In the global population, the non-alcoholic fatty liver disease (NAFLD) prevalence is estimated to be around 25 %, with the lowest rates in Africa (13 %) and the highest rates in Southeast Asia (42 %)(Reference Li, Zou and Yeo1). According to a study in 2016, the proportion of NAFLD in Iranian population was reported to be 33·9 %(Reference Moghaddasifar, Lankarani and Moosazadeh2). NAFLD is a clinical diagnosis, in which at least 5 % liver steatosis exists as determined by liver imaging or biopsy in the absence of any other known causes of liver dysfunction and probably presents with elevated liver enzymes(Reference Carr, Oranu and Khungar3,Reference Chalasani, Younossi and Lavine4) . It is noteworthy that NAFLD has been linked to a lot of metabolic diseases like insulin resistance (IR) and obesity which are the key properties of the metabolic syndrome(Reference Abenavoli, Milic and Di Renzo5). Therefore, NAFLD is often thought to be its hepatic manifestation(Reference Marchesini, Bugianesi and Forlani6). Additionally, according to recent studies, there exists a correlation between smoking and the risk of developing NAFLD(Reference Jang, Joo and Park7). Treating of individuals who are suffering from NAFLD usually includes multiple modes of treatment that targets various facets, such as weight reduction, lifestyle adjustments and optimisation of drug therapy(Reference Pouwels, Sakran and Graham8). There are many drugs on the pipeline that are reckoned as good candidates to cure NAFLD/non-alcohilic steatohepatitis (NASH), as evident in various recent papers, for example, pioglitazone, vitamin E and semaglutide(Reference Negi, Babica and Bajard9). NAFLD pathogenesis is defined by the TAG accumulation in the liver(Reference Ferramosca and Zara10). The role of fatty acid obtained through dietary intake as a key contributor to liver fat accumulation is widely recognised(Reference Ferramosca and Zara10). Indeed, the various dietary lipids have some unique characteristics, namely different degrees of saturation, which are divided into saturated, MUFA and PUFA(Reference Lian, Zhai and Li11). However, some dietary habits, like ‘western dietary pattern’ with low fibre and high saturated fat, are considered crucial in the commencement and development of NAFLD(Reference Berná and Romero-Gomez12–Reference Zelber-Sagi, Ratziu and Oren14). Ghee contains 60·4 % SFA, 31·4 % MUFA, 4 % PUFA and 1·5 % trans fatty acids (TFA), and that is clear with utilising a diet abundant in the SFA; liver fat increases(Reference Mohammadifard, Nazem and Naderi15–Reference Erfani, Ghavami and Shoeibi18). On the other hand, based on researches, the butyrate of butter could induce insulin sensitivity, and also the conjugated linoleic acid of that has beneficial impacts on metabolic illnesses(Reference Gao, Yin and Zhang19,Reference Malinska, Hüttl and Oliyarnyk20) . Additionally, it is evident that excessive consumption of TFA leads to notable hepatic steatosis, characterised by an increase in hepatic lipogenic gene expressions, heightened influx of free fatty acids into the liver and the accumulation of lipid peroxide(Reference Obara, Fukushima and Ueno21). The lipidomic properties involve the hepatic accumulation of TFA and a reduction in arachidonic acid content. These lipid species, including TFA, alongside their potential to induce local cytokines by Kupffer cells, may play a crucial role in the commencement and development of NAFLD(Reference Obara, Fukushima and Ueno21).
Rapeseed oil has the lowest amount of SFA (7·1 % of fatty acid content) and the highest concentration of n-3 fatty acid and MUFA (61 % oleic acid, 21 % linoleic acid and 11 % linolenic acid) of all the oils that are most popular in the USA(Reference Konuskana, Arsalan and Oksuz22). According to the myriad recommendations by public health organisations like the American Heart Association and National Cholesterol Education Program, which advised limiting the consumption of SFA and TFA, one way to meet this recommendation is to lower the consumption of oils that are rich in SFA(Reference Grundy23–Reference Johnson, Keast and Kris-Etherton25). Given the mentioned details, rapeseed has the most tremendous potential to reduce SFA usage by substituting oils in the diet(Reference Johnson, Keast and Kris-Etherton25). Considering the crucial influence of the gut microbiota in the development of fatty liver disease, altering the gut microbiota through nutritional supplements like probiotics or prebiotics, n-3 PUFA or other functional foods as complementary therapies can potentially reverse metabolic disorders associated with NAFLD(Reference Ji, Yin and Sun26,Reference Ma, Zhou and Li27) . Consequently, reports indicate simultaneous positive effects of a plant oil rich in n-3 and a prebiotic (like rapeseed oil that is rich in sinapine and n-3) in individuals with NAFLD(Reference Kavyani, Saleh-Ghadimi and Dehghan28). Furthermore, certain studies have indicated that the consumption of oils abundant in n-3, such as Camelina sativa oil or rapeseed oil, may enhance glycaemic control, alleviate inflammation and reduce oxidative stress in individuals with NAFLD(Reference Musazadeh, Dehghan and Saleh-Ghadimi29). Numerous curative agents have been attempted for the management of NAFLD; in any case, compelling treatment is still unavailable. Also, many physicians are attracted by using natural products to alleviate this very common liver disease, due to their safety, large availability and low cost, as evident in a lot of literature data(Reference Tarantino, Balsano and Santini30). Although researchers recognise the significance of oils in the diet, there is inadequate testimony regarding substituting ghee with rapeseed oil in the management of NAFLD. Starting from this background, the aim of this trial is to assess the impacts of substituting ghee with rapeseed oil on liver steatosis and enzymes, lipid profile, glycaemic variables and anthropometric measurements in patients with NAFLD.
Materials and methods
Recruitment and eligibility screening
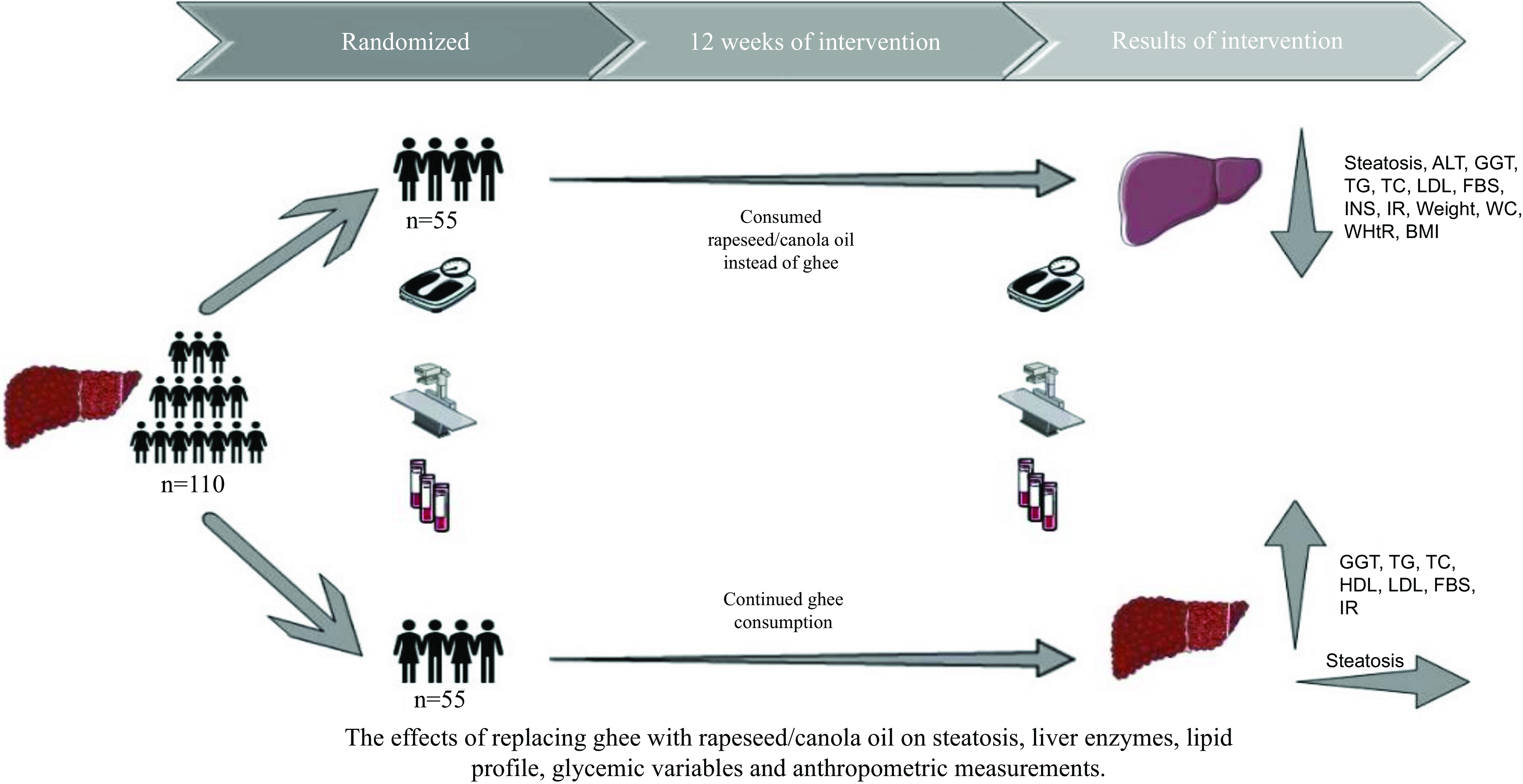
This parallel randomised controlled trial was conducted with the objective of studying the impact of substituting ghee with rapeseed oil for a period of 3 months on the outcomes of NAFLD. The primary objectives of this study involved assessing liver function including both fatty liver and liver enzyme levels. Additionally, secondary objectives encompassed the evaluation of glycaemic variables, lipid profile and anthropometric measurements. The procedure of the survey was approved by the Ethics Committee at the Urmia University of Medical Sciences and was registered at the Iranian Registry of Clinical Trials website (www.irct.ir) (IRCT20170206032417N5). The sample size was established based on the trial of Nigam et al. and the mean change of Homeostatic Model Assessment for Insulin Resistance (effect size = 1·3), and the 1-
![]() ${\raise0.7ex\hbox{$\alpha $} \!\mathord{\left/ {\vphantom {\alpha 2}}\right.}\!\lower0.7ex\hbox{$2$}}$
and 1-β were considered equal to 1·96 and 0·84, respectively(Reference Nigam, Bhatt and Misra31). The equation used to estimate the sample size was as follows:
${\raise0.7ex\hbox{$\alpha $} \!\mathord{\left/ {\vphantom {\alpha 2}}\right.}\!\lower0.7ex\hbox{$2$}}$
and 1-β were considered equal to 1·96 and 0·84, respectively(Reference Nigam, Bhatt and Misra31). The equation used to estimate the sample size was as follows:
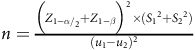 $n = {{{{\Big( {{Z_{{{{1 - \alpha }} {\left/ {\vphantom {{1 - \alpha } 2}}\right.}_{2}}}} + {Z_{1 - \beta }}} \Big)}^2} \times \left( {{S_1}^2 + {S_2}^2} \right)} \over {{{\left( {{u_1} - {u_2}} \right)}^2}}}$
. The timeframe was chosen based on earlier research that demonstrated the favourable impacts of the Dash diet on patients with NAFLD(Reference Razavi Zade, Telkabadi and Bahmani32). The study included 125 adult patients detected with NAFLD referred to the gastrointestinal and liver clinic in Imam Khomeini University Hospital in Urmia, Iran, during the months of February and August 2022, in order to recruitment in the trial. NAFLD was diagnosed by a gastroenterology and liver specialist via ultrasonography showing fatty liver, without another type of liver disease. All NAFLD patients were invited to contribute in the study and were enrolled if they satisfied the eligibility criteria and consented to take part. Before entry into the study, each patient was requested to provide written informed consent. The usual treatment in these patients was using pioglitazone and vitamin E medications, which was kept constant throughout the study. At the beginning of the study, people were selected who were consuming three to eight servings of ghee daily, and 5 g ghee was considered as a serving. As well as the usual treatment, the control group’s patients were instructed to keep up their ghee intake in the same amount. Also, in the intervention group, in parallel with the usual treatment, participants were recommended to change their consumed ghee to rapeseed oil in the same amount. To be included, participants had to be older than 18 years from both sexes, have first the visit to the hospital for NAFLD, consume three to eight servings of ghee daily and have a BMI under 30 kg/m2 with steatosis grade of 2 or 3. Patients with being on a particular diet, viral hepatitis, diabetes mellitus, psychiatric conditions, untreated hypothyroidism, kidney disease, heart disorders, bone disease, gastrointestinal illnesses (like celiac) and α-1 antitrypsin deficiency; drinking alcoholic beverages; with failure to adhere to our recommendations; taking herbal medicines, non-steroidal anti-inflammatory drugs, cholesterol-lowering drugs, barbiturates and antiepileptic drug; who were pregnant, breast-feeding and menopause women; and who were smokers were not included in the research. The research was conducted with a group of 110 participants, consisting of seventy men (thirty-five participants were allocated to each group) and forty women (twenty participants were allocated to each group). The stratified block randomisation was designed by an independent statistics specialist based on the steatosis grade, sex and age. The intervention and control groups were formed through a random allocation process of the patients by a blinded person. The block randomisation method was employed to randomise the participants, ensuring homogenised individuals allocate to each group. The laboratory personnel, radiologists and statisticians were blinded to the group allocation until the completion of the research. For controlling the intake of other foods, participants of the pair of groups were asked to pursue the guidelines provided by the FAO for Iranian(33). Initially, data related to sex, age, level of education, physical activity, energy intake, drug and supplement type and dosage, plant-based medicine, income, past chronic medical condition and familial history of NAFLD were obtained through a general questionnaire. In addition, anthropometric measurements and ultrasound imaging were conducted at the beginning and conclusion of the research. Participants were followed up by telephone weekly, and essential suggestions were made.
$n = {{{{\Big( {{Z_{{{{1 - \alpha }} {\left/ {\vphantom {{1 - \alpha } 2}}\right.}_{2}}}} + {Z_{1 - \beta }}} \Big)}^2} \times \left( {{S_1}^2 + {S_2}^2} \right)} \over {{{\left( {{u_1} - {u_2}} \right)}^2}}}$
. The timeframe was chosen based on earlier research that demonstrated the favourable impacts of the Dash diet on patients with NAFLD(Reference Razavi Zade, Telkabadi and Bahmani32). The study included 125 adult patients detected with NAFLD referred to the gastrointestinal and liver clinic in Imam Khomeini University Hospital in Urmia, Iran, during the months of February and August 2022, in order to recruitment in the trial. NAFLD was diagnosed by a gastroenterology and liver specialist via ultrasonography showing fatty liver, without another type of liver disease. All NAFLD patients were invited to contribute in the study and were enrolled if they satisfied the eligibility criteria and consented to take part. Before entry into the study, each patient was requested to provide written informed consent. The usual treatment in these patients was using pioglitazone and vitamin E medications, which was kept constant throughout the study. At the beginning of the study, people were selected who were consuming three to eight servings of ghee daily, and 5 g ghee was considered as a serving. As well as the usual treatment, the control group’s patients were instructed to keep up their ghee intake in the same amount. Also, in the intervention group, in parallel with the usual treatment, participants were recommended to change their consumed ghee to rapeseed oil in the same amount. To be included, participants had to be older than 18 years from both sexes, have first the visit to the hospital for NAFLD, consume three to eight servings of ghee daily and have a BMI under 30 kg/m2 with steatosis grade of 2 or 3. Patients with being on a particular diet, viral hepatitis, diabetes mellitus, psychiatric conditions, untreated hypothyroidism, kidney disease, heart disorders, bone disease, gastrointestinal illnesses (like celiac) and α-1 antitrypsin deficiency; drinking alcoholic beverages; with failure to adhere to our recommendations; taking herbal medicines, non-steroidal anti-inflammatory drugs, cholesterol-lowering drugs, barbiturates and antiepileptic drug; who were pregnant, breast-feeding and menopause women; and who were smokers were not included in the research. The research was conducted with a group of 110 participants, consisting of seventy men (thirty-five participants were allocated to each group) and forty women (twenty participants were allocated to each group). The stratified block randomisation was designed by an independent statistics specialist based on the steatosis grade, sex and age. The intervention and control groups were formed through a random allocation process of the patients by a blinded person. The block randomisation method was employed to randomise the participants, ensuring homogenised individuals allocate to each group. The laboratory personnel, radiologists and statisticians were blinded to the group allocation until the completion of the research. For controlling the intake of other foods, participants of the pair of groups were asked to pursue the guidelines provided by the FAO for Iranian(33). Initially, data related to sex, age, level of education, physical activity, energy intake, drug and supplement type and dosage, plant-based medicine, income, past chronic medical condition and familial history of NAFLD were obtained through a general questionnaire. In addition, anthropometric measurements and ultrasound imaging were conducted at the beginning and conclusion of the research. Participants were followed up by telephone weekly, and essential suggestions were made.
Biochemical measurements
At the start and end of the survey, after an overnight fast, patients got 5 mm of venous blood specimen drawn to execute biochemical assessments. Blood samples were subjected to centrifugation at a rate of 4000 rpm for a duration of 10 min. The resulting serum samples were then stored at a temperature of –80°C until biochemical examination. ELISA kits (Pars Azmoon Co.) were used to estimate serum fasting insulin levels. The analysis of the liver enzymes (alanine aminotransferase (ALT), aspartate aminotransferase, alkaline phosphatase (ALP) and γ-glutamyl transferase), lipid profile (total cholesterol, TAG, LDL-cholesterol and HDL-cholesterol) and fasting blood glucose (FBS) levels was conducted using BT1500 autoanalyzer (Biotecnica Instruments SpA). The suggested formulas were utilised to compute Homeostatic Model Assessment for Insulin Resistance and also to calculate Quantitative Insulin-Sensitivity Check Index (QUICKI)(Reference Sharma and Fleming34,Reference Katz, Nambi and Mather35) . As mentioned earlier, we conducted measurements for liver enzymes, lipid profile, FBS and insulin levels. Additionally, Homeostatic Model Assessment for Insulin Resistance and Quantitative Insulin-Sensitivity Check Index were calculated using the respective formulas.
Liver ultrasonography
At the beginning and end of the study, the assessment of fatty liver grade was performed by abdominal ultrasonography by a single operator and one expert radiologist (Siemens ACUSON S2000 Siemens Healthcare) with patients in a fasted state, before and end of the research project. NAFLD was identified based on the existence of a sonographic pattern in accordance with the subsequent criteria: including liver–kidney echo discrepancy, attenuated echo penetration, visibility of the diaphragm and narrowing of the lumen of the hepatic veins, as observed on ultrasonography. Fatty liver was further classified into normal, grades 1, 2 and 3, following the modified criteria outlined by Kurtz et al (Reference Kurtz, Dubbins and Rubin36). Although this imaging technique is low-cost and well-accepted, nevertheless, the case of diagnosing mild steatosis and steatosis in obese patients has low performance and sensitivity. As a result, patients with grade 1 NAFLD and those with a BMI >30 were not included in this study.
Anthropometric measurements
The measurements of height and weight were taken through the utilisation of digital scale and stadiometer with a precision of 0·1 cm and 100 gr, respectively, at baseline and week 12(Reference Casadei and Kiel37). While measuring, the participants were with negligible clothes and no footwear. The following formula was used to calculate the BMI: kg/m2; in this equation, kg represents the individual’s weight in kilograms and m2 is the square of their height in metres. The measurement of waist circumference (WC) was made just after the patient breathed out, by placing a flexible tape between the hip bones and the lowest rib. It was ensured that the tape was horizontal around the waist and did not compress the skin. The waist:height ratio is determined by dividing the waist measurement by the height measurement, both expressed in centimetres. For reliability, the measurements were conducted triplicate, and the mean of the three readings was employed.
As outlined previously, we obtained measurements for WC and height. Additionally, we calculated the BMI and waist:height ratio using the appropriate formula.
Dietary intake and physical activity assessment
To evaluate the consumption of rapeseed oil and ghee and the usage of other food groups, including cereal, dairy products, vegetables, fruit, grains, meat and sugar, before intervention and each month, four 3 d 24-h recalls (one weekend day and two non-consecutive week days) 12 d in total were performed. The metabolic equivalent of task questionnaire was used to evaluate the physical activities before intervention and each month(Reference Ainsworth, Haskell and Whitt38).
Primary and secondary outcomes
The principal purposes of our investigation were to evaluate the liver function including the levels of liver enzymes in the blood and the degree of liver steatosis as primary outcomes. Additionally, we examined the serum concentration of lipid profile, glycaemic variables and anthropometric measurements as secondary outcomes.
Statistical analysis
The statistical analysis was conducted using SPSS software version 26 (IBM Corp. IBM SPSS Statistics for Windows), and the threshold for accepting the statistical significance of the results was established as P value < 0·05. The homogeneity of individuals before and after the study remained relatively unchanged, and as a result, we conducted the analysis based on the protocol analysis. General characteristics between control and intervention groups before intervention were compared by independent sample t test for quantitative and χ 2 for qualitative variables and were reported as mean ± sd and frequency (%), respectively. To compare the differences within groups, the paired samples t tests were applied. Using the Kolmogorov–Smirnov test, we assessed the normality of the continuous values. To analyse the changes in dietary intakes and metabolic equivalent of task at baseline, 1st, 2nd and 3rd months, repeated-measures ANOVA was used. To evaluate the effects of replacing ghee with rapeseed oil on serum levels of lipid profile, liver enzymes, glycaemic index and anthropometric measurements, we applied ANCOVA test by adjusting weight changes and baseline value of the outcome. Ordinal generalised linear models were employed to evaluate changes in the severity of fatty liver (without change, aggravation or improvement) within the study population over the course of study.
Results
A random assignment process was used to divide 125 individuals into two groups. Following randomisation, fifteen participants were removed from the study because of not meeting inclusion criteria (n 5) and lost to follow-up (n 10). In total, the study was completed with 110 patients: the intervention group (n 55) and the control group (n 55) (Fig. 1). The age range of the patients was between 18 and 67 years. Regarding basic characteristics, no statistically significant variations were noted between the groups (Table 1). According to the 3 d 24-h dietary recalls obtained during the intervention, no significant differences were seen between the groups in terms of energy intake and food groups (Fig. 2). Using the metabolic equivalent of task questionnaire, the results did not indicate any significant differences between control and intervention groups (Fig. 2). Furthermore, Fig. 3 illustrates the composition of oils consumed by patients both before and during the study. As shown in Fig. 3, ghee and rapeseed consumption between the control and intervention group was significantly different (P group = < 0·001, P time = < 0·001, P group × time = < 0·001). About the other oil consumption, there was no significant difference between groups, before and during the study (P group = 0·075, P time = 0·231, P group × time = 0·652).

Fig. 1. The flow chart of study participants based on the CONSORT guidelines.
Table 1. Basic characteristics of individuals with non-alcoholic fatty liver disease (Mean values and standard deviations; frequencies and percentages)

Values are mean values and standard deviations for continuous variables and frequency (%) for categorical variables.
* P values were calculated by independent sample t test for continuous and χ 2 for categorical variables.

Fig. 2. Changes in dietary intake and physical activity of the individuals during the 12 weeks. The P values demonstrate the effect of group, time and time × group interaction (computed through the general linear model ANOVA for repeated measurements). MET, metabolic equivalent of task.

Fig. 3. Changes in the content of consumed oil by the individuals during the 12 weeks. The P values demonstrate the effect of group, time, and time × group interaction (computed through the general linear model ANOVA for repeated measurements).
Primary outcomes
The study concluded with a significant reduction in the serum levels of ALT (P = 0·014) and γ-glutamyl transferase (P = 0·024) in contrast with the control group. Adjustments for the impacts of the baseline value of the outcome and mean weight change did not affect the results. With regard to aspartate aminotransferase, there was a significant reduction in the intervention group in comparison with the control group only subsequent to adjusting for the baseline value of the outcome (P < 0·001). Regarding ALP, there was a significant decrease in the control in comparison with the intervention group (0·006) (Table 2). The intervention group demonstrated a significant reduction in grades of fatty liver in comparison with the control group (P < 0·001). The intervention group showed a reduction in the grade of steatosis in 41·81 % of patients, significantly (P < 0·001) (Table 3). Despite adjusting for the baseline value of the outcome and mean change in weight, statistically significant differences between the intervention and control group were still observed (P = 0·03) (Table 3).
Table 2. Changes in liver enzymes, lipid profiles, glycaemic variables and anthropometric measurements during the 12-week study in patients with NAFLD in the groups (n 55) (Mean values and standard deviations)

* Calculated using independent sample t test.
† Calculated using ANCOVA, adjusted for baseline value of the outcome.
‡ Calculated using ANCOVA, adjusted for baseline value of outcome and mean change in weight.
§ To change the measurement of total cholesterol in mg/dl to mmol/l, multiply the value by 0·0259. To change TAG in mg/dl to mmol/l, multiply the value by 0·0113. To change FBS in mg/dl to mmol/l, multiply the value by 0·0555. NAFLD, non-alcoholic fatty liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; FBS, fasting blood sugar; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; QUICKI, Quantitative Insulin Sensitivity Check Index; WHtR, waist:height ratio.
|| Calculated using paired sample t test.
Table 3. Comparison of liver steatosis grades assessed by ultrasound before and after the intervention in patients with non-alcoholic fatty liver disease in both the intervention and control groups for 12 weeks* (n 55) (Numbers and percentages)

* There were no significant variations between intervention and control group based on χ 2 test, with regard to baseline grades of liver steatosis (P = 0·589). P 1 was calculated by χ 2 test to compare fatty liver grades. P 2 was calculated by generalised linear models test after adjusting for baseline value of outcome. P 3 was calculated by generalised linear models test after more adjusting for mean change in weight. P 4 was calculated by generalised linear models test after more adjusting for mean change in weight and baseline value of outcome.
† Based on the χ 2 test, significant differences were observed between groups with regard to changes in grades of fatty liver steatosis. On the basis of generalised linear models test, there were no significant differences between groups with regard to changes in grades of fatty liver steatosis after adjusting for baseline value of the outcome (P = 0·057) and adjusting for mean change in weight (P = 0·144), but there were significant differences after adjusting for baseline value of the outcome and mean change in weight (P = 0·03).
Secondary outcomes
With regard to the impacts of the intervention on lipid profile, the intervention group compared with the control group had lower concentrations of TAG (P < 0·001), total cholesterol (P < 0·001) and LDL (P = 0·001), even after adjusting for the initial value of the outcome and mean change in weight (Table 2). In the matter of serum concentration of HDL, the results showed a significant reduction in intervention group at the end of the trial in male patients (0·04) but not in female individuals (0·085). Also, in male patients, there was a significant reduction in HDL levels after adjustment for baseline value of the outcome (0·005) (Table 4).
Table 4. Changes in HDL and WC during the 12-week study in patients (male and female) with NAFLD in the groups (n 55) (Mean values and standard deviations)

NAFLD, non-alcoholic fatty liver disease; WC, waist circumference.
* The difference between groups calculated using independent sample t test.
† The mean change difference between groups calculated using ANCOVA, adjusted for baseline value of the outcome.
‡ The mean change difference between groups calculated using ANCOVA, adjusted for baseline value of outcome and mean change in weight.
§ Within group changes calculated using paired samples t test.
On the subject of glycaemic variables, the intervention in contrast with the control group showed a lower serum level of FBS (P < 0·001), insulin (P < 0·001), Homeostatic Model Assessment for Insulin Resistance (P < 0·001) and higher level of Quantitative Insulin-Sensitivity Check Index (P < 0·001). Adjustments for the effects of baseline value of the outcome and mean change in weight did not change the results (Table 2).
In anthropometric measurements, the intervention group in comparison with the control group showed a significant decrease in weight (P < 0·001), BMI (P < 0·001) and waist:height ratio (P < 0·001). These significant differences were still observed even after adjusting for the baseline values (Table 2). About the WC, there was a significant reduction in WC in male and female patients even after adjustment for baseline value of the outcome (< 0·001) (Table 4).
Discussion
To date, no prior investigation has studied the potential impact of substituting ghee with rapeseed oil on clinical parameters of individuals diagnosed with NAFLD. Our study demonstrated that substituting ghee with rapeseed oil through 12 weeks resulted in significant improvement in the severity of steatosis, some of liver enzyme levels, lipid profile, glycaemic variables and anthropometric measurements. Significant variations in liver steatosis were seen among the groups, even subsequent to adjustments made with covariates. These findings showed that rapeseed oil, by itself and independent to any associated weight loss and baseline value of outcome, contributed to the significant improvements in the outcomes as mentioned earlier. Currently, there is insufficient information available regarding the impact of rapeseed oil on hepatic steatosis in individuals with NAFLD.
In a randomised, parallel, open-label design study by Nigam et al.(Reference Nigam, Bhatt and Misra31), it was found that rapeseed oil improved hepatic steatosis significantly. Results from a study by Li et al.(Reference Li, Li and Su39) strongly confirm the fact that consuming sinapine, as a prebiotic agent of rapeseed oil, could prevent IR and NAFLD. In a separate study conducted by Li et al., consistent with our findings, the results indicated that sinapine could serve as a prebiotic, enhancing the nutritional properties of vegetable oils and potentially preventing obesity-related chronic diseases, including NAFLD(Reference Li, Li and Cao40). As previously noted, rapeseed oil is primarily comprising MUFA, with reduced amounts of SFA. Additionally, it contains higher amounts of PUFA, which comprise α-linolenic acid and linoleic acid. This efficacy can at least be mainly attributed to α-linolenic acid regulation of molecular mechanisms involved in the metabolism of lipids in the liver. Specifically, this can be attributed to the effects of the α-linolenic acid on increasing the DNA binding of PPARα and reducing the DNA binding of sterol regulatory element-binding protein 1c, which are transcription factors that play a role in lipid oxidation and synthesis, respectively(Reference Foretz, Guichard and Ferré41,Reference Marx, Duez and Fruchart42) . However, sinapine is a crucial factor in preventing the initiation of obesity and inflammation induced by a diet. This is achieved by modulating the composition of the intestinal microflora (decrease in the ratio of Firmicutes:Bacteroidetes and augmented presence of probiotics, such as Lactobacillaceae, Akkermansiaceae and Blautia), which produces SCFA and ultimately inhibits NAFLD. Additionally, the SCFA/G protein-coupled receptor 43 pathway seems crucial for inhibiting inflammation and NAFLD by gut microbes(Reference Li, Li and Su39). Additionally, recent research has indicated that n-3 supplementation may lead to a reduction in serum levels of inflammatory markers such as TNF-α, IL-6 and CRP. This, in turn, has the potential to ameliorate conditions associated with chronic inflammation, including NAFLD(Reference Kavyani, Musazadeh and Fathi43). Moreover, the results of the current research indicated that the intake of rapeseed oil improved liver enzymes such as ALT, aspartate aminotransferase and γ-glutamyl transferase and increased ALP levels. In a report from Capanni et al.(Reference Capanni, Calella and Biagini44), supplementation of n-3 PUFA (EPA and DHA in the ratio of 0·9:1·5, respectively) was inversely related to aspartate aminotransferase, ALT, γ-glutamyl transferase, TAG and FBS. Consistent with our trial, contemporary studies have demonstrated that the intervention of n-3 PUFA results in the improvement of liver enzymes and reduction in liver fat(Reference Musazadeh, Karimi and Malekahmadi45). In line with the current trial, Hasan et al.(Reference Hasan, Tamanna and Haque46) revealed that rapeseed oil could significantly increase the ALP levels. Similarly, an increase in ALP was found after supplementation of rapeseed oil in Wistar rats(Reference Sharif, Tamanna and Mosaib47). An elevation in the amount of ALP is indicative of liver cell damage, intrahepatic cholestasis or infiltrative liver disease. It is assumed that an increase in the mentioned enzyme may be due to the presence of erucic acid in rapeseed oil; nonetheless, further investigations are needed.
In the current study, we observed a reduction in fasting blood sugar and insulin concentration following the consumption of rapeseed oil. A survey of the health benefits of MUFAs by Gillingham et al.(Reference Gillingham, Harris-Janz and Jones48) disclosed that MUFA could modulate insulin sensitivity and glycaemic variables when substituted for SFA. The study accomplished by Södergren et al.(Reference Södergren, Gustafsson and Basu49) showed that a diet based on rapeseed oil yielded reduced levels of fasting plasma glucose as contrasted with a SFA-rich diet. However, the levels of fasting insulin were not significantly different between the two diets. According to the findings of Gustafsson et al.(Reference Gustafsson, Vessby and Ohrvall50), a diet based on rapeseed oil led to a reduction of FBS levels by 6 % when compared with diet containing more than 15 % SFA. However, previous studies have shown that replacing saturated fats with MUFA and PUFA could decrease blood lipid levels and had positive effects on glucose and insulin homoeostasis(Reference Imamura, Micha and Wu51,Reference De Lorgeril, Salen and Martin52) . The underlying mechanism for this phenomenon may involve the amelioration of postprandial TAG and glucagon-like peptide-1 responses in individuals with IR, as well as the up-regulation of GLUT-2 expression in the liver. As referred to earlier, a study has shown that the intake of MUFA could lower blood TAG levels through two mechanisms: activation of PPARα and reduction in sterol regulatory element-binding protein. MUFA can activate both PPARα and PPARγ, leading to an elevation in lipid oxidation and a reduction in IR, which can ultimately reduce the occurrence of hepatic steatosis(Reference Soriguer, Morcillo and Cardona53). An additional mechanism may be associated with the up-regulation of G protein-coupled receptor 43 induced by sinapine, which leads to the production of SCFA that can inhibit inflammation in the intestines. These actions serve to prevent IR in adipose tissue.
In the present clinical trial, the intake of rapeseed oil caused a significant reduction in lipid profile. Conversely, the control group displayed an increase in lipid profiles upon consumption of ghee. In line with present study, Amiri et al.(Reference Amiri, Raeisi-Dehkordi and Sarrafzadegan54) conducted a meta-analysis and demonstrated that rapeseed oil intake was associated with improvements in total cholesterol, TAG and LDL-cholesterol, as well as a decrease in HDL-cholesterol levels. Earlier, Engel et al.(Reference Engel and Tholstrup55) observed that consuming butter fat/ghee, when compared with the impacts of olive oil intake and a habitual diet, resulted in increased levels of total cholesterol, LDL-cholesterol and HDL-cholesterol. The underlying mechanisms responsible for rapeseed oil’s ability to lower cholesterol levels may be attributed to its high content of MUFA, phytosterols and stanols. Rapeseed oil’s phytosterols and stanols have been found to reduce concentrations of LDL-cholesterol(Reference Heggen, Granlund and Pedersen56). These non-lipid constituents impede cholesterol metabolism by their structural resemblance to cholesterol, thereby curtailing cholesterol absorption in the intestine and inhibiting cholesterol esterase enzymes(Reference Salar, Faghih and Pishdad57). Moreover, MUFA has been shown to enhance insulin and lipoprotein lipase activity, ultimately leading to decreased levels of TAG(Reference Salar, Faghih and Pishdad57).
Our study revealed that the intervention group exhibited significant improvements in anthropometric measurements. Based on the findings of Dehkordi et al.’s(Reference Raeisi-Dehkordi, Amiri and Humphries58) meta-analysis, it has been demonstrated that the intake of rapeseed oil leads to a significant loss of weight. Furthermore, subgroup analysis disclosed that rapeseed oil intake led to a decrease in WC compared with a typical diet. Previous research has established that the storage and oxidation properties of fatty acids play a pivotal role in the controlling body weight(Reference Raeisi-Dehkordi, Amiri and Humphries58). n-3 fatty acids are efficacious in treating obesity and have the capacity to regulate the proliferation, differentiation and apoptosis of adipocytes(Reference Martínez-Fernández, Laiglesia and Huerta59). Moreover, it is suggested that PUFA may contribute to weight loss by modulating the gene expression that promotes oxidation in adipose tissue, liver and other organs, leading to lower fat storage(Reference Buckley and Howe60). Additionally, rapeseed oil has been shown to enhance the sense of satiety and decrease hunger by stimulating the secretion of cholecystokinin, which has a satiating effect on the ileum(Reference Maljaars, Romeyn and Haddeman61). The passage highlights the nutritional qualities of rapeseed oil, emphasising its significance as a source of n-3 fatty acids with a favourable n-6 : n-3 ratio (2:1). Additionally, it underscores the substantial polyphenol content in rapeseed oil, particularly sinapine and sinapic acid, known for their diverse physiological functions such as antioxidative, anti-tumour and hypoglycaemic properties. The suggestion is that these polyphenols, especially sinapine and sinapic acid, might contribute to improving glucose and lipid metabolism disorders as well as IR in individuals with NAFLD. Furthermore, the anti-inflammatory attributes of rapeseed polyphenols are proposed to be linked to SCFA through the regulation of intestinal flora(Reference Li, Li and Su39). Consistent with our results, Musazadeh et al. in a study indicated that adding Camellia sativa oil, which is abundant in n-3 like rapeseed oil, may lead to reductions in anthropometric measurements such as weight, BMI, waist:hip ratio and WC, as well as ALT levels and lipid profile levels (excluding HDL levels). Additionally, in line with our results, Farhangi et al. in a trial showed that the combination of Camellia sativa oil with resistant dextrin and an energy-restricted diet resulted in decreased weight, BMI, liver enzymes and lipid profile among patients with NAFLD(Reference Musazadeh, Dehghan and Khoshbaten62,Reference Farhangi, Dehghan and Musazadeh63) .
The study’s main advantage is that using rapeseed/rapeseed oil as a substitute for ghee or other oils high in SFA is an affordable approach to managing NAFLD. A limitation of the trial is that the study’s diagnostic method for fatty liver grade relied on liver ultrasonography, which is low-cost, non-invasive and widely available. Second, the study could not be blinded due to its design, and the intervention duration was short.
In conclusion, this randomised controlled trial demonstrated that replacing ghee with rapeseed oil improved NAFLD symptoms and could potentially benefit metabolic disorders. However, additional clinical trials with increased sample sizes and extended intervention periods are required. These trials would provide more accurate and reliable proof to endorse the consumption of rapeseed oil for improving health outcomes.
Acknowledgements
There is no acknowledgement.
This trial was supported by the Urmia University of Medical Sciences.
The authors’ responsibilities were as follows: M. A. and F. M. S. conceived and designed the study and analysed the data, M. M. H provided material and technical support, F. M. S. wrote the manuscript, M. A. critically revised the manuscript for important intellectual content and M. A. had primary responsibility. All authors read and approved the final manuscript.
There are no conflicts of interest to declare.