INTRODUCTION
Q fever is a globally occurring zoonosis caused by Coxiella burnetii, an intracellular Gram-negative bacterium. The main reservoirs for Q fever in humans are domestic ruminants, such as cattle, goats and sheep. Transmission to humans mainly occurs through inhalation of contaminated aerosols, released when infected animals give birth [Reference Maurin and Raoult1].
From 2007 to 2010, The Netherlands faced increasingly large annual and seasonal outbreaks of Q fever. More than 4000 acute human Q fever cases were notified, making this the largest recorded Q fever epidemic worldwide to date [Reference Van der Hoek2]. Although no outbreaks of Q fever had been reported in The Netherlands prior to 2007, historical data suggest high endemicity in preceding decades. In sera of blood donors from various regions of The Netherlands in 1968 and 1983, high seroprevalences were found [Reference Richardus3], significantly higher than the 2·4% seroprevalence in the general population of The Netherlands in 2006–2007, just prior to the first Q fever outbreak [Reference Schimmer4].
However, comparison of findings from different surveys may be problematic, due to discrepancies in the populations surveyed and the laboratory methods used. For humans, population-based seroprevalence studies routinely measure IgG phase II antibodies. Indirect immunofluorescence assay (IFA) is regarded as the reference test for serological detection of Q fever. However, performing IFA is labour intensive and there is no uniform consensus for the cut-off titres. For large studies, ELISA is a convenient test as it can be easily automated. However, ELISA may be less sensitive than IFA [Reference Blaauw5].
We analysed the seroprevalence dynamics of Q fever before and during the recent Dutch epidemic. We used historical and recently acquired seroprevalence data, taking into account the dilemma of using different diagnostic tests and cut-off definitions.
MATERIALS AND METHODS
Study design and study area
We performed a cross-sectional study using historical samples from 1987, analysed with a commercial IFA. The results were compared with historical results from 1983 to assess the accuracy of the historical data. Furthermore, the results were compared with recently acquired data from 2008 and 2010. All samples and data were collected from the same geographical area, i.e. South Limburg, the most southern part of the province of Limburg, The Netherlands (Fig. 1).

Fig. 1. Map of South Limberg region with the municipality of Maastricht highlighted.
This region had 606 000 inhabitants in 2014 [data from Statistics Netherlands (CBS)] combining both urban and rural areas. With 917 population/km2, it is one of the most densely populated regions of The Netherlands. The largest cities in the region are Maastricht, Heerlen and Sittard. In 2009, the South Limburg region faced a large outbreak of Q fever around the municipality of Voerendaal originating from a dairy goat farm [Reference Hackert6].
Study population
The samples for 1987 were available from the biobank of the Monitoring Project on Cardiovascular Risk Factors. This project was conducted in The Netherlands from 1987 to 1991. More than 36 000 persons aged 20–59 years were screened for cardiovascular risk factors, using questionnaires and blood samples [Reference Verschuren7]. The survey was conducted in the municipalities of Amsterdam, Doetinchem and Maastricht. Stored plasma samples from this survey were available for further research and had not been tested previously for Q fever or other infectious diseases. A random selection of plasma samples from 1987 was taken from the Maastricht subgroup. Assuming an estimated seroprevalence of 50% with a confidence level of 95%, 385 samples would be required for adequate power. The samples had been stored at –35 °C in the biobank at the centres for Nutrition and Health (CVG) and Prevention and Health Services Research (PZO) at the National Institute for Public Health and the Environment (RIVM). The use of the anonymous plasma samples was approved by the Scientific Advisory Group of the PZO.
For the 1987 samples, antibodies against C. burnetii were determined in plasma by IFA (Focus Diagnostics, USA) measuring IgG against C. burnetii phases I and II according to the manufacturer's instructions. Seropositivity was defined as an IgG phase II titre ⩾1:32. The plasma samples were analysed at RIVM.
Comparison populations
Data from 1983 were available from the Maastricht subgroup in a seroprevalence survey performed by Richardus et al. [Reference Richardus3]. In this survey, plasma samples from 248 not randomly selected blood donors were screened using an in-house developed IFA. Seroprevalence data for 2008 and 2010 were available from a survey that was conducted in 2010 in response to the large outbreak of Q fever in South Limburg [Reference Hackert6]. In this study, 847 samples from 2008 of adults vaccinated for healthcare-related hepatitis B risk and 633 samples from 2010 of persons attending the regional sexual health clinic from January to April 2010 were screened for antibodies against C. burnetii, using a commercial ELISA (Institute Virion/Serion GmbH, Germany). Positive and intermediate results were confirmed using an IFA (Fuller Laboratories, USA) at a cut-off value of 1:16, according to the manufacturer's instructions.
Statistical analysis
The samples for 1987, 2008 and 2010, were divided into the same five age groups that were used in the 1983 survey of Richardus et al. [Reference Richardus3]. For each year, the results were directly standardized for age group and gender, using demographic data for The Netherlands for the respective year as published by Statistics Netherlands. For the years 1983, 1987 and 2008 (the years without recent outbreaks), the seroprevalence figures by age were also visualized using error bar diagrams, representing the confidence interval (CI) of the mean seroprevalences. For data analysis SPSS Statistics v. 21 (IBM Corp., USA) was used.
RESULTS
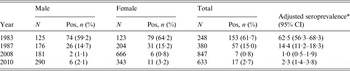
Of the 385 selected plasma samples from 1987, 380 were suitable for analysis. The samples were from 176 (46%) male and 204 (54%) female participants. Of the 380 samples, 57 tested positive for antibodies against C. burnetii. When standardized for age and gender, this resulted in a seroprevalence of 14·4% (95% CI 11·2–18·3).
The 1983 study included 125 (50%) male and 123 (50%) female participants [Reference Richardus3]. Of the 248 samples, 153 tested positive for antibodies against C. burnetii, resulting in an age- and sex-standardized seroprevalence of 62·5% (95% CI 56·3–68·3).
The 2008 samples included 181 (21%) male and 666 (79%) female participants. Of the 847 samples, seven samples tested positive for antibodies against C. burnetii, resulting in an age- and sex-standardized seroprevalence of 1·0% (95% CI 0·5–1·9).
The 2010 samples included 290 male (46%) and 343 (54%) female participants. Seventeen of the 633 samples tested positive for antibodies against C. burnetii, resulting in an age- and sex- standardized seroprevalence of 2·3% (95% CI 1·4–3·8).
Table 1 shows the crude and standardized seroprevalence results for all four populations. None of the surveys had statistically significant differences in seroprevalence between age groups or between males and females. A graphical comparison of the seroprevalence estimates from 1983, 1987 and 2008 is shown in Figure 2. The seroprevalence estimates for 1987 are significantly lower than the seroprevalence estimates from 1983 across all age groups. The seroprevalence in 1987 was significantly higher than the seroprevalence in 2008 for all age groups, except the 20–24 years age group (Fig. 2). The difference in seroprevalence between 2010 and 2008 was not statistically significant.

Fig. 2. Comparison of seroprevalence of IgG phase II-specific antibodies to Coxiella burnetii (Q fever) in Maastricht in 1983, 1987, and 2008.
Table 1. Prevalence of IgG antibodies against phase II of Coxiella burnetii in samples from Maastricht and South Limburg
CI, Confidence interval.
* Seroprevalence standardized for age distribution and gender, based on population data from Statistics Netherlands (CBS).
DISCUSSION
The present study, using stored samples from 1987, confirms that seroprevalence of antibodies against C. burnetii was high in South Limburg in the 1980s, even though no Q fever outbreaks were described in that period. Between 1987 and 2008, only three cases of acute Q fever were notified in South Limburg (1999, 2002, 2007). During this period, the seroprevalence declined in this geographical area. Since 2008 the number of notifications increased with a peak of 238 cases in 2009 (Table 2). We suggest that waning immunity in the population may have contributed to the size of the Q fever outbreak that occurred in South Limburg in 2009.
Table 2. Number of notifications of acute Q fever in the South Limburg region, 1988–2014
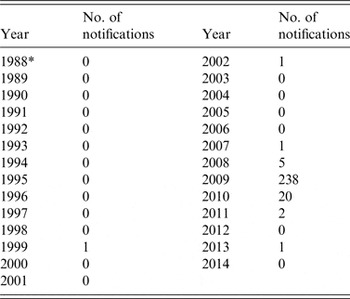
Data from RIVM-OSIRIS.
* Surveillance data for acute Q fever is only available from 1988 onwards.
In interpreting these findings, several limitations have to be considered. First, by comparing results from historical seroprevalence surveys, we must account for different methodologies and study populations. Original sera from the published 1980s’ seroprevalence studies were no longer available and the in-house IFA employed at the time could not be reproduced. Therefore, we tested historical samples from 1987 using a contemporary commercial IFA. We used a cut-off of 1:32, whereas a cut-off of 1:16 was used in the 1983 survey. Assuming that the performance of both IFAs was otherwise comparable, a lower cut-off with increased sensitivity and decreased specificity may have led to more false-positive results and an overestimation of seroprevalence in 1983. Moreover, the samples from 1983 and 1987 were collected in different ways. Whereas the samples from 1987 were (retrospectively) randomly selected, the 1983 specimens were selected such that each age group would include 50 samples. However, no significant differences were found in the age/gender distribution for both 1983 (P = 0·999) and 1987 (P = 0·856). The plasma samples we used for retrospective testing were stored for many years at a temperature of –35 °C. The effect of this temperature on the survival of antibodies is unknown, but degradation of antibodies could have led to underestimation of the true seroprevalence.
The low cut-off that was used in 1983 and the unknown test qualities of the in-house IFA, suggest that the 1983 seroprevalence of 60% might have been overestimated. However, the seroprevalence of 14·4% we found for 1987 is still markedly high. By contrast, during the Dutch Q fever epidemic, in high-incidence areas seroprevalence surveys in pregnant women in 2008, blood donors in 2009, and persons with risk factors for chronic Q fever found a mean seroprevalence of 9·1%, 12·2% and 10·7%, respectively [Reference Van der Hoek8–Reference Kampschreur10].
The seroprevalence estimate for 1987, based on the present analysis, is also significantly higher than the estimates based on samples from 2008 and 2010 from the same region. The samples from 2008 and 2010 were tested using a different IFA with a lower cut-off of 1:16, but this was according to the manufacturer's instructions. Further, the low seroprevalence estimates for 2008 and 2010 are comparable with the estimate in the study of Schimmer et al. [Reference Schimmer4], in which the IFA of Focus Diagnostics was used. In the Schimmer et al. study, the estimated seroprevalence increased from 1·5% to 2·4% after correction for false-negative ELISA results. Therefore, such a correction of the 2008 and 2010 results might have led to a small increase in seroprevalence.
We have no clear explanation for the high C. burnetii seroprevalence in the 1980s, without much clinical Q fever disease at the time. One possibility is that the high seroprevalence was due to exposure to a C. burnetii cattle genotype that might have been less virulent than the C. burnetii goat genotype that caused the large 2007–2010 epidemic in The Netherlands [Reference Tilburg11–Reference Dijkstra14].
Using PCR testing, DNA from the cattle C. burnetii genotype can be found in retail milk and a possible hypothesis is that a daily dose of fragments of C. burnetii in retail milk could cause a seroconversion and immunize people rather than making them ill. In this context it would be interesting to study milk consumption patterns over time and changes that have taken place in milk pasteurization practices that in certain years may have been insufficient to kill C. burnetii. Another hypothesis is the spreading of the less virulent C. burnetii strain by dairy cattle farms. In The Netherlands, the number of dairy cattle declined while the dairy goat sector expanded rapidly after introduction of the European milk quota system in 1984 [Reference Roest15]. From 1984 to 2008, the number of cattle in South Limburg did decrease from 73 366 to 36 011 (data from Statistics Netherlands). This could have resulted in a lower exposure to the cattle C. burnetii genotype.
As shown in animal experiments, antibodies against C. burnetii protect against the development of Q fever after exposure to viable C. burnetii, probably due to enhanced macrophage activity [Reference Humphres and Hinrichs16]. Therefore, the decreasing seroprevalence of antibodies against C. burnetii in the regional population of South Limburg could have played a role in the regional outbreak that occurred in the region in 2009. The question remains whether the results for South Limburg are representative for the entire Dutch population. The high seroprevalence found in 1983 in other regions of The Netherlands [Reference Richardus3] and the lack of differences in seroprevalence between regions in 2007 [Reference Schimmer4], suggest that seroprevalence decreased in the entire country. This could have made the entire Dutch population vulnerable for the almost nationwide outbreak of Q fever in the years 2007–2010.
Assuming a high risk for exposure and infection in the 1980s, followed by a period of low exposure, one would expect an age-cohort effect with higher seroprevalence in the older age groups, which indeed was demonstrated in a 2006/2007 survey [Reference Schimmer4]. The age-cohort effect is further supported by the lack of an effect of age in the seroprevalence studies that were performed in the 1980s including our survey from 1987.
An interesting comparison can be made with the situation in the United States, where a national survey showed a seroprevalence of 3·1%, indicating that millions of people have been exposed to C. burnetii [Reference Anderson17]. However, there are fewer than 100 notifications per year of human Q fever in the United States, suggesting gross underreporting and/or circulation of a less virulent (cattle) strain. Unfortunately, no historical seroprevalence results are known for the United States. For some European countries, historical data exist, as shown in Table 3. In some countries, a similar decrease in seroprevalence can be seen as for The Netherlands. However, the comparison of different seroprevalence surveys remains difficult, due to differences in the populations surveyed and developments in laboratory methods used since the 1980s. In contrast, in some high endemic areas, there seems to be an increase in seroprevalence.
Table 3. The seroprevalence of antibodies against Coxiella burnetii in other European countries

ih-IFA, in-house developed indirect immunofluorescence assay; cIFA, commercial IFA; ih-ELISA, in-house developed enzyme-linked immunosorbent assay; cELISA, commercial ELISA; CFT, complement fixation test.
* Source: J. W. Brockman et al., unpublished observations.
CONCLUSION
C. burnetii seroprevalence in South Limburg declined from the 1980s to 2008. This might indicate waning population immunity that could have contributed to the 2009 Q fever outbreak is this region. A similar declining trend in the seroprevalence for other regions in The Netherlands, could possibly have contributed to the 2007–2010 epidemic. For a better understanding of the infection dynamics of Q fever and other infectious diseases, longitudinal studies over a longer period or repeated seroprevalence surveys with standardized serological methods will be necessary. We advocate a large multi-country study, with analyses performed by one reference centre.
ACKNOWLEDGEMENTS
This study was financed from the regular budget of the Centre for Infectious Disease Control of The Netherlands. We thank the technicians, Marsha Hesp and Carla Nijhuis from the Centre for Infectious Disease Control for testing the plasma samples. We also thank Robert Jan de Klein and colleagues for assisting in collection of plasma samples.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.








