Introduction
Breast cancer is the most prevalent malignancy in women worldwide. The global incidence of breast cancer was estimated at more than 1.3 million new cases in 2007, and nearly 465 000 women were expected to die because of this disease [Reference Garcia, Jemal and Ward1]. The incidence of breast cancer increases with age – in the United States, the 10-year probability of developing invasive breast cancer increases from one in 234 for a 30-year-old woman to one in 28 for a 60-year-old woman [2]. In spite of the striking correlation between advanced age and risk of invasive breast cancer, approximately one quarter of all breast cancers are diagnosed in premenopausal women [Reference Anderson, Chatterjee, Ershler and Brawley3]. The last few decades have seen great improvements in the early detection of breast tumors, and as a result, the majority of breast cancers are diagnosed at early stages, allowing for earlier and more effective therapy [2]. However, clinical outcomes can be improved further. For instance, the 5-year survival rate is lower for women with breast cancer diagnosed before they are 40 years old (80%) compared with those diagnosed at ages 40 and older (89%), potentially because younger women present with more aggressive disease at the time of diagnosis [2].
The expression of estrogen and/or progesterone receptors in tumor cells is a major determinant of therapeutic options for breast cancer. Hormone receptor-negative breast cancers, which are more common in premenopausal compared with postmenopausal women, are typically treated using cytotoxic chemotherapy. However, approximately 60% of breast cancers detected in premenopausal women are hormone receptor-positive (HR+), and will likely respond to endocrine therapy [Reference Anderson, Chatterjee, Ershler and Brawley3]. Although the efficacy of surgical ovarian ablation (oophorectomy) for targeted treatment of breast cancer has been known for more than a century, progress in improving endocrine therapies in the premenopausal setting has been slow [Reference Pritchard4].
Ovarian ablation in premenopausal women with breast cancer
Estrogen production in premenopausal women is predominantly ovarian, with a small component contributed by aromatization reactions in extragonadal tissues. In contrast, aromatase activity in peripheral tissues is the main source of estrogen in postmenopausal women [Reference Emens and Davidson5]. As a result, aromatase inhibitors (AIs) effectively suppress estrogen production in postmenopausal women, whereas suppression of estrogen production in premenopausal women requires ovarian ablation. Permanent ovarian ablation can be achieved by surgery (bilateral oophorectomy) or radiation therapy; cytotoxic agents such as cyclophosphamide can also induce ovarian dysfunction [Reference Emens and Davidson5]. Bilateral oophorectomy is the preferred means of achieving permanent ovarian ablation because it leads to a rapid and irreversible decrease in circulating estrogen levels and also reduces the risk of ovarian cancer in women who are at elevated risk for that disease [Reference Emens and Davidson5].
Although early trials of ovarian ablation did not reveal an immediate benefit in the premenopausal breast cancer setting, a meta-analysis of data from various trials in patients with early breast cancer (with or without chemotherapy; total N > 2200) revealed that ovarian ablation resulted in significant improvements in recurrence-free survival (RFS) and overall survival (OS) compared with patients who did not undergo ovarian ablation [6]. Overall improvement in RFS in the ovarian ablation groups was 18.5% (P = 0.0007). The effect of ovarian ablation was much more dramatic in patients who did not receive chemotherapy: ovarian ablation improved RFS by 25% (P = 0.0005) in this cohort vs. 10% (statistically not significant) in the chemotherapy cohort [6]. Overall survival was similarly improved by ovarian ablation. The benefits of ovarian ablation were more substantial in patients with node-positive disease compared with node-negative: relative improvements in disease-free survival (DFS) and OS were 13.4% and 12.5%, respectively, for node-positive cancers and 8.9% and 5.6%, respectively, for node-negative cancers (P ⩽ 0.01 vs. no ablation for all) [6]. Conversely, improvements in RFS and OS because of ovarian ablation were not significant in 1354 women aged 50 years or older (perimenopausal population) [7]. In another trial in 762 premenopausal women with node-positive, HR+ breast cancer, ovarian ablation and chemotherapy provided similar DFS and OS benefits [Reference Ejlertsen, Mouridsen and Jensen8]. However, chemotherapy was associated with more adverse events: 57% of patients in the chemotherapy arm experienced at least one episode of myelosuppression, and 33% of patients had moderate to severe nausea and vomiting in spite of prophylactic antiemetics [Reference Ejlertsen, Mouridsen and Jensen8]. Thus, in this study, ovarian ablation provided survival benefits similar to those of cytotoxic chemotherapy with less toxicity.
It is also important to note that adjuvant chemotherapy frequently leads to ovarian shutdown in premenopausal women. The rate of chemotherapy-induced ovarian shutdown is influenced by the chemotherapy regimen used, the number of cycles, and the patient’s condition and age [Reference Walshe, Denduluri and Swain9]. For example, 96% of women between the ages of 40 and 49 years experienced ovarian failure after doxorubicin-based chemotherapy compared with only 33% of women between the ages of 30 and 39 years [Reference Walshe, Denduluri and Swain9]. Similarly, among women receiving epirubicin-based chemotherapy, rates of amenorrhea were 88% in women 40 years of age or older vs. 32% in women between the ages of 32 and 39 years [Reference Walshe, Denduluri and Swain9]. Therefore, it is likely that improved clinical outcomes after adjuvant chemotherapy are at least partly attributable to the indirect endocrine effects of these treatments, especially in older pre- and perimenopausal women. Furthermore, a retrospective analysis of 3700 premenopausal women who underwent adjuvant chemotherapy for breast cancer showed that clinical outcomes were significantly worse in younger women (<35 years old): 10-year DFS was 35% for women under the age of 35 years vs. 47% for older women (P < 0.001) [Reference Aebi, Gelber and Castiglione-Gertsch10]. Interestingly, DFS was substantially lower in young premenopausal women receiving chemotherapy for HR+ (hormone-responsive) tumors compared with hormone receptor-negative tumors [Reference Aebi, Gelber and Castiglione-Gertsch10]. Taken together with the lower rates of chemotherapy-induced ovarian shutdown in younger women, these data indicate that young premenopausal women with HR+ breast cancer may benefit from endocrine therapy.
Reversible ovarian suppression as adjuvant therapy for premenopausal women with breast cancer
Reversible ovarian suppression (sometimes also called medical castration) can be achieved through treatment with luteinizing hormone-releasing hormone (LHRH) analogues. These agents act via pituitary LHRH receptors to suppress gonadotropin secretion, leading to dramatic reductions in the production of steroid hormones by the ovary [Reference Emens and Davidson5]. This modality of ovarian suppression is generally reversible, thereby preserving fertility in young women undergoing adjuvant therapy for breast cancer. The efficacy of goserelin was similar to that of ovarian ablation (oophorectomy or radiation) in a small prospective trial in 85 perimenopausal women with metastatic breast cancer [Reference Boccardo, Rubagotti and Perrotta11]. As a result, more recent studies have included treatment with goserelin or other LHRH inhibitors (e.g., leuprolide and triptorelin) as a means of suppressing ovarian function in pre- and perimenopausal women with breast cancer.
Trials comparing reversible ovarian suppression to chemotherapy in the adjuvant setting for premenopausal breast cancer
The Zoladex Early Breast Cancer Research Association (ZEBRA) trial
The ZEBRA study evaluated the efficacy of ovarian suppression with goserelin (3.6 mg subcutaneous (SC) every 28 days; n = 817) compared with six cycles of CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy (n = 823) as adjuvant therapy for node-positive breast cancer in premenopausal women [Reference Jonat, Kaufmann and Sauerbrei12]. After a median follow-up at 6 years, DFS and OS were similar in both treatment arms for patients with estrogen receptor-positive (ER+) tumors (goserelin vs. CMF: hazard ratio (HR) = 1.01 for DFS and 0.99 for OS). However, goserelin was significantly inferior to CMF in the estrogen receptor-negative cohort (HR = 1.76, P = 0.0006 for DFS and HR = 1.77, P = 0.0043 for OS) [Reference Jonat, Kaufmann and Sauerbrei12]. Amenorrhea was prevalent in the goserelin arm during treatment but was reversed in more than 75% of patients within 1 year after stopping goserelin. Although fewer patients in the CMF arm developed amenorrhea during treatment, the amenorrhea in this group persisted for years after completion of therapy [Reference Jonat, Kaufmann and Sauerbrei12].
The Takeda Adjuvant Breast Cancer Study with Leuprorelin Acetate (TABLE) trial
This study evaluated the efficacy of ovarian suppression using the LHRH agonist leuprorelin acetate (SC every 3 months for 2 years; n = 299) with six cycles of standard CMF chemotherapy (n = 300) in premenopausal women with node-positive breast cancer [Reference Schmid, Untch and Wallwiener13,Reference Schmid, Untch and Kosse14]. At a median follow-up of 5.8 years, RFS was similar in the two treatment arms (HR = 1.19; P = 0.15), whereas exploratory analysis of OS favors the endocrine therapy arm (HR = 1.50; P = 0.005) [Reference Schmid, Untch and Kosse14]. These data show that ovarian suppression using LHRH agonists has efficacy comparable with that of chemotherapy in premenopausal women with endocrine-responsive breast cancer.
Tamoxifen as adjuvant therapy for premenopausal women with breast cancer
Tamoxifen is a selective estrogen receptor modulator that has been used extensively to treat both metastatic and early stage hormone-responsive breast cancer. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) compounded a recent overview of several trials of tamoxifen in the adjuvant setting. The ‘control’ arms in these studies varied from no adjuvant therapy in some to active chemotherapy in others, but all were characterized by the absence of adjuvant tamoxifen. Outcomes of these meta-analyses revealed that 5 years of adjuvant tamoxifen treatment reduces the annual death rate attributable to breast cancer by approximately 31% vs. control in patients with ER+ disease, and this effect is largely independent of age, adjuvant chemotherapy, progesterone-receptor status, or other tumor characteristics [15]. In pooled data from 44 trials (total N > 33 000), tamoxifen treatment for 1 to 2 years significantly decreased breast cancer recurrence and mortality in women with ER+ disease (tamoxifen vs. control: HR = 0.74 for recurrence and 0.82 for OS; P < 0.00001 for both) [15]. Data from 12 trials of tamoxifen treatment for 5 years (total N > 15 000) yielded even greater improvements in clinical outcomes (tamoxifen vs. control: RFS HR = 0.59 and OS HR = 0.66; P < 0.00001 for both) [15]. Tamoxifen treatment for this duration also significantly decreased the annual incidence of contralateral breast carcinomas from 0.6% (in control arm) to 0.4% (P < 0.00001). Approximately 5 years of tamoxifen treatment improved RFS and OS in women with ER+ disease irrespective of age: HRs for RFS were 0.56 for age less than 40 years, 0.71 for ages 41 to 49 years, 0.66 for ages 50 to 59 years, and 0.55 for ages 60 to 69 years (P < 0.00001 for tamoxifen vs. control in all strata) [15].
It is therefore evident from the data described that tamoxifen substantially improves clinical outcomes and survival for premenopausal women with hormone-responsive breast cancer compared with controls. However, tamoxifen treatment is associated with a significant increase in uterine cancers: in the pooled analysis described above, the annual rate of uterine cancer in patients receiving 5 years of tamoxifen (n = 7512) was 0.19% compared with 0.06% in controls (n = 7505; P < 0.00001) [15]. In the same analysis, there was also a trend toward an increased incidence of stroke in patients receiving tamoxifen for 5 years (54 vs. 29 controls; P = 0.07) [15]. Therefore, other options for adjuvant endocrine therapy should be investigated in the premenopausal breast cancer setting.
Sequential and combination adjuvant endocrine therapies in the premenopausal breast cancer setting
Ovarian suppression and adjuvant chemotherapy individually decrease disease recurrence and improve survival in premenopausal women with hormone-responsive breast cancer. Further, pooled data from several randomized clinical trials projected that sequential therapy with six cycles of anthracycline-based adjuvant chemotherapy followed by 5 years of tamoxifen will reduce 15-year mortality because of HR+ breast cancer by approximately 50% [15]. In addition, a meta-analysis of four trials (combined N = 506) of tamoxifen plus LHRH agonists vs. LHRH agonists alone in the advanced breast cancer setting in premenopausal women revealed that the combined endocrine therapy significantly improved OS (HR = 0.78; P = 0.02) and progression-free survival (HR = 0.70; P = 0.0003) [Reference Klijn, Blamey, Boccardo, Tominaga, Duchateau and Sylvester16]. However, the effects of adjuvant therapies plus ovarian suppression (concomitant or sequential) in the early breast cancer setting were less clear. The following section will summarize key findings from trials (Table 1) that evaluated sequential and combination chemoendocrine therapy regimens.
Table 1 Clinical trials evaluating the efficacy of ovarian suppression as combination or sequential therapy for hormone receptor-positive breast cancer in premenopausal women.
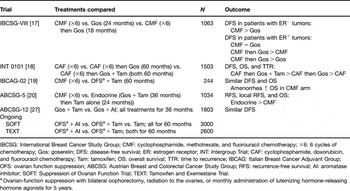
IBCSG: International Breast Cancer Study Group; CMF: cyclophosphamide, methotrexate, and fluorouracil chemotherapy; ×6: 6 cycles of chemotherapy; Gos: goserelin; DFS: disease-free survival; ER: estrogen receptor; INT: Intergroup Trial; CAF: cyclophosphamide, doxorubicin, and fluorouracil chemotherapy; Tam: tamoxifen; OS: overall survival; TTR: time to recurrence; IBCAG: Italian Breast Cancer Adjuvant Group; OFS: ovarian function suppression; ABCSG: Austrian Breast and Colorectal Cancer Study Group; RFS: recurrence-free survival; AI: aromatase inhibitor; SOFT: Suppression of Ovarian Function Trial; TEXT: Tamoxifen and Exemestane Trial.
aOvarian-function suppression with bilateral oophorectomy, radiation to the ovaries, or monthly administration of luteinizing hormone-releasing hormone agonists for 5 years.
Trials evaluating sequential chemo- and endocrine therapies
International Breast Cancer Study Group (IBCSG) trial VIII
The IBCSG trial VIII investigated the efficacy of six cycles of CMF chemotherapy (n = 360) vs. ovarian suppression with goserelin for 2 years (n = 346) vs. CMF (six cycles) followed by goserelin (for 18 months; n = 357) in pre- and perimenopausal women with node-negative breast cancer [Reference Castiglione-Gertsch, O’Neill and Price17]. At a median follow-up of 7 years, DFS in the hormone receptor-negative subgroup was higher with CMF (alone or followed by goserelin) compared with goserelin alone. In contrast, in the HR+ cohort, CMF and goserelin each provided similar DFS benefits (5-year DFS = 81% for both groups) and the sequential therapy improved DFS (5-year DFS = 86%) compared with either modality alone [Reference Castiglione-Gertsch, O’Neill and Price17]. The improvement in DFS with sequential therapy was especially marked in younger women (⩽39 years old) with HR+ tumors. In this group, the DFS HR for sequential therapy vs. monotherapy was 0.34 (P = 0.02) [Reference Castiglione-Gertsch, O’Neill and Price17].
Eastern Cooperative Oncology Group/ US Intergroup Trial INT 0101
This study compared the efficacy of chemotherapy (six cycles of cyclophosphamide, doxorubicin, and fluorouracil (CAF)) alone (n = 494), CAF followed by 5 years of monthly goserelin (n = 502), or CAF followed by 5 years of monthly goserelin and daily tamoxifen (n = 507) in premenopausal women with HR+ breast cancer [Reference Davidson, O’Neill and Vukov18]. At a median follow-up of 9.6 years, sequential therapy with CAF and goserelin produced non-significant improvements in DFS, OS, and time to recurrence compared with CAF alone. In contrast, sequential therapy with CAF, goserelin, and tamoxifen significantly improved DFS (HR = 0.74; P < 0.01) and time to recurrence (HR = 0.73; P < 0.01) compared with CAF followed by goserelin alone [Reference Davidson, O’Neill and Vukov18].
Trials evaluating combination endocrine therapies vs. chemotherapy
Italian Breast Cancer Adjuvant Group 02 (IBCAG-02) randomized trial
The IBCAG-02 trial compared the efficacy of six cycles of standard CMF chemotherapy (n = 120) with 5 years of ovarian suppression plus daily tamoxifen (n = 124) [Reference Boccardo, Rubagotti and Amoroso19]. Ovarian suppression was achieved by oophorectomy (n = 6), irradiation (n = 31), or goserelin injections (n = 87). At a median follow-up of 76 months, DFS and OS were comparable between groups. No significant differences in DFS or OS were observed between treatments after controlling for age, tumor size, and nodal status. However, treatment-induced amenorrhea was associated with significantly better OS within the CMF arm (P = 0.05) [Reference Boccardo, Rubagotti and Amoroso19].
Austrian Breast and Colorectal Cancer Study Group Trial 5 (ABCSG-5)
The ABCSG-5 trial randomized 1034 premenopausal women with hormone-responsive breast cancer to either six cycles of CMF chemotherapy (n = 523) or endocrine therapy (3 years of goserelin plus tamoxifen followed by 2 years of tamoxifen alone; n = 511) [Reference Jakesz, Hausmaninger and Kubista20]. At a median follow-up of 60 months, RFS and local recurrence-free survival differed significantly in favor of endocrine therapy (HR = 1.40, P = 0.019 for RFS; HR = 1.98, P = 0.008 for local recurrence-free survival; both HRs are for chemotherapy vs. endocrine therapy). Overall survival was also improved in the endocrine-therapy arm compared with CMF, but the difference was not statistically significant [Reference Jakesz, Hausmaninger and Kubista20].
A recent meta-analysis of data from 16 trials involving 11 906 premenopausal women with early breast cancer confirmed that LHRH agonists (such as goserelin) have similar efficacy to chemotherapy in ER+ tumors but are ineffective against estrogen receptor-negative tumors [Reference Cuzick, Ambroisine and Davidson21]. The addition of LHRH agonists to tamoxifen, chemotherapy, or both significantly reduced disease recurrence and death after recurrence vs. each regimen without LHRH agonists (12.7% decrease, P = 0.02 and 15.1% decrease, P = 0.03, respectively) [Reference Cuzick, Ambroisine and Davidson21]. Thus, LHRH agonists represent an important addition to adjuvant therapy for hormone-responsive breast cancer in the premenopausal setting.
Aromatase inhibitors in the premenopausal breast cancer setting
Aromatase inhibitors have now become the standard option for adjuvant endocrine therapy in postmenopausal women with HR+ breast cancer, although questions regarding the optimal timing and duration of AI therapy in this setting remain [Reference Winer, Hudis and Burstein22]. In the Breast International Group (BIG) 1-98 study (a randomized, double-blind, phase III trial in 8010 postmenopausal women), the highly active third-generation AI, letrozole, significantly improved DFS (HR = 0.81, P = 0.003 at a follow-up of 26 months; HR = 0.82, P = 0.007 at a follow-up of 51 months) and reduced the incidence of early distant metastases (HR = 0.73, P = 0.001 at 26 months follow-up) [Reference Coates, Keshaviah and Thurlimann23,Reference Thurlimann, Keshaviah and Coates24]. The use of AIs in the premenopausal setting has yet to be established. A major concern about AI therapy in premenopausal women is their potential for hyperstimulation of ovarian function – reduction in estrogen levels because of AI therapy can lead to a reflex increase in gonadotropin production, resulting in ovarian stimulation [Reference Winer, Hudis and Burstein22]. However, the efficacy of AIs combined with chronic ovarian suppression is being evaluated. Recently, the HOBOE (Hormonal Adjuvant Treatment Bone Effects) study completed analysis of the endocrine effects of letrozole in combination with ovarian suppression (using triptorelin administered every 4 weeks) vs. tamoxifen plus triptorelin in the premenopausal cohort (N = 81 women with operable, HR+ breast cancer) [Reference Rossi, Morabito and De Maio25]. After 6 months of adjuvant endocrine therapy, median serum estradiol levels were significantly lower in the letrozole-plus-triptorelin arm vs. tamoxifen plus triptorelin (<5 vs. 7.95 pg/ml; P = 0.0008) [Reference Rossi, Morabito and De Maio25]. Clinical outcomes and skeletal health data from this study will be forthcoming. The combination of goserelin and the AI, anastrozole, brought about a similar substantial reduction in circulating estradiol levels in a small pilot study (n = 16) [Reference Forward, Cheung, Jackson and Robertson26]. Recently, the ABCSG-12 trial evaluated the safety and efficacy of the goserelin–anastrozole combination in >1800 premenopausal women with early stage, hormone-responsive breast cancer [Reference Gnant, Mlineritsch and Schippinger27].
Austrian Breast and Colorectal Cancer Study Group Trial 12 (ABCSG-12)
The ABCSG-12 study randomized 1803 premenopausal women with early stage (I/II) breast cancer to 3 years of treatment with monthly goserelin plus daily tamoxifen or anastrozole, alone or in combination with twice-yearly doses of zoledronic acid (a bisphosphonate) [Reference Gnant, Mlineritsch and Schippinger27]. The primary endpoint was two pairwise comparisons of DFS: the first was the goserelin-plus-anastrozole group vs. goserelin-plus-tamoxifen group, whereas the second was the endocrine therapy-plus-zoledronic-acid group vs. the endocrine therapy-alone group. Secondary endpoints included RFS and OS. Although event-driven analysis at a median follow-up of 47.8 months revealed that anastrozole was not superior to tamoxifen, there was no significant difference in efficacy between the anastrozole and tamoxifen arms in this patient population (HR = 1.10, P = 0.59 for DFS) [Reference Gnant, Mlineritsch and Schippinger27]. However, in keeping with the known safety profiles of these agents, patients receiving anastrozole experienced fewer endometrial abnormalities (e.g., uterine polyps) and thromboembolic adverse events compared with tamoxifen. Longer follow-up of this trial is needed to elucidate possible differences between anastrozole and tamoxifen in long-term efficacy and safety outcomes. Interestingly, the addition of twice-yearly zoledronic acid to adjuvant endocrine therapy not only prevented bone loss [Reference Gnant, Mlineritsch and Luschin-Ebengreuth28] but also significantly improved DFS (HR = 0.64; P = 0.012) and RFS (HR = 0.65; P = 0.014) compared with endocrine therapy alone [Reference Gnant, Mlineritsch and Schippinger27]. These results indicate that concomitant treatment with bisphosphonates such as zoledronic acid to preserve bone health during adjuvant endocrine therapy for premenopausal breast cancer may provide additional benefits including substantially improved clinical outcomes, potentially because of the inherent antitumor properties of zoledronic acid and other bisphosphonates [Reference Winter, Holen and Coleman29].
The efficacy of ovarian suppression combined with AIs in the premenopausal setting is currently being further evaluated. Ongoing phase II studies in the premenopausal metastatic (stage IV) breast cancer setting include trials NCT00498901 in the United States (sample size, 25) comparing letrozole plus goserelin with letrozole plus leuprolide [30] and NCT00532272 in Korea (sample size, 70) comparing letrozole plus goserelin in premenopausal women with letrozole alone in postmenopausal women [31]. In the adjuvant setting for early stage breast cancer, ongoing phase III trials evaluating ovarian suppression plus AIs involve >5000 premenopausal women. The SOFT (Suppression of Ovarian Function) trial is ongoing and will enroll 3000 patients [32]. This study will compare the efficacy of ovarian suppression plus the AI exemestane vs. ovarian suppression plus tamoxifen vs. tamoxifen alone. Another ongoing trial with similar design, TEXT (Tamoxifen and Exemestane Trial), will compare ovarian suppression plus exemestane with ovarian suppression plus tamoxifen in more than 2600 premenopausal women with early stage breast cancer [33]. Data from these trials will further elucidate the therapeutic potential of AIs in the adjuvant as well as advanced settings for premenopausal breast cancer.
Conclusions
A large body of data shows that adjuvant endocrine therapy for premenopausal women with hormone-responsive breast cancer can achieve clinical outcomes similar to that of cytotoxic chemotherapy while sparing these patients the toxicities and adverse events associated with chemotherapy. Moreover, in recent years, it has also become evident that the combination of standard adjuvant therapy with chronic, reversible ovarian suppression (e.g., using LHRH agonists) can further improve DFS and OS in this setting. Although the adjuvant role of AIs is yet to be established in premenopausal women undergoing chronic ovarian suppression, it is possible that potent, third-generation AIs may provide benefits beyond those obtained with tamoxifen in this setting. Future data from ongoing trials will help to further understand the utility of AIs in the adjuvant setting for premenopausal breast cancer. Interestingly, recent data reveal that the addition of bisphosphonates to adjuvant endocrine therapy not only prevents AI-associated bone loss but also substantially improves clinical outcomes for premenopausal women with early stage breast cancer. Therefore, such combination therapies might, in future, provide the best overall outcomes in this setting.
Acknowledgements
The ABCSG-12 clinical trial was supported by AstraZeneca and Novartis Pharmaceuticals. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Shalini Murthy, PhD, for her medical editorial assistance with this manuscript.
Conflict of interest: Dr Gnant has served on advisory boards for and received consulting fees from AstraZeneca, Novartis, and Pfizer and lecture fees from Roche, Schering, Pfizer, Novartis, AstraZeneca, sanofi-aventis, and Amgen.



