The association between deficiencies in central nervous system (CNS) serotonergic functioning and adult impulsive aggression, particularly among males, is well established, whether indexed as aggressive behaviour leading to incarceration, lifetime history of aggressive acts as measured by semi-structured interview, or self-ratings of dispositional aggression (see Reference Manuck, Kaplan, Lotrich and NelsonManuck et al, 2005a for a review). Results from a parallel body of research with non-human primates support this association (e.g. Reference Mehlman, Higley and FaucherMehlman et al, 1994; Reference Higley, Mehlman and HigleyHigley et al, 1996). Sociopathy is also associated with dysregulated serotonergic function (e.g. Reference O'Keane, Moloney and O'NeillO'Keane et al, 1992), although people with antisocial personality disorder do not always exhibit aggressive behaviour (i.e. antisocial personality disorder and impulsive aggression are not completely overlapping constructs).
In contrast to this large and consistent body of work, the evidence linking dysregulated serotonergic function and aggression among children is equivocal (Reference Kruesi, Rapoport and HamburgerKruesi et al, 1990; Reference Stoff, Pasatiempo and YeungStoff et al, 1992; Reference Castellanos, Elia and KruesiCastellanos et al, 1994; Halperin et al, Reference Halperin, Sharma and Siever1994, Reference Halperin, Newcorn and Schwartz1997; Reference Pine, Coplan and WassermanPine et al, 1997). One reason might be the exclusive focus on cross-sectional studies of childhood and early adolescence when aggression is temporally unstable (Reference MoffittMoffitt, 1993); longitudinal research that examines the neurobiological correlates of temporally persistent aggression might yield more consistent findings. In support of this view, one prospective study links lower 5-hydroxyindole acetic acid (5-HIAA) levels in the cerebrospinal fluid (CSF) of boys with disruptive behaviour disorders to aggression scores/arrests measured 2 years later (Reference Kruesi, Hibbs and ZahnKruesi et al, 1992). The current study capitalised on the unique opportunity to observe the prospective relationship between serotonergic function assessed in childhood and the emergence of antisocial personality disorder in late adolescence/young adulthood. We predicted that a lower prolactin response to fenfluramine in childhood would be associated with the development of antisocial personality disorder assessed 9 years later.
METHOD
Baseline evaluation
Between July 1990 and May 1997, 7– to 11-year-old children were referred by clinicians to a research programme examining the relationship between serotonergic function and aggression in children with disruptive behaviour disorders. Children were screened using the IOWA Conners Teacher Rating Scale (Reference Loney and MilichLoney & Milich, 1982). Subsequently, parents completed the Child Behavior Checklist (CBCL; Reference AchenbachAchenbach, 1991) and were interviewed with the Diagnostic Interview Schedule for Children (Reference Shaffer, Fisher and DulcanShaffer et al, 1996).
The current report is based on the total number of participants with attention-deficit hyperactivity disorder (ADHD) who had undergone the childhood evaluation including a fenfluramine challenge and who were later interviewed for identification of Axis II psychopathology between August 2001 and February 2005. The sample included 52 males and 6 females and represents 53% of the total number of children who were administered the fenfluramine challenge and were eligible for follow-up in February 2005 (n=110). Participation in the follow-up evaluation was not associated with gender, socio-economic status, age at initial evaluation, IQ, or parent and teacher ratings of psychopathology (P<0.18). At baseline, all 58 children (mean age 9.24, s.d.=1.19 years) met diagnostic criteria for ADHD and 47 were diagnosed with oppositional-defiant disorder; 21 met criteria for conduct disorder.
Fenfluramine challenge
Following the clinical evaluation and prior to the day of the challenge protocol, participants followed a low monoamine diet for 3 days and reported to the laboratory at 08.00 h after fasting overnight. An indwelling catheter was inserted into a forearm vein. Following an adaptation period, baseline blood samples were drawn at 09.45 h and 09.55 h. At 10.00 h, a 1 mg/kg dose of d,l-fenfluramine hydrochloride was administered orally. Blood samples were drawn 60, 120, 180, 240 and 300 min later for determination of plasma prolactin, fenfluramine and norfenfluramine concentrations. All samples were placed on ice prior to centrifugation (within 2 h). After separation, samples were frozen at –80° C until analysis. The lower limit of detection for the prolactin assay is <1.0 ng/ml. Intra- and interassay variability are less than 6.7% and 8.4% respectively. Blood samples for determination of plasma fenfluramine and norfenfluramine were drawn hourly. After separation, these samples were frozen at –20° C prior to assay by gas chromatography with electrical detection. The lower limit of sensitivity for these assays is 2 ng/ml for fenfluramine and 3 ng/ml for norfenfluramine. Intra- and interassay variability for the two assays are less than 7%. Participants remained awake and fasting during the entire procedure, reclining in a bed and watching videotapes. All fenfluramine studies were completed prior to September 1997.
Follow-up evaluation
Participants who were evaluated for ADHD in childhood were contacted to participate in a study of the longitudinal course of ADHD. The mean (s.d.) age at follow-up was 18.44 (1.23) years; follow-up took place on average 9.4 years later (s.d.=1.9 years). Seventeen participants identified themselves as African American, 18 as non-Hispanic White, 18 as Black Hispanic and 5 as mixed race. The participants were generally of lower- to lower-middle socio-economic status (SES) (mean SES score 44.59, s.d.=15.62; Reference Nakao and TreasNakao & Treas, 1994). At follow-up, all participants and a parent or adult relative (informant) were administered the Structured Clinical Interview for DSM–IV Axis II Personality Disorders (SCID–II; Reference First, Spitzer and GibbonFirst et al, 1997).
Although diagnoses of personality disorder have not traditionally been assigned to people under 18, there is growing acceptance that personality disorder diagnoses can be made reliably in adolescence (e.g. Reference Bernstein, Cohen and VelezBernstein et al, 1993; Reference Johnson, Brent and ConnollyJohnson et al, 1995) and DSM–IV–TR (American Psychiatric Association, 2000) allows for this if the symptoms are persistent and present for at least 1 year. In the case of antisocial personality disorder, a prior diagnosis of conduct disorder is also required and, by definition, it is the only personality disorder diagnosis that cannot be made in individuals under 18. In the current study, because the SCID–II interview was used for research purposes it was administered to all participants regardless of age; if they were found to meet criteria for conduct disorder based on the interview, the criteria for antisocial personality disorder were also evaluated. Interviewers were masked to baseline data, including whether children had been given a diagnosis of conduct disorder during the baseline study, and the interviews were conducted independently (i.e. the same interviewer did not interview the participant and informant). Proband and informant interviews were not conducted for all participants; one proband died prior to evaluation and three probands did not identify an informant. If either the participant or the informant endorsed criteria for antisocial personality disorder, the participant was considered to carry the diagnosis. Concordance (Pearson's r) between ratings was 0.62.
Signed informed consent was obtained from participants over 18 years and from parents for participants under 18, in whom assent was obtained. The protocol was approved by the institutional review boards at Queens College, City University of New York and Mount Sinai School of Medicine.
Data reduction and statistical analyses
Baseline prolactin level was calculated as the mean of two blood samples collected prior to administration of fenfluramine and area under the curve (AUC PRL[fen]) was calculated using trapezoidal integration (Reference Pruessner, Kirschbaum and MeinlschmidPruessner et al, 2003). Both baseline prolactin and AUC PRL[fen] were log-transformed prior to analyses to normalise the distributions. Owing to the moderate association between baseline prolactin and AUC (r=0.57), log AUC PRL[fen] was regressed on log baseline prolactin to obtain a baseline-independent measure of serotonergic responsivity. This served as the major outcome measure of serotonergic responsivity. Fenfluramine and norfenfluramine levels during the challenge were averaged across time but were unavailable for three participants. These averages were found to be correlated with log AUC PRL[fen] (r=0.45 and 0.39 respectively); log AUC PRL[fen] was thus adjusted for covariation with log baseline prolactin concentration and drug levels using linear regression. The hypothesis that lower serotonergic responsivity would predict the development of antisocial personality disorder was evaluated using two-tailed Student's t-tests.
RESULTS
Seventeen participants (29%) met criteria for antisocial personality disorder according to self-report or informant report and results indicated that lower serotonergic responsivity in childhood predicted the development of antisocial personality disorder (t (56)=2.25, P=0.028). Unadjusted prolactin levels throughout the 5 h challenge are shown in Fig. 1. The difference between groups was greater after also adjusting for drug concentrations (t (53)= 2.71, P<0.001). Gender is associated with responsivity to fenfluramine among adults (Reference McBride, Tierney and DeMeoMcBride et al, 1990) and children (Reference Koda, Halperin and NewcornKoda et al, 1996), with females showing greater hormonal responsivity. Although there were no gender differences in serotonergic responsivity in the current sample (t (56)= –0.26, P=0.79), no females in the current sample met criteria for antisocial personality disorder. Thus, it might be argued that gender accounts for the group differences reported here. We therefore conducted post hoc analyses excluding girls, but the results were unchanged after adjusting for log-baseline prolactin (t (50)=2.27, P=0.028) and after adjusting for log baseline prolactin and drug concentrations (t (48)=2.93, P=0.005).
The two diagnostic groups were then compared for other characteristics measured in childhood and were not found to differ in age (P=0.76), full-scale IQ (P=0.42), parent ratings on the CBCL of attentional difficulties (P=0.75) or aggression (P=0.39). There was a tendency for parents to endorse higher levels of delinquent behaviour in childhood among those who later developed antisocial personality disorder (t (49)=–1.88, P=0.066) and teachers rated these children as more aggressive (t (53)=–2.23, P=0.03) on the IOWA Connors Aggression Scale. The two groups did not differ in the number of years between the fenfluramine challenge and evaluation of antisocial personality disorder (P=0.10) or in SES score measured at the second evaluation (P=0.25). Interviews to ascertain family history were conducted at the initial evaluation for 47 participants (81%). Results indicated that participants who met criteria for antisocial personality disorder had a higher percentage of relatives with the disorder, (t (45)=3.69, P=0.001).
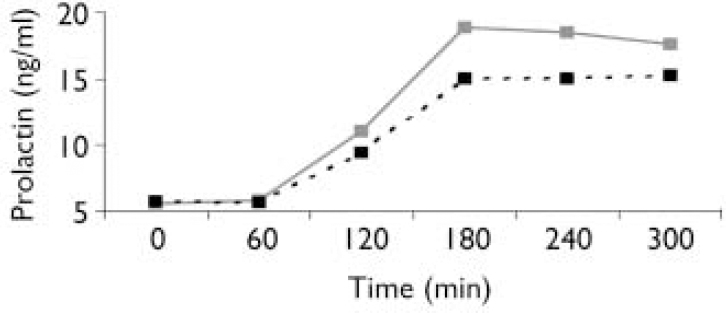
Fig. 1 Plasma prolactin concentrations following administration of a 1 mg/kg dose of d,l-fenfluramine hydrochloride. - - ▪ - -, Antisocial personality disorder; — ![]() —, no antisocial personality disorder.
—, no antisocial personality disorder.
Criteria for antisocial personality disorder were evaluated if participants met criteria for conduct disorder on the SCID–II, even if they were under age 18. However, post hoc analyses included only participants who were 18 years or over at the time of the interview assessment (n=33). Despite loss in statistical power, results indicated that lower serotonergic responsivity was associated with the development of antisocial personality disorder, (t (31)=2.32, P=0.03).
Finally, an exploratory forward selection stepwise logistic regression analysis using maximum likelihood estimates was conducted to examine the relative influence of childhood conduct disorder, childhood aggression (IOWA Connors Aggression Scale) and childhood serotonergic function (baseline-adjusted log AUC PRL[fen]) in predicting a diagnosis of antisocial personality disorder. Results indicated that a model that included serotonergic functioning and childhood aggression was significant (χ2(2)=13.22, P=0.001, –2LL=53.79). Serotonergic functioning entered the model first (B=–3.05, 95% CI –5.43 to –0.68), z=6.4, P=0.012), followed by childhood aggression (B=0.21 (95% CI 0.04–0.38); z=6.2, P=0.013). Odds ratios are not reported because serotonergic functioning is represented by a log transformation of AUC, which is not a meaningful unit of measurement.
DISCUSSION
Main findings
These results are the first to demostrate that dysregulated serotonergic function measured during childhood (i.e. lower prolactin responsivity to fenfluramine) predicts the emergence of antisocial personality disorder in early adulthood. Although consistent with a considerable body of adult human and non-human primate research showing that dysregulated serotonergic function is associated with impulsive aggression, these are remarkable findings given that serotonergic responsivity was assessed 9 years prior to the assessment of antisocial personality disorder and was not at the time correlated with the severity of childhood aggression (Reference Schulz, Newcorn and McKaySchulz et al, 2001).
Moreover, these results provide a critical link between the child and adult literature on the covariation of impulsive aggression and serotonergic function because this is the only study that spans the developmental period between childhood and early adulthood.
Other studies
A potential explanation for inconsistencies in the childhood literature is that aggressive and delinquent behaviour across childhood and adolescence is temporally unstable and less tightly linked to individual variation in serotonergic function than in adulthood. Developmental theories of antisocial personality disorder propose alternative trajectories from childhood to adulthood, including continuous or life-course-persistent and transitory or adolescence-limited antisocial behaviour (Reference DiLalla and GottesmanDiLalla & Gottesman, 1989; Reference MoffittMoffitt, 1993). These theories propose that delinquent behaviour in children that persists into adulthood is a more heritable or biologically mediated form of behaviour that likely includes aggression and violence. This view is supported by a twin study showing that age at onset moderates the association between genetic and environmental influences on antisocial behaviour (Reference Slutske, Heath and DinwiddieSlutske et al, 1997) and a meta-analysis of twin studies of aggression (Reference Miles and CareyMiles & Carey, 1997), which concluded that heritability estimates of aggression increase from childhood to adulthood, whereas the relative magnitude of environmental influences decreases. In the current study, we observed that boys who went on to develop antisocial personality disorder had more relatives with such a diagnosis, which is consistent with our previous report that aggressive children with higher familial aggregation of aggressive and antisocial behaviours show a lower prolactin response to fenfluramine (Reference Halperin, Schulz and McKayHalperin et al, 2003).
In contrast to life-course-persistent antisocial behaviour, many adolescents engage in more ‘normative’ forms of delinquent behaviour, including substance use. Because the base rate of this behaviour is so high, it is correlated with neither childhood nor adult behaviour. Of the 21 children diagnosed with conduct disorder in childhood in the current sample, only about half (n=11) went on to develop antisocial personality disorder, corresponding to the stability data reported by Lahey et al (Reference Lahey, Loeber and Burke2005) in a longitudinal study of conduct disorder first assessed in childhood. In addition, seven individuals who met criteria for antisocial personality disorder did not carry a diagnosis of conduct disorder at the time of the childhood evaluation, although six of the seven met criteria for oppositional defiant disorder, another precursor for antisocial personality disorder. In these, features of conduct disorder emerged after the childhood evaluation, and indeed, were apparent at the time of assessment of antisocial personality disorder. It should also be noted that children who did not meet full criteria for conduct disorder during childhood would not necessarily be considered ‘non-aggressive’. Because all children met criteria for a disruptive behaviour disorder upon study entry, they might have exhibited features of conduct disorder that did not reach the threshold level for diagnosis.
The results from the current study are not informative regarding the origins of the covariation between dysregulated serotonergic activity and antisocial personality disorder. Pedigree studies suggest that indices of serotonin (e.g. whole blood, CSF 5-HIAA levels) are heritable (Reference Higley, Thompson and ChampouxHigley et al, 1993; Reference Abney, McPeek and OberAbney et al, 2001), as are measures of aggression and antisocial behaviour (Reference Miles and CareyMiles & Carey, 1997; Reference Slutske, Heath and DinwiddieSlutske et al, 1997). Environmental conditions suggestive of social adversity (e.g. peer rearing in non-human primates (Reference Shannon, Schwandt and ChampouxShannon et al, 2005) and poverty and unemployment (Reference Manuck, Bleil and PetersenManuck et al, 2005b )) are associated with dysregulated serotonergic function and these social factors may interact with functional variants of serotonin-regulating genes to confer greater risk for antisocial personality disorder (Reference Caspi, McClay and MoffittCaspi et al, 2002). Because the current sample included only children with disruptive behaviour disorders, in whom we would expect to see a full complement of genetic and environmental risk predictors for antisocial personality disorder, we cannot disentangle the relative impact of these factors on serotonergic function prior to age 7. Moreover, we do not posit that dysregulated serotonergic function is the sole feature that leads to antisocial personality disorder, but consider it one aspect of a complex interplay between biological and psychosocial variables.
Limitations of the study
Limitations of the current study should be acknowledged. The sample size was small and prospective replication of the association is warranted. However, the extended length of follow-up and the fact that the association between serotonergic functioning and antisocial personality disorder could not be explained by common risk factors (e.g. gender, IQ, SES) suggests that the finding is robust. The sample was predominantly male and the findings may not generalise to girls, but we note that the results were unchanged upon exclusion of females from the analyses. In addition, although it is of great interest to identify neurobiological features that are associated with specific aspects of antisocial personality disorder (e.g. Reference Yang, Raine and LenczYang et al, 2005), the number of people meeting the criteria for such a diagnosis was considered too small to conduct these analyses.
Limitations of the method used for measuring central serotonergic function should be acknowledged. Throughout the 1980s and 1990s hormonal responsivity to fenfluramine was a well-established and frequently-used measure for assessing serotonergic function in the hypothalamic–pituitary axis and the procedure is considered to reflect ‘net’ serotonergic transmission from the raphe nuclei to the hypothalamus, including both presynaptic and postsynaptic functioning (Reference Coccaro, Siever and KlarCoccaro et al, 1989; Reference Yatham and SteinerYatham & Steiner, 1993), although data suggest that the findings may generalise to the prefrontal cortex (Reference Soloff, Meltzer and GreerSoloff et al, 2000). This method of assessment has been largely supplanted by neuroimaging which affords greater regional specificity of central serotonergic pathways and receptor activity. In addition, it should be noted that fenfluramine was withdrawn from the US market in 1997 when safety concerns curtailed its continued use in neurobiological research, which prevented us from repeating biological assessment in our young adult sample.
Antisocial personality disorder is a heterogeneous entity that includes overlapping constructs: criminal behaviour, impulsive aggression and a lack of remorse for transgressions. The literature suggests that dysregulated serotonergic function will be associated prospectively with impulsive aggression rather than other aspects of antisocial personality disorder, but this hypothesis awaits verification.
The age range of the current sample spans the time of the developmental transition from adolescence into young adulthood and it will be critical to follow these individuals over the next 5–10 years to determine whether their current levels of behaviour persist. The longitudinal nature of this study will enable us to examine specific childhood neurobiological and psychosocial predictors of the persistence of aggression and related forms of antisocial behaviour.
Acknowledgements
This work was supported in part by grants RO1MH046448 (to J.M.H.) and and KO1069979 (to J.D.F.) from the National Institute of Mental Health.




eLetters
No eLetters have been published for this article.