INTRODUCTION
Nosocomial bloodstream infections (BSIs) constitute a serious health problem worldwide and are associated with increased length of hospital stay and health-care costs, and, most importantly, with high morbidity and mortality [Reference Wenzel and Edmond1, Reference Pittet2]. The incidence of hospital-acquired BSIs varies with the type of population studied, the size of institution, and the ward location. Critically ill patients carry much higher rates of BSIs than those in general wards, with a reported incidence ranging from 2·7 to 10 episodes/100 intensive-care unit (ICU) admissions and a mortality rate ranging from 32% to 82% [Reference Wenzel and Edmond1–Reference Harbarth7]. Differences in patients' characteristics, including the severity of acute illness, comorbidities, hospital and ICU type and microbiological pattern, account at least to some degree, for the wide range in both incidence and mortality. In addition, epidemiological differences in microbiological and/or resistance patterns vary considerably over time in different world areas and hospitals. Furthermore, the increasing prevalence of antimicrobial-resistant nosocomial pathogens has made the monitoring and prevention of ICU-associated BSIs a health-care priority and is highly recommended by many organizations such as the American Society of Microbiology [Reference Jones8]. Knowledge of local epidemiology could optimize the empirical antibiotic choice before results of samples, obtained on suspicion of infection, become available. Although data about pathogens and antibiotic resistance in Greece are available [9], studies concerning the incidence and the relation between clinical and microbiological features of BSIs in ICU patients are limited.
The main objectives of the present study were to determine the risk factors for BSIs in ICU patients and to assess their influence in mortality; secondary objectives were to evaluate factors associated with BSIs and to describe the microbiological profile of BSIs.
METHODS
The present prospective, observational study was carried out in the ICU of Evangelismos Hospital in Athens from September 2004 to August 2005. This ICU is a 25-bed multidisciplinary, university unit in a tertiary care hospital of 1000 beds, and has an occupancy rate of 100%. It serves critically ill medical and surgical patients with the exception of those with acute coronary syndromes and transplantation, managed in separate units. Cardiac surgery patients are transferred from the post-operative cardiac surgery unit to our ICU only in case of a complicated post-operative course. Nurse:patient ratio was generally 1:2 falling to 1:4 during the night hours during the year of the study.
Study design and data collection
All patients consecutively admitted to the ICU for >48 h, during the study period, were included. The first 112 of these patients had also been included in a previous study from our unit examining the ability of the illness severity scores to predict the occurrence of bacteraemia and its outcome [Reference Routsi10]. The data were prospectively collected and included age, gender, underlying disease, admission category (classified as medical or surgical), hospitalization before ICU admission, laboratory and microbiological data, and severity of illness as estimated by the Acute Physiology and Chronic Health Evaluation (APACHE II) score [Reference Knauss11], and the sequential organ failure assessment (SOFA) score [Reference Kajdacsy-Balla12]. Both scores were calculated using the worst values on the first day of admission, as recommended, and also on the day the first positive blood culture specimen was drawn for those patients who developed BSI.
Definitions
All cultures were ordered by the attending physicians in the presence of signs and symptoms of systemic inflammatory response syndrome (SIRS) [13]. No surveillance blood cultures were performed during the study period. Blood cultures were obtained via peripheral venous puncture using a standard sterile technique. Ten millilitres of blood was drawn from each patient and was equally divided in into aerobic and anaerobic Bactec blood culture bottles (Becton Dickinson, MA, USA). Positive blood cultures were diagnosed by isolation of organisms from any blood culture with the exception of coagulase-negative staphylococci or other common skin contaminants (e.g. diphtheroids, Bacillus spp., Propionibacterium spp. or micrococci), which required isolates from more than one blood culture obtained from different venous puncture sites [Reference Garner14, Reference Correa and Pittet15].
An ICU-acquired BSI was defined when one or more blood culture drawn at least 48 h after ICU admission yielded one or more microorganisms. Multiple blood cultures yielding the same pathogen were considered as a single infection. Polymicrobial BSI was recorded if more than one microorganism were isolated from a single culture. For patients with two or more BSIs, only the first BSI episode for each patient was included in the analysis [Reference Pittet, Tarara and Wenzel3, Reference Harbarth7]. A BSI episode was defined as any positive blood culture result associated with two or more SIRS criteria [13]. Mortality was assessed at the time of discharge from the ICU.
Sources of BSIs were recorded according to the Centers for Disease Control and Prevention (CDC) definitions [Reference Garner14]. When an organism isolated from blood culture was compatible with an infection at another site, the BSI was classified as secondary. In the absence of an identified source, the BSI was classified as primary. In case of several possible sources, the BSI was classified as ‘multiple sources’.
Microbiological methods
Blood cultures were performed using the Bactec 9240 system (Becton Dickinson). Identification of the blood isolates was performed by standard methodology and using the commercially available ID panels of the semi-automated Wider I system (Francisco Soria Melguizo S.A., Madrid, Spain). Identification of Candida isolates was performed by the germ tube test and the API 32C AUX test (bioMérieux, Marcy l'Etoile, France). Antimicrobial susceptibility was performed by determination of the minimum inhibitory concentrations (MICs) using MIC panels of the Wider I system. Interpretive criteria followed the National Committee for Clinical Laboratory Standards (NCCLS) recommendations [16]. Antibiotic-resistant organisms were defined as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecalis or E. faecium or any Gram-negative organisms resistant to carbapenems.
Statistical analysis
Data are reported as mean±standard deviation (s.d.) when normally distributed and as median with interquartile range (IQR) otherwise. The Student's t test or the non-parametric Mann–Whitney U test for non-normally distributed data, were used to compare continuous variables, and χ2 test to compare categorical variables. To identify the independent risk factors for incidence and mortality, a backward stepwise logistic regression analysis was used. All examined variables were initially entered into the model. Furthermore, a multiple backward stepwise logistic regression analysis was also performed for patients with BSI to identify risk factors for mortality. The variables initially entered into the model were those that were statistically significant in the univariate analysis. P values of <0·05 were considered statistically significant. The SPSS software package was used for all analyses (version 11.5; SPSS Inc., Chicago, IL, USA).
RESULTS
Study population
Between 1 September 2004 and 31 August 2005, 572 patients, out of 693 admissions in the ICU, had an ICU stay >48 h. Of this group, 159 patients developed one or more episodes of BSI; 11 had a positive blood culture within the first 48 h after ICU admission and were excluded. Thus, a total of 148 patients who developed BSI after 48 h of ICU stay were included in the analysis. The remaining 413 ICU patients (who did not develop any BSI episode during their ICU stay) served as controls. The incidence of BSIs was 16·3 episodes/1000 patient-ICU days. All patients had at least one central venous catheter, a peripheral arterial catheter and a urinary catheter. The median length of ICU stay for all patients, both with and without BSI, was 9 days (IQR 4–22 days). Patients who developed BSI had a median time from ICU admission to first episode of BSI of 9 days (IQR 5–17 days). Diagnosis at admission was post-surgical in the majority of patients (322, 56%) who had a mean age of 56±19 years (range 16–90). The remaining 239 patients (44%) were medical having a mean age of 54±20 years (range 15–85). Thirty-one patients were on corticosteroids or other immunosuppressive agents prior to ICU admission.
Of the 148 patients 17 (11·5%) developed catheter-related BSI and 44 (29·5%) developed BSI not related to infection at another site, thus being classified as primary; the remaining 87 patients had secondary BSIs, the main source being ventilator-associated pneumonia in 46 (31%), whereas in 41 (28%) patients more than one source was detected including respiratory tract, urine, intra-abnominal, skin/soft tissue and central nervous system infections.
Risk factors for developing ICU-acquired BSI
A number of factors present within the first day of ICU admission were associated with subsequent development of ICU-acquired BSI (univariate analysis, Table 1). Patients with BSIs, compared to those without, were more severely ill on admission, as judged by higher APACHE II and SOFA scores (19·4±6·7 vs. 15·6±6·9, P<0·001 and 8·6±2·9 vs. 7·1±5·4, P<0·002, respectively). In addition, patients with BSIs were significantly more likely to have a history of diabetes mellitus, need for mechanical ventilation, diagnosis of acute respiratory distress syndrome (ARDS), and lower haematocrit (Ht) and haemoglobin (Hb) values on admission than patients without BSIs. Furthermore, they had a prolonged length of ICU stay and were significantly more likely to die in the ICU. Multivariable logistic regression analysis showed that the independent risk factors most predictive for the development of BSI were: APACHE II score on admission (OR 1·07, 95% CI 1·04–1·1, P<0·001), the presence of ARDS (OR 3·57, 95% CI 1·92–6·64, P<0·001), and a history of diabetes mellitus (OR 2·37, 95% CI 1·36–4·11, P=0·002).
Table 1. Demographic and clinical data of ICU patients with and without BSIFootnote *
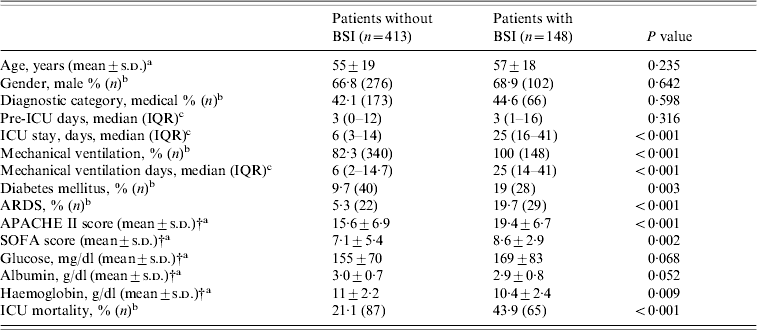
APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, Acute Respiratory Distress Syndrome; BSI, bloodstream infection; ICU, intensive-care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
* Comparisons were made using: at test, b χ2 test, c Mann–Whitney U test.
† At ICU admission.
Mortality and risk factors for death in all studied ICU patients
The ICU mortality for patients who developed BSIs was 43·9% compared with 21% in the control group (P<0·001, Table 1). The median survival time after the first episode of BSI was 15 days (IQR 4·3–27 days). By multivariable logistic regression analysis, independent predictors of mortality for all patients were: APACHE II score (OR 1·14, 95% CI 1·10–1·18, P<0·001), the presence of BSI (OR 1·76, 95% CI 1·11–2·78, P=0·015), and the presence of ARDS (OR 5·02, 95% CI 2·52–9·98, P<0·001).
Mortality and risk factors for death in the 148 ICU patients with BSI
Characteristics of the ICU patients with BSI upon ICU admission and on the day of the first BSI episode, according to outcome, are shown in Table 2. Patients who died were older, more severely ill on both ICU admission and day of first BSI episode, as shown by APACHE II and SOFA scores, compared to those patients with BSI who survived. In addition, they had lower values of albumin, Ht and Hb, both on admission and BSI day. The level of glucose, the white cell blood count and the temperature on BSI day, were not significantly different between the two groups. Multivariable logistic regression analysis showed that an increased SOFA score on BSI day (OR 1·44, 95% CI 1·22–1·67, P<0·001) and the level of albumin (OR 0·47, 95% CI 0·23–0·97, P=0·04) were associated with an increased probability of death.
Table 2. Characteristics of ICU patients with BSI (n=148), according to the outcomeFootnote *
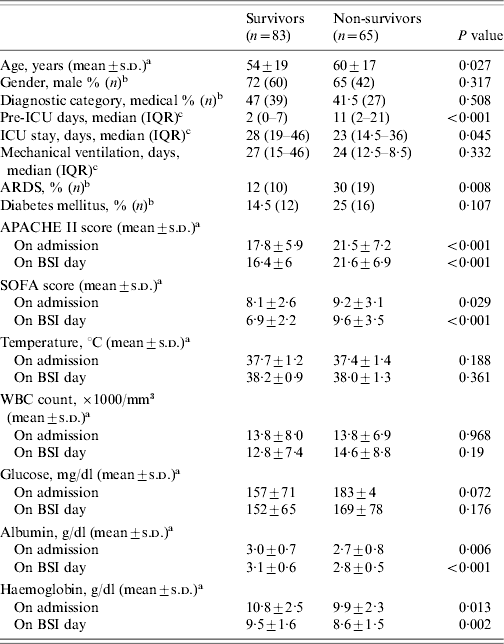
APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, Acute Respiratory Distress syndrome; BSI, bloodstream infection; ICU, intensive-care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; WBC, white cell blood cell count.
* Comparisons were made using: at test, b χ2 test, c Mann–Whitney U test.
Microbiological findings
During the study period the 148 patients who developed ICU-acquired BSI had a total of 232 episodes of bacteremia and/or fungaemia. Ninety-four patients (63·5%) developed one episode of BSI and 54 patients (36·5%) had two or more episodes. Of the 232 episodes, 90% were monomicrobial and 10% polymicrobial. There was no difference in mortality between patients with one and those with two or more BSI episodes (40·4% vs. 50%, P=0·259). All isolated organisms are shown in Table 3. The most frequent group of pathogens was Gram-negative accounting for 67·8% of all isolates. Acinetobacter baumannii was the most frequent (25·7%), followed by Klebsiella pneumoniae (16·2%) and Pseudomonas aeruginosa (12·2%). Of 23 Candida isolates from BSIs, 15 (65%) were non-albicans Candida spp. The proportion of methicillin resistance in S. aureus isolates was 90% and of 38 K. pneumoniae isolates, 35 (92%) produced extended spectrum β-lactamases. Antimicrobial resistance levels for the Gram-negative organisms are shown in Table 4. There was no statistically significant difference in mortality between patients with BSI due to sensitive or resistant strains (47% vs. 30%, P=0·07).
Table 3. Microorganisms responsible for ICU-acquired BSIs
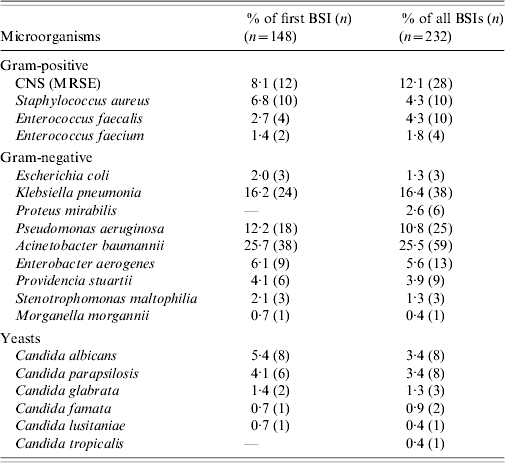
BSI, Bloodstream infection; CNS, coagulase-negative staphylococcus; ICU, intensive-care unit; MRSE, methicillin-resistant Staphylococcus epidermidis.
Table 4. Antimicrobial resistance of the Gram-negative blood cultures isolates
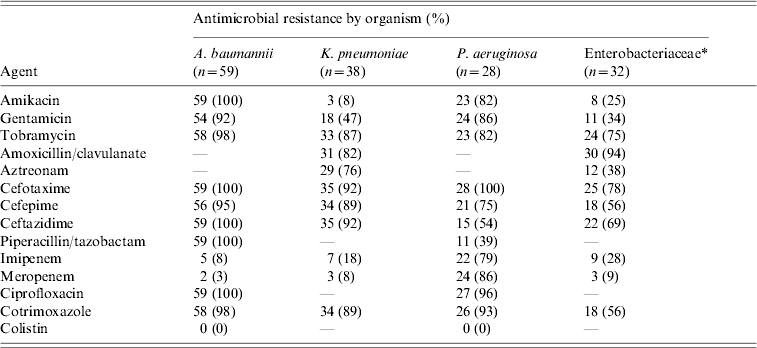
* Other than Klebsiella pneumoniae, Escherichia coli, Proteus mirabilis, Enterobacter aerogenes, Providencia stuartii, Morganella morgannii.
DISCUSSION
The main findings of the present study are: first, a high incidence of BSIs of 16 episodes/1000 ICU patient-days was found; second, Gram-negative bacteria with a high level of resistance predominated; third, the illness severity on admission, the presence of ARDS and a history of diabetes mellitus were risk factors for the occurrence of BSI; finally, the illness severity on ICU admission and the development of an ICU-acquired BSI were independent risk factors for death. Furthermore, in the group of patients who developed BSIs, the severity of organ dysfunction on the day of the first BSI episode and the level of serum albumin independently affected the outcome.
The high incidence of BSIs found here might be attributable to several factors. A growing incidence of BSIs has been reported in ICU patients over the last decades [Reference Pittet and Wenzel17] and has been linked to various factors including an increase in the number of debilitated patients and the use of invasive procedures. Indeed, all patients included in this study had at least one central venous and one peripheral arterial catheter in place. In addition, according to the results of the present study, similar to those of others [Reference Routsi10, Reference Laupland18], the illness severity on ICU admission and, additionally, the presence of ARDS, were factors independently associated with the occurrence of ICU-acquired BSI. Therefore, the high incidence of BSIs found, at least in part, reflects the illness severity the patients had. Furthermore, full-bed occupancy combined with the high workload in our ICU during the study period, due to limited personnel, probably contributed to this increase. As previously reported, the higher the workload, the lower the compliance with precaution measures, thus resulting in a high rate of nosocomial infections [Reference Pittet19, Reference Borg20]. However, it should be noted that the incidence of BSIs reported here, refers to patients with an ICU stay of >48 h and not to the whole number of ICU admissions, since, by definition, patients who stayed in the ICU for <48 h, i.e. only for post-operative monitoring, were excluded. Finally, the finding of diabetes mellitus as an independent risk factor for BSI development is plausible and consistent with other studies showing that diabetic patients may have an increased incidence of BSI [Reference Tsai21, Reference Sreeramoju22].
The clinical significance of BSIs in ICU patients is controversial. Several studies have shown that bacteraemic patients are at greater risk of death than those with comparable severity of illness and without this complication [Reference Pittet, Tarara and Wenzel3, Reference Routsi10, Reference Laupland18]. On the contrary, other studies did not find any significant difference in mortality between bacteraemic and non-bacteraemic critically ill patients [Reference Rello23, Reference Blot, Vandewoude and Colardyn24]. The findings here showing that the development of an ICU-acquired BSI is independently related to the clinical outcome with an odds ratio of 1·76 are in accordance with previous studies. The mortality rate of 44% in patients with BSI, significantly higher than that of patients without BSI, corresponds to that reported in other ICU studies [Reference Pittet, Tarara and Wenzel3, Reference Valles, Leon and Alvarez-Lerma5–Reference Harbarth7] and could be attributable to several factors including the severity of illness. Indeed, we found a strong correlation between the SOFA score on BSI day and outcome. This accords with previous studies and emphasizes the role of the underlying illness. Unsurprisingly, mortality was higher if bacteraemia was associated with shock [Reference Rello4, Reference Brun-Buisson, Doyon and Carlet6], ARDS [Reference Valles, Leon and Alvarez-Lerma5, Reference Fein25, Reference Kaplan, Sahn and Petty26], severity of illness at the onset of BSI and the number of evolving organ failures thereafter [Reference Harbarth7]. The additional finding of the relation of a low serum albumin level with mortality could also represent a surrogate marker of the illness severity rather than a specific aetiological factor per se.
The predominance of Gram-negative over Gram-positive organisms, although in agreement with studies reported in the early 1990s [Reference Bueno-Cavanilas27], is inconsistent with subsequent reports showing an increased incidence of Gram-positive bacteria in ICU patients with BSI [Reference Valles, Leon and Alvarez-Lerma5–Reference Harbarth7, 28]. However, a number of factors including prior antibiotic use, increased age of hospitalized patients and the severity of comorbid illness have contributed to a steady increase in Gram-negative infections and they remain an ever-present threat particularly to patients in ICUs [Reference Waterer and Wunderink29–Reference Kohlenberg32].
Multidrug-resistant A. baumannii was the most frequent single organism isolated from this series of BSIs. A. baumannii isolates have an increasing resistance to many antimicrobials, and exhibit a propensity for clonal spread. Indeed, the present study was performed 1 year after our unit had experienced an outbreak of multidrug-resistant A. baumannii [Reference Kraniotaki33] which has since become endemic in the unit. Data regarding molecular typing of the A. baumanni isolates are not available for the study period and this is a limitation of the study. In accordance with this finding recent microbiological data derived from the ongoing Greek System for the Surveillance of Antibiotic Resistance [9] have shown an increase in the proportion of imipenem-resistant A. baumannii isolates [Reference Falagas34]. Similarly, multidrug-resistant Gram-negative pathogens including Acinetobacter spp. predominated in high numbers in isolates causing BSIs particularly in ICUs, in two recent studies from Asia [Reference Wu35, Reference Jerassy36]. These findings along with the reported increased proportion of Acinetobacter spp. associated with ICU pneumonia in the United States [Reference Gaynes and Edwards30] indicate that this is a worldwide issue.
Multidrug-resistant strains have become a major clinical problem in many countries, especially in ICUs [Reference Bueno-Cavanilas27, Reference Flournoy37, Reference Jones38] but the contribution of antibiotic resistance to outcome in infected hospitalized patients remains unclear. Multidrug resistance was associated with an increased risk of death in some studies [Reference Raymond39] but this has not been confirmed by others [Reference Harbarth40]. A relation between multidrug resistance and mortality was not apparent in the present study; however, the barely significant P value of 0·07 does not permit a definitive conclusion. Furthermore, this is an observational study, not designed to elucidate the factors associated with the development of multidrug resistance and its effect on outcome. Nevertheless, it illustrates the epidemiology and the clinical impact of BSIs in our ICU and, although it may not be applicable to all ICU populations because of the high incidence of Gram-negative pathogens, is a potential alarm for the imminent appearance of pan-drug-resistant strains.
In summary, we report an increased incidence of BSIs in ICU patients with a predominance of multidrug-resistant Gram-negative strains. The severity of illness on admission correlated with the incidence, and the severity of organ dysfunction on BSI day are associated with the outcome of affected patients. Finally, the presence of ICU-acquired BSI independently influences the outcome. Thus, surveillance programmes and rigorous infection control strategies in our ICU can contribute to reduce both the incidence and resistance of microbial pathogens, with better patient outcomes.
ACKNOWLEDGEMENTS
This study was supported in part by the Thorax Foundation and by the Special Account for Research Grants of the University of Athens.
DECLARATION OF INTEREST
None.






