Inflammation is the natural response of an organism to injury or infection(Reference Minihane, Vinoy and Russell1). However, when the inflammatory response occurs constantly, acute inflammation can progress to chronic inflammation(Reference Noland2). Previous studies have shown that chronic inflammation can directly affect health(Reference Leon-Pedroza, Gonzalez-Tapia and del Olmo-Gil3–Reference Elinav, Nowarski and Thaiss6). Changes in cardiometabolic markers, such as lipids, plasma glucose, insulin resistance and blood pressure (BP), are closely related to the inflammatory response(Reference Canto-Osorio, Denova-Gutierrez and Sanchez-Romero7). These disturbances express a cascade of excessive amounts of reactive oxygen species and vascular endothelium dysfunction(Reference Welty, Alfaddagh and Elajami8), contributing to the metabolic syndrome and inflammation(Reference Leon-Pedroza, Gonzalez-Tapia and del Olmo-Gil3), leading to several diseases, such as obesity and diabetes(Reference Galassetti4), CVD(Reference Ruiz-Canela, Bes-Rastrollo and Martinez-Gonzalez5), and cancer(Reference Elinav, Nowarski and Thaiss6).
Dietary patterns and components play a central role in regulating chronic inflammation. Adherence to unhealthy dietary patterns, such as the Western diet, characterised by high intakes of refined grains, sugar, processed foods, and saturated fat from red meat and dairy products, is associated with metabolic disorders and high levels of inflammatory markers(Reference Thorburn, Macia and Mackay9). On the other hand, the Mediterranean diet, which emphasises intake of whole grains, vegetables, fruits, fish, and moderate alcohol and olive oil, has been related to lower levels of inflammation(Reference Bonaccio, Cerletti and Iacoviello10).
The dietary inflammatory index (DII) was developed in 2009 as a tool to assess the overall inflammatory properties of a diet, allowing a quantitative classification of dietary patterns ranging from maximally anti-inflammatory to maximally pro-inflammatory(Reference Cavicchia, Steck and Hurley11). In 2014, the DII was revised and improved after an extensive literature review(Reference Shivappa, Steck and Hurley12). The new version of the DII considers a total of forty-five food parameters, including whole foods, nutrients and other bioactive compounds(Reference Shivappa, Steck and Hurley12). Scores obtained from the DII have been frequently associated with inflammation in child and adult populations(Reference Shivappa, Steck and Hurley12,Reference Phillips, Shivappa and Hebert13) . A recent study explored the inflammatory potential of diets among adults and concluded that pro-inflammatory diets are associated with unfavourable lipoprotein and inflammatory profiles. Moreover, an increased risk for the metabolic syndrome was observed among those with higher DII scores(Reference Phillips, Shivappa and Hebert13). Additionally, data published in a recent meta-analysis indicated that higher DII categories were associated with an increased risk of cancer, where breast and colorectal cancer were the most prevalent(Reference Fowler and Akinyemiju14).
However, studies exploring the relation between dietary inflammatory potential and cardiometabolic risk factors among adolescents are scarce(Reference Shivappa, Hebert and Marcos15,Reference Kenia, Débora and Nathalie16) . Most of the data for this age group show an association between the DII and inflammatory markers, like TNF-α, IL-1 and IL-2, interferon-γ, and C-reactive protein, although levels of markers are divergent among studies(Reference Kenia, Débora and Nathalie16–Reference Kurklu, Torun and Kucukcetin18). Furthermore, there are few data linking DII scores and metabolic markers in adolescents, with inconsistent results(Reference Kurklu, Torun and Kucukcetin18,Reference Sethna, Alanko and Wirth19) . Thus, we aimed to investigate the association between dietary inflammatory potential, assessed by the DII, and cardiometabolic risk factors in a large sample of Brazilian adolescents.
Methods
Study design
The Study of Cardiovascular Risks in Adolescents (ERICA) is a multicentre cross-sectional school-based study that assessed Brazilian adolescents aged 12–17 years old, enrolled in public and private schools, to estimate the prevalence of the metabolic syndrome and cardiovascular risk factors. A complex sampling approach was performed. Thus, the population target was divided into 32 geographic strata: all 26 state capitals, the Federal District, and 5 more strata representing other municipalities with 100 000 inhabitants in each region of Brazil. The schools were selected based on probability proportional to size (number of students per school) and inversely proportional to the distance between the school municipality and the state capital. In total, 1247 schools in 124 municipalities were selected. Three classrooms were randomly selected from each school, and all students in these classes were invited to participate in ERICA. Further details regarding the sampling and design of the ERICA project can be found in previous publications(Reference Bloch, Szklo and Kuschnir20,Reference Vasconcellos, Silva and Szklo21) . Data were collected between February 2013 and November 2014.
The participation rate for adolescents who completed the questionnaires, anthropometric measures and blood sampling in ERICA was 52 %(Reference da Silva, Klein and Souza Ade22). For the present study, we used data from morning-shift students, including those in the integral or semi-integral system, as overnight fasting was required prior to blood sampling. Participants with pre-existing cardiometabolic disease such as diabetes, hypertension or dyslipidemia were excluded from the analyses. All participants provided a written agreement to participate, and their parents or legal guardians signed an informed consent form. ERICA was approved by the Research Ethics Committees in all twenty-seven research centres in Brazil.
Cardiometabolic risk factors
Cardiometabolic markers were evaluated from a fasting blood sample. Before the exam, a questionnaire was applied to confirm the fasting state. Plasma glucose, glycated Hb (HbA1c), insulin, TAG, total cholesterol and HDL-cholesterol were evaluated; LDL-cholesterol was estimated indirectly by the Friedewald equation(Reference Friedewald, Levy and Fredrickson23).Insulin resistance was evaluated through the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (HOMA-IR = (insulin × fasting glucose)/22·5). All blood samples were analysed by a single laboratory following a standardised protocol(Reference Cureau, Bloch and Henz24). The reference values used to determine abnormal values of these variables are as follows: fasting plasma glucose ≥ 100mg/dl, HbA1c ≥ 5·7 %, TAG ≥ 150 mg/dl, total cholesterol ≥ 170 mg/dl, HDL-cholesterol ≤ 45 mg/dl and LDL-cholesterol ≥ 130 mg/dl(25,Reference Xavier, Izar and Faria Neto26) . Abnormal values of HOMA-IR (females ≥ 2·32 and males ≥ 2·87) were classified using cut-off points for Brazilian adolescents(Reference Chissini, Kuschnir and de Oliveira27).
BP was measured with a digital monitor (Omron 705-IT – Omron Healthcare, Bannockburn, IL) from the right arm using individual cuff sizes after 5 min of sitting still and with an interval of 3 min between each measure. Three consecutive measurements were taken for each individual, with an interval of 3 min between them. The first measurement was excluded and the average of the last two measurements was used in the analyses(Reference Bloch, Szklo and Kuschnir20). High BP was defined as values of systolic or diastolic BP higher than 95th percentile for sex, age and height(28).
Dietary measurements
Dietary intake was assessed by a 24-h dietary recall collected in person by trained interviewers using the multiple-pass method(Reference Conway, Ingwersen and Vinyard29). The food intake data were entered directly into the ERICA-REC24-h dietary assessment software developed specifically for ERICA(Reference Barufaldi, Abreu Gde and Veiga30). This software consisted of a list containing 1626 food items, based on data from the Brazilian Household Budgets Survey 2008–2009(Reference Barufaldi, Abreu Gde and Veiga30,Reference Souza Ade, Pereira and Yokoo31) . Photographs were included in the software to help with estimation of portions consumed. All information provided by the adolescents was recorded in detail, including preparation and quantity of food consumed. Food items that were not included in the software database were inserted by the researchers during the interview. To estimate energy intake, the Brazilian Food Composition Table(32) and the Brazilian Portion Size Table(33) were considered. Three adolescents who had an energy intake < 100 kJ/d were excluded from the analyses(Reference da Silva, Klein and Souza Ade34). This cut-off point was adopted since lower values are implausible, considering that the sample is composed of schoolchildren and only the consumption of school meals, distributed free of charge in schools, would be able to provide more than 100 kcal. Dietary recalls were conducted between Tuesdays and Fridays at schools, and a similar number of adolescents were enrolled on each day of the week. After collection, data were immediately available in a central database.
Dietary inflammatory index
The complete details as to how the DII scores were calculated have been published elsewhere(Reference Shivappa, Steck and Hurley12). In brief, a systematic review including 1943 publications assessing the relation between inflammatory markers and diet was performed. After reviewing the literature, a total of forty-five food parameters were consistently related (positively or negatively) to inflammatory markers and, thus, were selected to compose the DII. Finally, for each nutrient or food component, a specific inflammatory effect score was assigned based on the number of studies that reported an anti-inflammatory, pro-inflammatory or no inflammatory effect; scores were then weighted by study design and number of papers for each dietary parameter–inflammatory marker relationship. The energy-adjusted DII (E-DII) was calculated by converting each subject’s nutrient intake to 1000 kJ, taking into account different amounts of energy consumption among people.
In our study, twenty-five of the forty-five DII dietary parameters were available in the food intake data. These included mean intakes of pro-inflammatory nutrients (carbohydrate, protein, total fat, trans fat, saturated fat, energy and cholesterol), anti-inflammatory nutrients (fibre, MUFA, PUFA, n-3 and n-6 fatty acids, folic acid, thiamin, riboflavin, vitamin B6, niacin, vitamin C, vitamin D, vitamin E, vitamin B12, and vitamin A) and minerals (Mg, Fe and Zn).The nutrients and foods that were not considered for the calculation were as follows: alcohol, β-carotene, caffeine, eugenol, garlic, ginger, onion, saffron, serine, turmeric, green/black tea, flavan-3-ol, flavones, flavonols, flavonones, anthocyanidins, isoflavones, pepper, thyme/oregano and rosemary.
Covariates
The participants completed a self-filled questionnaire applied in the classroom, using a personal digital assistant (PDA – model LG GM750Q). Self-reported data included sex, age, skin colour (white, black, brown or other), school area (urban or rural), smoking, screen time and physical activity habits. An economic index, similar to the one used in the Brazilian Demographic Census(35), was calculated to assess the socio-economic status. It included possession of specific goods and the presence of a housekeeper at home. For the analyses, this economic index was categorised in tertiles.
Time spent in moderate-to-vigorous physical activity was assessed using a questionnaire cross-culturally adapted and validated in Brazilian adolescents(Reference Farias Júnior, Lopes and Mota36). To determine the weekly amount of time spent in physical activity, we multiplied self-reported duration and frequency for each activity listed and then dichotomised into < 60 or ≥ 60 min/d(37). Screen time was measured using the following question: ‘during an ordinary weekday, how many hours do you spend watching TV, using a computer, or playing video games?’ Thus, screen time was obtained in hours per d and categorised into ≤ 2 or > 2 h/d(38).
Anthropometric measurements were performed by trained researchers, following standardised written protocols(Reference Bloch, Szklo and Kuschnir20), while the adolescents were wearing light clothing and no shoes. Body weight was measured using a digital scale (Líder, model P200M) and height was measured twice using a calibrated portable stadiometer (Alturexata). The weight and height measures were used to calculate the BMI (BMI = weight(kg)/height(m))2).
Statistical analyses
All estimates were stratified by sex, considering that the diet and prevalence of abnormal cardiometabolic risk factors can differ among adolescents by sex in this age group. Descriptive analyses were reported as means and 95 % CI for continuous variables and percentages for categorical variables. The E-DII was categorised in quartiles according to sex and the values within them were presented as median plus ranges. The consumption of each individual dietary parameter included in DII calculation was described as amount per d (i.e. grams, milligrams or micrograms per d). Energy adjustment was performed using the residual method. Differences between quartiles of E-DII score were estimated with the ANOVA test.
We used Poisson regression to model the association between E-DII quartiles and cardiometabolic risk factors. The model was adjusted by age, skin colour, socio-economic status, school area, smoking, physical activity, screen time, BMI (kg/m2) and total energy intake (kcal). These variables were chosen a priori in accordance with the literature and kept in the model after inclusion. There was no suspicion of collinearity between the adjustment variables. To obtain population representative findings, the ERICA’s sample weights and complex sample design were considered in all analyses(Reference Vasconcellos, Silva and Szklo21). All data analyses were conducted in Stata software (version 14.0, Stata Corporation). All tests were bi-caudal, and a P-value below 0·05 was regarded as statistically significant.
Results
The analyses included a total of 31 684 adolescents (59·9 % females) without a pre-existing diagnosis of hypertension, dyslipidemia or diabetes. The overall mean age in this sample was 14·6 (sd = 1·6). The E-DII score ranged from –2·8 to +4·3, where higher scores indicate a more pro-inflammatory diet. Table 1 shows the characteristics of the included adolescents by quartiles of the E-DII score and sex. Girls with a moderately pro-inflammatory diet (Q3) spend more time in front of the screens, and those with a more pro-inflammatory diet (Q4) present a lower prevalence of meeting the physical activity recommendations and the mean of BMI was slightly lower than girls with a more anti-inflammatory diet (Q1). Girls with a better socio-economic status seem to keep a more pro-inflammatory diet (Q4). However, boys with a highly pro-inflammatory diet (Q4) spent more time in front of screens and have slightly lower BMI than those with an anti-inflammatory diet (Q1).
Table 1. Characteristics of the Brazilian adolescents across sex-specific quartiles of the energy-adjusted dietary inflammatory index (E-DII), ERICA 2013–2014
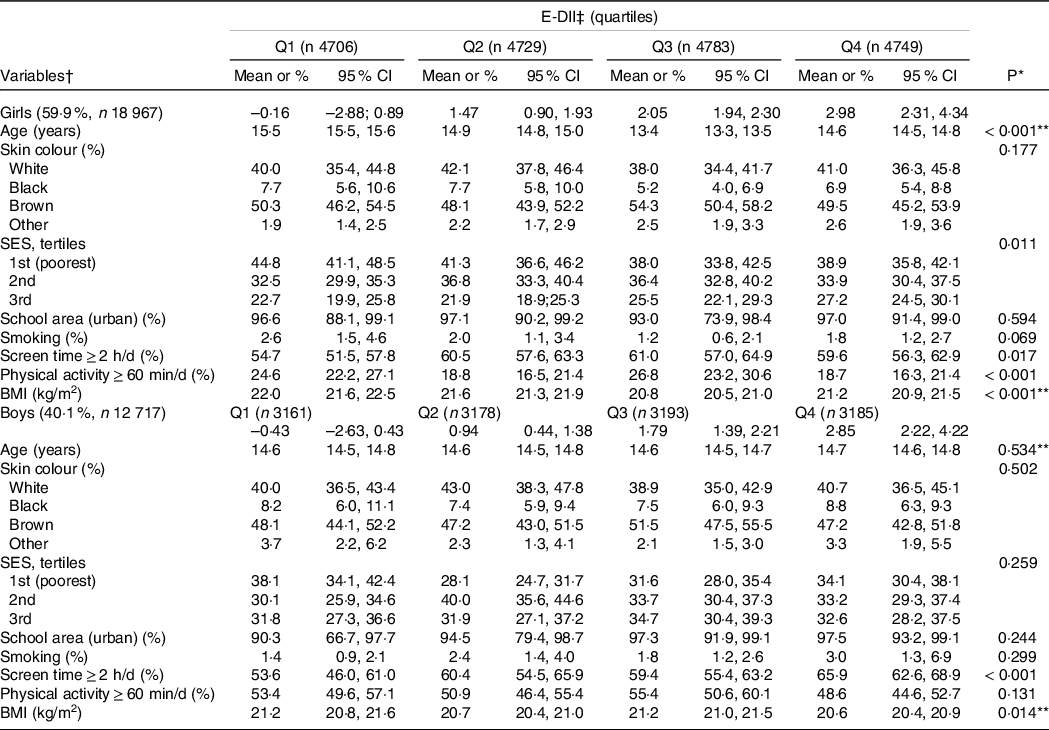
SES, socio-economic status.
* Wald’s test for heterogeneity.
† Values are means or percentages and 95 % CI.
‡ Energy-adjusted dietary inflammatory index; quartiles are shown as median and ranges.
** ANOVA test.
Table 2 shows the distribution of macro and micronutrients through quartiles of the E-DII. After adjusting for energy intake, we observed that participants with highly pro-inflammatory diets (Q4) presented higher means for most of the pro-inflammatory nutrients, except for protein intake; and lower intake of anti-inflammatory nutrients and minerals, with the exception of monounsaturated fat, the consumption of which was higher in those with highly pro-inflammatory diets (Q4).
Table 2. Nutritional data through sex-specific quartiles of the energy-adjusted dietary inflammatory index (E-DII) and by sex, ERICA 2013–2014
(Mean values and 95 % confidence intervals)
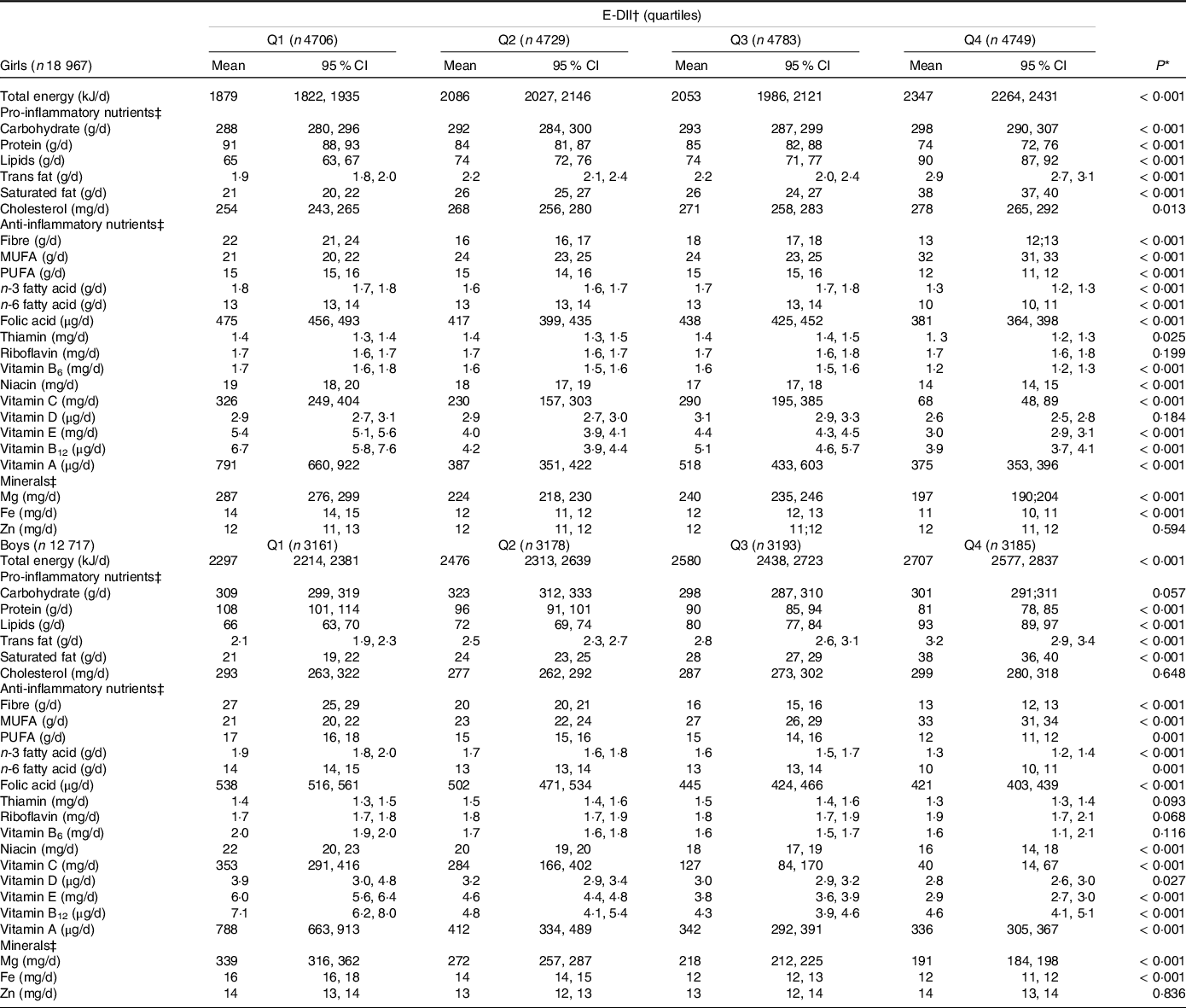
Values are means and 95 % CI.
* Differences for nutrients between sex-specific quartiles of dietary inflammatory index were estimated with ANOVA tests.
† Energy-adjusted dietary inflammatory index.
‡ Data were energy-adjusted.
Table 3 shows the prevalence and prevalence ratios (PR), respectively, for abnormal concentrations of metabolic variables and BP by sex-specific E-DII quartiles. In the adjusted model and considering a high anti-inflammatory diet (Q1) as the reference, following a high pro-inflammatory diet increases the PR of high HOMA-IR among males (PRQ4 = 1·37, 95 % CI: 1·04, 1·79). In girls, a high pro-inflammatory diet was associated with high fasting glucose values(PRQ4 = 1·96, 95 % CI: 1·02, 3·78), high TAG (PRQ4 = 1·92, 95 % CI: 1·06, 3·46), low HDL-cholesterol (PRQ4 = 1·16, 95 % CI: 1·02, 1·32) and high LDL-cholesterol (PRQ4 = 1·93, 95 % CI: 1·12, 3·33). Additionally, moderately pro-inflammatory diets were associated with high HOMA-IR (PRQ2 = 1·14, 95 % CI: 1·02, 1·29) among females and with high total cholesterol (PRQ3 = 1·56, 95 % CI: 1·20, 2·01) among males. Only among males was a moderately pro-inflammatory diet inversely associated with high BP levels (PRQ3 = 0·65, 95 % CI: 0·48, 0·88).
Table 3. Prevalence and PR for cardiometabolic risk factors through sex-specific quartiles of the energy-adjusted dietary inflammatory index (E-DII), ERICA 2013–2014
(Percentages and 95 % confidence intervals)
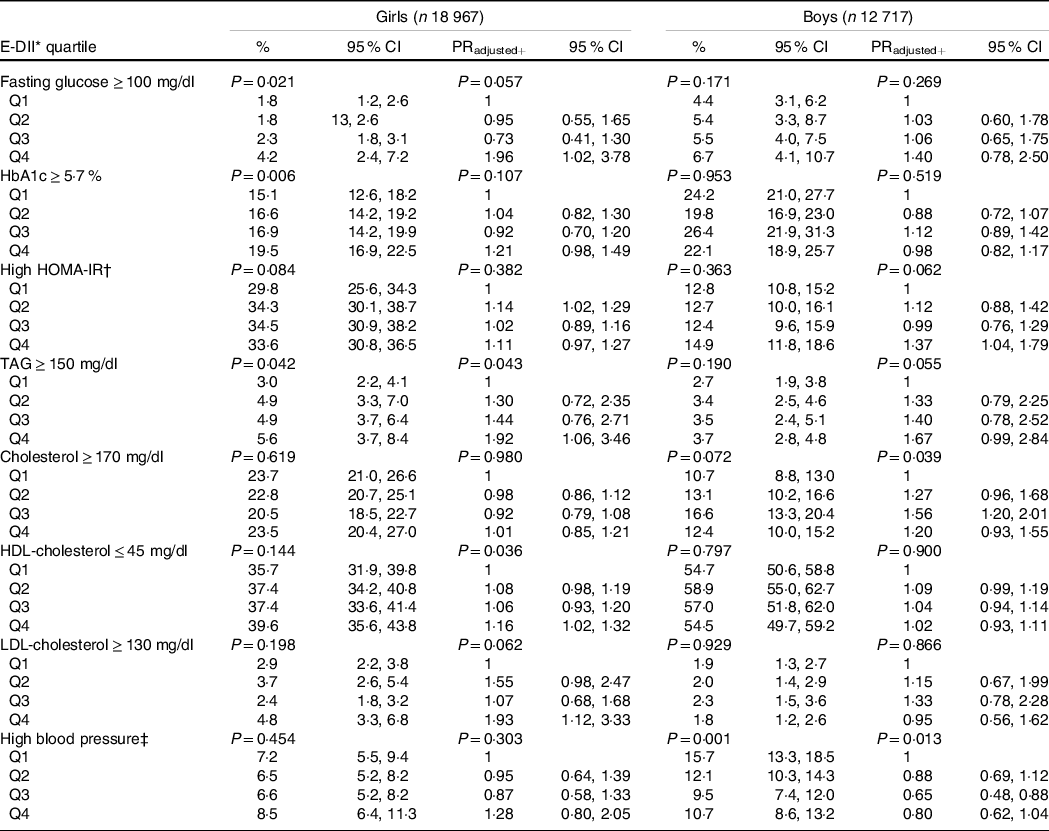
PR, prevalence ratios; HbA1c, glycated Hb; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance.
Models+ were adjusted by: age, skin colour, socio-economic status, school area, smoking, physical activity, screen time, BMI and energy intake.
Sample size across E-DII sex-specific quartiles: girls, Q1 (n 4706), Q2 (n 4729), Q3 (n 4783), Q4 (n 4749); boys, Q1 (n 3161), Q2 (n 3178), Q3 (n 3193), Q4 (n 3185).
P-values: Wald’s test for linear trends.
* Energy-adjusted dietary inflammatory index.
† High HOMA-IR: females ≥ 2·32 and males ≥ 2·87(Reference Chissini, Kuschnir and de Oliveira27).
‡ High blood pressure: ≥ 95th percentile of National High Blood Pressure Education Program(28).
Discussion
The present study is the first to evaluate the association between inflammatory potential of diet, as measured by the E-DII, and cardiometabolic health among a representative sample of Brazilian adolescents. The results revealed a positive association between highly pro-inflammatory diets, an increase in HOMA-IR in males and a worse lipid and glucose profiles among females. Curiously, an inverse association was observed between a moderately pro-inflammatory diet and high BP among males.
A recent systematic review including studies from different continents (Europe, Asia and North America) analysed dietary inflammatory potential, cardiometabolic risk and inflammation in children and adolescents(Reference Suhett, Hermsdorff and Cota39). Only two studies assessed BP and only one assessed other metabolic markers (fasting glucose, HOMA-IR and lipid profile)(Reference Correa-Rodriguez, Gonzalez-Jimenez and Rueda-Medina40,Reference Sen, Rifas-Shiman and Shivappa41) . Both studies could not find an association between pro-inflammatory diet changes and the evaluated cardiometabolic risk factors(Reference Suhett, Hermsdorff and Cota39,Reference Correa-Rodriguez, Gonzalez-Jimenez and Rueda-Medina40) , possibly because of different populations studied and a lower number of patients evaluated, as compared with the present study, where we could observe that a pro-inflammatory diet can be slightly associated with a poor cardiometabolic profile, especially among females.
Our main results show that the E-DII is associated with selected cardiometabolic risk factors, but not with all of them. In adults, the results are consistent, showing that a more pro-inflammatory diet is associated with a poorer cardiometabolic profile, metabolic syndrome and CVD(Reference Ruiz-Canela, Bes-Rastrollo and Martinez-Gonzalez5,Reference Phillips, Shivappa and Hebert13,Reference Mazidi, Shivappa and Wirth42) .This profile is probably also associated with environmental exposure throughout life, such as poor diet quality, sedentary behaviour, sleep deprivation, exposure to cigarette smoke and psychosocial stress(Reference Mazidi, Shivappa and Wirth42). Possibly in childhood and adolescence, the evidence is not as strong as in adulthood because youth are a healthier population, with less life-long exposition to unhealthy dietary patterns, allowing us to observe health changes in some risk factors only. In spite of that, there is emerging recognition that CVD originates in childhood, showing us the importance of better understanding the role of diet in inflammatory systemic process early in life.
In our study, females represented a high risk group. These differences in results between girls and boys can be in part explained by lifestyle behaviours. For example, in ERICA, boys were more physically active than girls, and physical activity is a well-known behaviour associated with adopting a healthier diet and, consequently, having a better cardiometabolic profile(Reference Poitras, Gray and Borghese43). However, girls often report concerns about weight and body image, which can lead to the adoption of restrictive diets with low nutrition quality and poor in anti-inflammatory nutrients(Reference Madalosso, Schaan and Cureau44). Furthermore, in this study, girls with better socio-economic conditions ingest more pro-inflammatory nutrients. In this context, a recent study reports that young Americans still follow a diet of low nutritional quality regardless of socio-economic status(Reference Liu, Rehm and Onopa45).
Another recent population-based study(Reference Sethna, Alanko and Wirth19), conducted with 6101 North American children and adolescents (aged 12–18 years old) observed, after stratifying by weight status, that a highly pro-inflammatory diet was associated with high albuminuria and dyslipidemia in overweight adolescents. Moreover, they did not observe any association between inflammatory diet and cardiometabolic risk factors when including normal-weight youths in the analysis. These results suggest that being overweight/obese can drive this association, which can be explained by behavioural and metabolic changes among this group. Also, changes in stages of physical development and sexual maturation of the adolescents can contribute to the observed differences on abnormal values of cardiometabolic risk factors among boys and girls(Reference Kelsey, Pyle and Hilkin46).
Several dietary strategies have been used to reduce BP levels in adults and in children(Reference Saneei, Salehi-Abargouei and Esmaillzadeh47–Reference Couch, Saelens and Levin49). In spite of this, our finding fails to find association between higher E-DII and high BP among adolescents. This is in accordance with the National Health and Nutrition Examination Survey (NHANES) findings(Reference Sethna, Alanko and Wirth19). Among boys, unexpectedly, we observed an inverse association between a moderately inflammatory diet and high BP. In ERICA, BP was collected once, increasing false-positive cases of high BP. Also, nutritional status of the adolescents may play a role, as reported before(Reference Sethna, Alanko and Wirth19). Prospective studies, which include multiple measurements of BP on different days, are important to understand this possible association.
Our observations may have implications for public health. A highly pro-inflammatory diet appears to be associated with a poor cardiometabolic profile in adolescents, especially among females. Although calculation of the E-DII is not simple, the main results of this study can indicate that nutrients with a greater anti-inflammatory potential should be preferred and recommended for youth instead of nutrients with pro-inflammatory characteristics. Interventions aimed at reducing the pro-inflammatory diet score may be more important among females; moreover, it is important to combine this recommendation with other healthy behaviours (eating habits and physical activity). Finally, to understand the overlap of the DII and other dietary evaluation approaches (i.e. NOVA classification for food processing or other scores that assess the global quality of the diet), it is important to understand their potential negative impact on dietary pattern and health(Reference Canto-Osorio, Denova-Gutierrez and Sanchez-Romero7,Reference Ronca, Blume and Cureau50) .
The present study has some potential limitations. First of all, because it is a cross-sectional study, it is not possible to establish a causal relationship between the E-DII and cardiometabolic risk factors. Reverse causality remains a possibility, but it seems more likely that a highly pro-inflammatory diet precedes a worsening of the cardiometabolic profile than the opposite. Besides that, food consumption was assessed by only one 24-h dietary recall which could interfere in results considering that our data were collected on school days and dietary pattern varies among week days and weekends. In addition, the dietary recall software has not been validated, which may compromise the dietary pattern description of the study population. However, the ERICA-REC24h program (software) proved to be an adequate tool in terms of time and simplicity of application, with the system having friendly features that facilitated the report of food consumption. The training of supervisors and field researchers, the use of figures from home measurements and alert messages in the program were strategies that ensured the quality of food consumption data. The developed software proved to be suitable for use in population studies, even in a country like Brazil where there is a great difference in dietary patterns among regions(Reference Barufaldi, Abreu Gde and Veiga30). In addition, only twenty-five of the forty-five parameters of the DII were included in the analyses, which can compromise the best interpretation of the results. However, most published studies show the same limitation. Consequently, further studies using a more accrued dietary report and including more food parameters are needed in this field. Due to the big sample size, results with P-values higher than 0·01 should be interpreted with caution.
On the other hand, a strength of the present study is the inclusion of a representative sample of adolescents of a large-sized country. The large sample size also allowed for analyses stratified by sex. We corrected all analyses for the complex sampling design and adjusted our analyses for a number of potentially important confounding factors, including BMI and healthy behaviours. Another important point is that all participants that previously reported diseases like diabetes, dyslipidemia and hypertension were excluded from the analyses, since dietary patterns could have introduced bias. Finally, all data collection was standardised including that for cardiometabolic risk factors, and all biomarkers were analysed by standardised biochemical procedures in one certified laboratory.
In conclusion, this study provides new evidence on the association between inflammatory diets and the presence of cardiometabolic risk factors among adolescents. Future studies from other countries are important to better understand the contribution of regional diets to the DII and its association with cardiometabolic risk factors among the young population.
Acknowledgements
Brazilian Ministry of Health (Department of Science and Technology), Brazilian Ministry of Science and Technology (Financiadora de Estudos e Projetos (FINEP 01090421)), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 565037/2010-2, 405009/2012-7 and 457050/2013-6), and Research and Events Incentive Fund (FIPE 2009–0098, 2015–0400) of the Hospital de Clínicas de Porto Alegre. Thisstudy was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
P. F. T. and F. V. C. designed the study, performed the statistical analyses and interpreted the results; N. S. and J. R. H. calculated the inflammatory index of the diet; P. F. T., R. S., J. R., F. V. C. and B. D. S. wrote the manuscript and had primary responsibility for the final content. All authors have critically reviewed the manuscript.
The authors declare no conflicts of interest.






