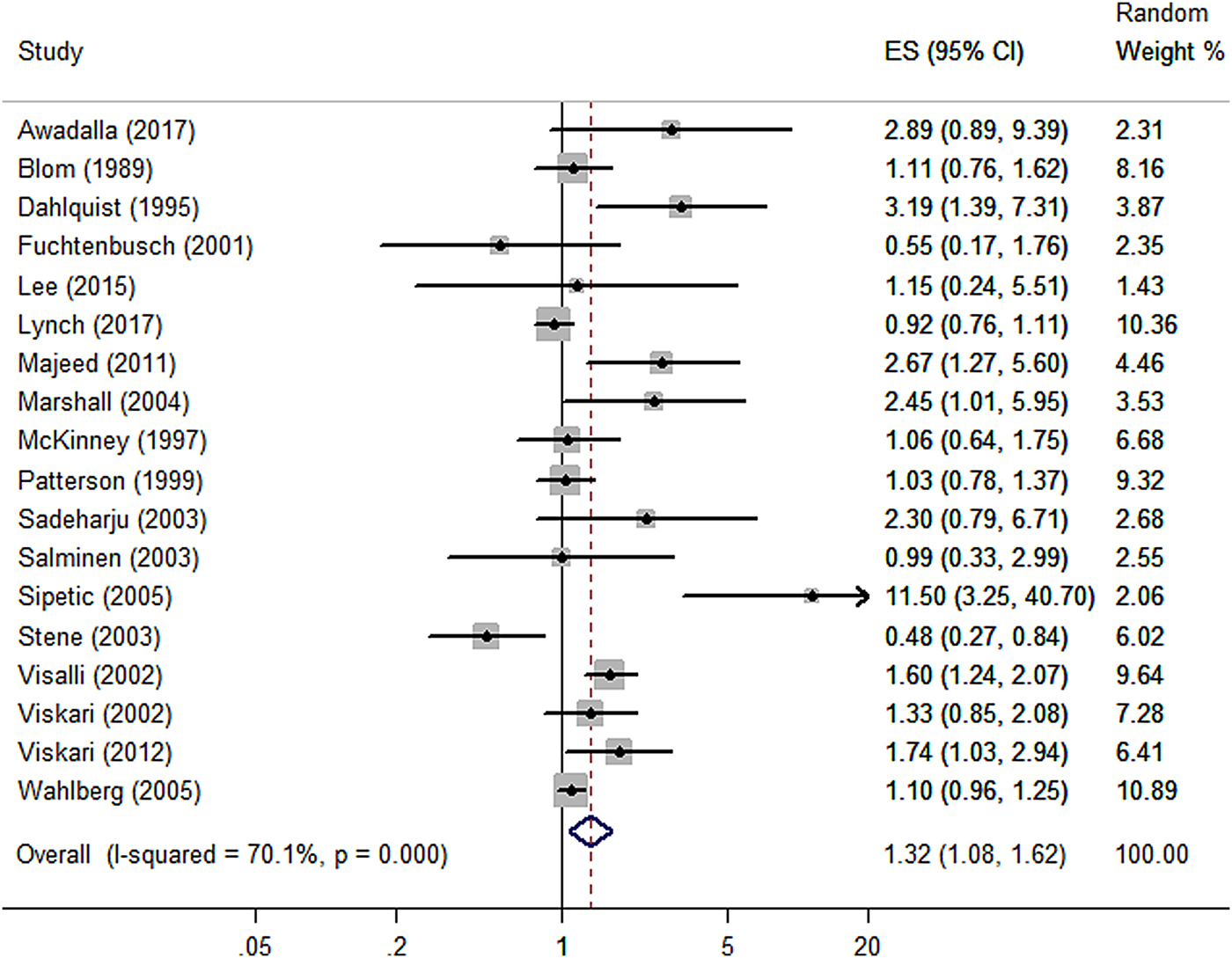
Fig. 2. Forest plot of odds ratios for the association between maternal infection during pregnancy and T1DM or islet autoimmunity in the offspring. Weights are from the random-effects analysis. ES, effect size.
Subgroup and sensibility analysis
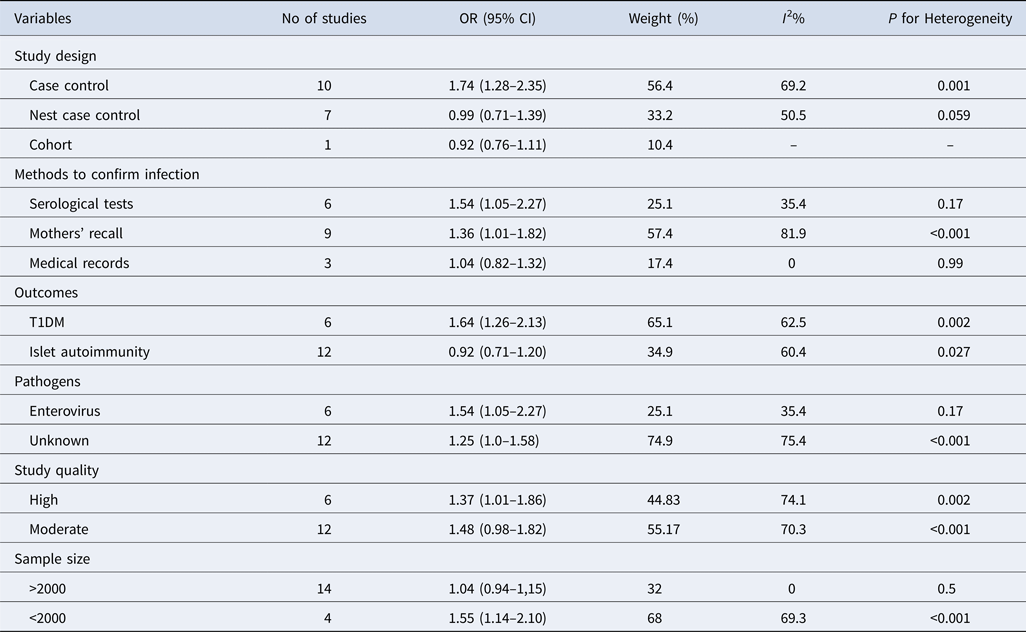
Subgroup analyses by study design, islet autoimmunity or T1DM, methods to confirm gestational infection, pathogens and study quality are presented in Table 2. Subgroup analysis stratified by study design demonstrated that the association was only significant in the case-control studies with a pooled OR of 1.74 (95% CI 1.28–2.35). The association was not significant among nest case-control studies and in the cohort study (Supplementary Fig. S1). We completed subgroup analysis according to the different outcomes for islet autoimmunity or T1DM. Twelve studies measured T1DM as the outcome demonstrated by a pooled OR of 1.64 (95% CI 1.26–2.13). For six other studies that estimated islet autoimmunity as the outcome, the pooled OR was 0.92 (95% CI 0.71–1.20), indicating that the association between maternal gestational infection and islet autoimmunity in the offspring was not significant (Supplementary Fig. S2). The subgroups in which maternal infection was detected by mothers’ recall or serological tests demonstrated a pooled OR of 1.36 (95% CI 1.01–1.82) and 1.54 (95% CI 1.05–2.27), respectively. However, in studies that examined the mothers’ medical records, the association was not significant, with a pooled OR of 1.04 (95% CI 0.82–1.32) (Supplementary Fig. S3). Notably, there were six studies that tested the IgG, IgM and viral RNA of enterovirus or subtypes of enterovirus in cord blood or mothers’ serum samples, yielding a pooled OR of 1.54 (95% CI 1.05–2.27) with no significant heterogeneity (I 2 = 35.4%; P = 0.171) (Supplementary Fig. S4). Furthermore, we conducted a subgroup analysis based on study quality. It demonstrated that the pooled OR of studies with a NOS score of <7 is 1.34 (95% CI 0.98–1.82), While for the studies with scores of ⩾7, the pooled OR among studies was 1.37 (95% CI 1.01–1.86) (Supplementary Fig. S5).
Table 2. Pooled OR and heterogeneity in subgroups and sensibility analysis
Sensitivity analyses show that no single study affected the overall result after individually removing each study (Supplementary Table S2). In addition, we performed a sensitivity analysis based on the study size: four studies had participants >2000, with two studies conducted in a multicenter setting, yielding a pooled OR of 1.04 (95% CI 0.94–1.15) with no significant heterogeneity (I 2 = 0%; P = 0.51). The combined OR among studies with <2000 participants was 1.55 (95% CI 1.14–2.10), with significant heterogeneity (I 2 = 69%; P < 0.0001) (Supplementary Fig. S6). In addition, we removed three small studies with cases <50 and the overall result did not substantially change.
Several studies reported that specific HLA genotypes could affect the risk of T1DM and the incidence of infection in children [Reference Sadeharju32]. For example, newborn infants bearing the HLA-DR-DQB1 genotype are prone to develop T1DM and have more enterovirus infections than children without this genotype [Reference Sadeharju32]. Previous findings demonstrated an association of HLA-DR3 with increased beta cell responses to enterovirus [Reference Füchtenbusch25]. Thus, we estimated the pooled OR of the five studies that matched for HLA-DR genotypes in cases and controls demonstrates a pooled OR of 1.16 (95% CI 0.76–1.77) (Supplementary Fig. S7). In addition, eight studies that investigated children with islet autoimmunity or T1DM under the age of 10 demonstrated a combined OR of 1.01(95% CI 0.79–1.29) (Supplementary Fig. S8).
Publication bias
The funnel plot indicated no significant asymmetry among studies (Fig. 3), in accordance with the results of the Begg's test and Egger's test (all P > 0.05), which revealed no evidence of publication bias.
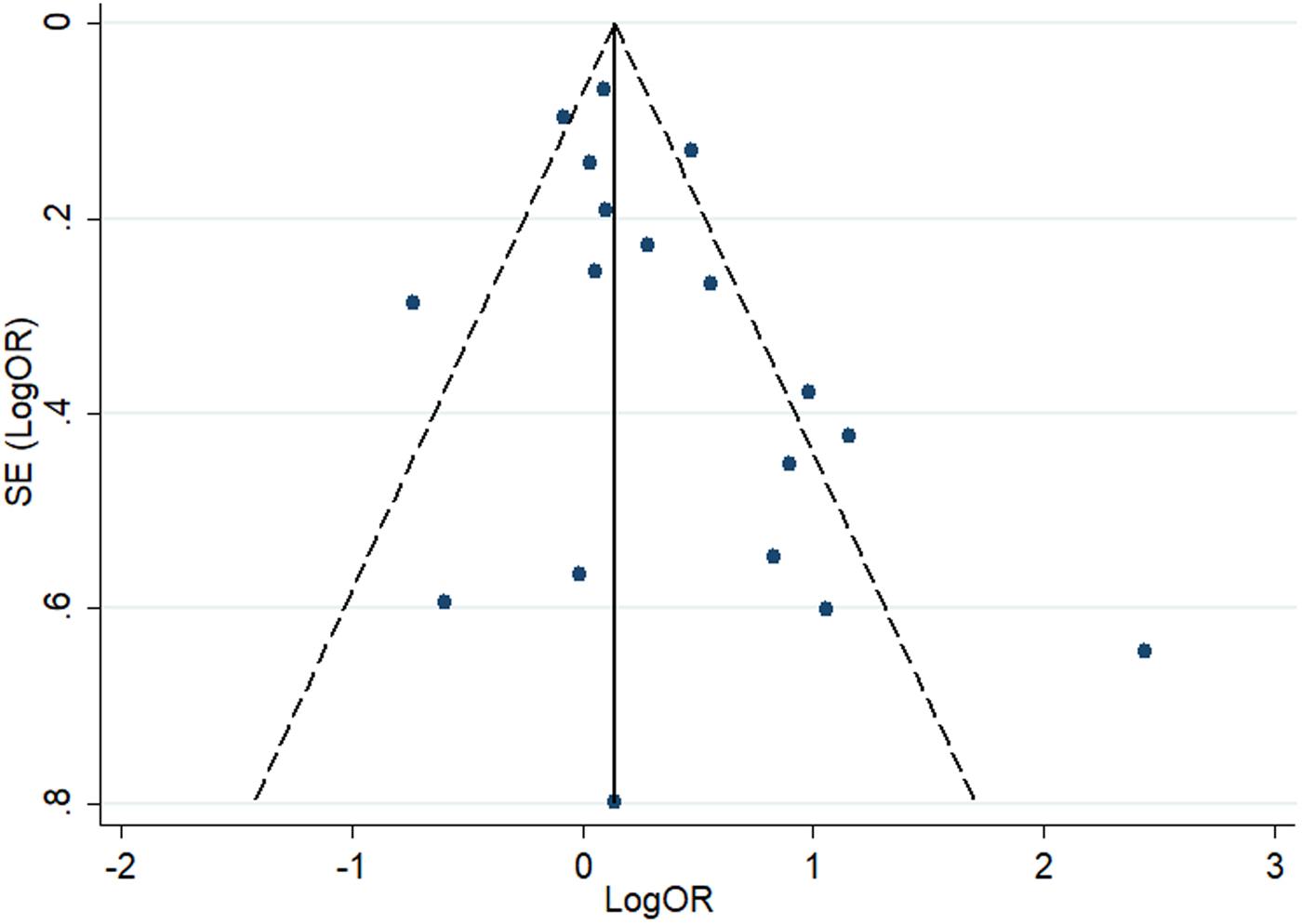
Fig. 3. Funnel plot of the association between maternal infection and T1DM or islet autoimmunity in the offspring.
Discussion
Eighteen studies were included in this meta-analysis, comprising 4304 cases and 25 846 participants. Most of the studies were conducted in Finland and Sweden, where T1DM incidence is high. The results demonstrated that maternal gestational infection is associated with 32% increased odds of T1DM or islet autoimmunity in the offspring in the pooled estimate, with significant heterogeneity across studies.
Subgroup analysis was conducted according to study design and no significant association was found in the cohort study and among the seven nest case-control studies. Subgroup analysis performed according to the different methods of determining maternal infection demonstrated a non-significant association among the studies using medical records and low pooled OR depending on mothers’ recall, which may account for selection or recall bias in those studies.
We analysed studies that matched for HLA-DR genotypes, resulting in a pooled OR of 1.16 (95% CI 0.76–1.77). Some studies reported that while gestational infection together with HLA-DR genotypes did not influence the overall incidence of beta-cell autoantibodies, they may delay the appearance of these autoantibodies [Reference Lynch27, Reference Ziegler38]. Thus, for infants with HLA-DR genotypes combined with maternal infection, the onset of T1DM may occur after several years. All five studies that matched for HLA-DR genotypes investigated children <10-years-old or a short follow-up time. Therefore, the islet autoantibodies may not yet have appeared at the end of the studies, which may have resulted in the low OR and non-significant association between maternal infection and childhood T1DM among these five studies.
Our results demonstrate that the pooled OR of the studies in children <10-years-old was 0.98 (95% CI 0.79–1.23). It is suggested that patients often undergo a long prodromal stage before clinical onset of T1DM [Reference Daneman2, Reference Devendra, Liu and Eisenbarth39]. Recent studies have indicated that only approximately 60% patients with T1DM are younger than 10-years-old [Reference Daneman2]. Thus, studies that investigated the incidence of T1DM in children <10-years-old yielded an insignificant effect. The subgroup analysis conducted according to different sample sizes yielded a summary OR of 1.04 (95% CI 0.94–1.15) in the studies with >2000 participants. The obtained non-significant association may also be a result of the younger age of participants, considering that three of the four studies included children <10-years-old. In this study, we conducted subgroup analysis according to different outcomes (T1DM or islet autoimmunity). We observed that the association was only significant in the group with T1DM but not islet autoimmunity. In the six studies that investigated the incidence of islet autoimmunity in the offspring, four matched for HLA genotypes and all studies had a short follow-up time, this may have caused missed diagnosis in some patients in whom the onset of T1DM started later and resulted in a low effect size.
It is noteworthy that studies that tested the enterovirus or subtypes of enterovirus infection in pregnancy yielded a pooled OR of 1.54 (95% CI 1.05–2.27), indicating that maternal enterovirus infection during pregnancy results in 54% increased risk of T1DM in the offspring. Evidence has demonstrated that some pathogens can cause cross-reactive immune responses against islet beta cells and thus generate beta-cell autoantibodies [Reference Wells7, Reference Maclaren and Atkinson40]. Therefore, the possibility that maternal enterovirus infection could cause childhood T1DM should be considered. If necessary, specific vaccines should be used to prevent such infections, to reduce the incidence of T1DM in the offspring [Reference Ilonen41].
However, the question of whether the association between maternal infection and the incidence of T1DM in the offspring is sex-dependent remains [Reference Viskari23, Reference Stene35]. But the pooled OR of studies investigating the association between different sexes could not be performed because only one study reported a negative association among girls but not boys [Reference Stene35].
Our study has several merits. To reduce potential bias, we prepared pre-defined inclusion criteria, searching eligible studies from several approaches without geographical and linguistic restrictions and the process was conducted by two reviewers. The random-effects models were used to provide more conservative effect estimates. In addition, this analysis includes 25 846 participants and all included studies were of moderate to high quality, which provides high reliability for our findings. However, our study has several limitations that should be considered. First, our study demonstrated significant heterogeneity. After performing subgroup analyses and sensitivity analyses, heterogeneity still existed among the studies (Table 2). We noted that three factors that may explain the presence of heterogeneity: (i) different study designs (case-control, nest case-control and cohort); (ii) different methods of determining maternal infection during pregnancy (serological tests, extracted from medical records or mothers’ recall); (iii) the greatly varied sample size among different studies. Second, some studies performed serological tests to determine maternal infection using samples taken only at the end of the first trimester [Reference Viskari22] or at delivery [Reference Dahlquist21, Reference Füchtenbusch25], instead of selecting multiple sampling points, which may result in missed diagnoses. Third, studies using the method of medical records or mothers’ recall were prone to selection or recall bias. Moreover, some other confounders such as birth weight, birth order and caesarean section may modify the risk of T1DM [Reference Blom20, Reference Visalli36], but it is impossible to control all of these potential confounders.
In conclusion, this meta-analysis demonstrated an association between maternal gestational infection and the risk of childhood T1DM. We recommend further studies investigating how T1DM risk varies by different pathogens and by different HLA genotypes. More studies with a longer follow-up time are needed to determine the interactions between environmental and genetic factors with the development of diabetes, to provide a guideline for preventing the development of T1DM and curb expenses related to health care.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818002455.
Acknowledgements
We would like to thank Editage (http://www.editage.cn) for English language editing.
This work was supported by the National Science Foundation of China (No.81330016, 81630038, 81771634, 81300524), the National Key R&D Program of China (2017YFA0104200), the Grants from Ministry of Education of China (IRT0935), the Grants from Science and Technology Bureau of Sichuan Province (2016TD0002) and the Grant of clinical discipline program (Neonatology) from the Ministry of Health of China (1311200003303).
Conflict of interest
None.








