In addition to its long-established role in blood clotting, current evidence suggests that phylloquinone (vitamin K1) is the most important form of vitamin K in relation to extrahepatic functions and, as such, may impact on bone and cardiovascular health (Shearer, Reference Shearer, Garrow, James and Ralph2000a, Reference Shearerb; Weber, Reference Weber2001). Phylloquinone is the predominant form of vitamin K in the circulation and is thought to be derived exclusively from the diet (Shearer, Reference Shearer, Garrow, James and Ralph2000a), and not by metabolic conversion from other forms of vitamin K.
Plasma phylloquinone concentration has been shown to be highly correlated with carboxylation status of serum or plasma osteocalcin (Sokoll & Sadowski, Reference Sokoll and Sadowski1996; Binkley et al. Reference Binkley, Krueger, Engelke, Foley and Suttie2000; McKeown et al. Reference McKeown, Jacques, Gundberg, Peterson, Tucker, Kiel, Wilson and Booth2002; Yan et al. Reference Yan, Zhou, Greenberg, Wang, Nigdikar, Prynne and Prentice2004; Beavan et al. Reference Beavan, Prentice, Stirling, Dibba, Yan, Harrington and Shearer2005), a protein involved in bone mineralisation owing to its high affinity for binding Ca when in its vitamin K-dependent carboxylated state. Serum undercarboxylated osteocalcin has been shown to be a risk factor for hip fracture in elderly women (Szulc et al. Reference Szulc, Chapuy, Meunier and Delmas1993). There is also increasing evidence that adequate vitamin K intake, and correspondingly adequate plasma concentration, may also help to maintain bone health by positively affecting Ca balance through reduced urinary excretion (Weber, Reference Weber2001). Another vitamin K-dependent protein, matrix Gla protein, may help to prevent soft tissue, including vascular, calcification when in its carboxylated state and so reduce the risk of atherosclerosis and arteriosclerosis (Shearer, Reference Shearer2000b; Braam et al. Reference Braam, Hoeks, Brouns, Hamulyák, Gerichhausen and Vermeer2004). Assessment of vitamin K status in populations may therefore be of public health significance.
Plasma phylloquinone concentrations are sensitive to changes in recent dietary intake (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer1998; Booth & Suttie, Reference Booth and Suttie1998), responding rapidly to diets that have been depleted and subsequently repleted of phylloquinone content (Suttie et al. Reference Suttie, Mummah-Schendel, Shah, Lyle and Greger1988; Ferland et al. Reference Ferland, Sadowski and O'Brien1993; Booth et al. Reference Booth, Lichtenstein, O'Brien-Morse, McKeown, Wood, Saltzman and Gundberg2001), or rise rapidly in response to supplementation (Sokoll et al. Reference Sokoll, Booth, O'Brien, Davidson, Tsaioun and Sadowski1997; Binkley et al. Reference Binkley, Krueger, Engelke, Foley and Suttie2000). Although plasma phylloquinone concentration it is not a functional biomarker of status (such as undercarboxylated osteocalcin and undercarboxylated prothrombin, PIVKA-II), there is evidence that plasma concentrations broadly reflect tissue and body stores (Usui et al. Reference Usui, Tanimura, Nishimura, Kobayashi, Okanoue and Ozawa1990; Shearer, Reference Shearer, Garrow, James and Ralph2000a; Olson et al. Reference Olson, Chao, Graham, Bates and Lewis2002). Since it can be measured reliably by HPLC, it is likely that plasma phylloquinone concentration can be used as a biochemical index of vitamin K status.
To the best of our knowledge, the present study is only the second to report plasma phylloquinone concentration in a representative sample of British adults; the first reported values from a national sample of adults aged 65 years and over living in mainland Britain (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a). Plasma phylloquinone concentrations are reported in a nationally representative sample of adults aged 19–64 years living in the UK. The variation in plasma phylloquinone concentration with socio-demographic factors, smoking status and season is reported, together with its association with phylloquinone intake, other biochemical indices of nutritional status and blood analytes of interest to nutrition and health.
Subjects and methods
The present study used data from a nationally representative sample of adults living in mainland Britain, who participated in the 2000–1 National Diet and Nutrition Survey of people aged 19–64 years. Full details and methods relevant to the present paper are provided in two of the survey reports (Henderson et al. Reference Henderson, Gregory and Swan2002; Ruston et al. Reference Ruston, Hoare, Henderson, Gregory, Bates, Prentice, Birch, Swan and Farron2004), and so only a brief description is given here.
After obtaining ethical approval for all aspects of the survey from the National Health Service Local Research Ethics Committees for each of the 152 postcode sectors involved, a stratified random sample of participants living in private households was obtained. The survey fieldwork was conducted from July 2000 to June 2001, with approximately equal numbers of participants in each season. Socio-demographic and lifestyle information was obtained by trained fieldworkers (Henderson et al. Reference Henderson, Gregory and Swan2002).
Blood samples and plasma phylloquinone concentration
After obtaining consent from participants, blood samples were obtained, by venepuncture, by trained phlebotomists. Unlike most blood samples obtained from free-living older adults in the 1994–5 National Diet and Nutrition Survey (Finch et al. Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke1998), participants in the 2000–1 National Diet and Nutrition Survey were not asked to fast overnight before providing a blood sample nor was the blood sample necessarily obtained early in the morning. The timings of blood samples were distributed throughout the day; being taken between 05.50 and 21.50 hours, with most taken between about 07.00 and 14.00 hours.
Full details of the analytical procedures used to quantify plasma phylloquinone concentration are given elsewhere (Wang et al. Reference Wang, Bates, Yan, Harrington, Shearer and Prentice2004). Briefly, plasma phylloquinone concentration was analysed from heparinised plasma samples and stored at − 80°C until analysis. A modified HPLC method, based on Davidson & Sadowski (Reference Davidson and Sadowski1997), was used with fluorescence detection after Zn post-column reduction. Compensation for procedural losses of phylloquinone was made by the method of internal standardisation using a proprietary vitamin K derivative. Increased sensitivity of detection was enabled by the use of the high-sensitivity Waters 440 fluorescence detector (Waters Corp., Milford, MA, USA), while optimised chromatography conditions increased the sensitivity to 4 fmol phylloquinone. This assay had a detection limit of 2·0 pg injection and a lower limit of quantification for 0·25 ml plasma of 0·04 nmol/l (equivalent to 0·02 ng/ml). Concentrations below this limit were arbitrarily assigned a value of 0·02 nmol/l, in order to include these detectable, but not reliably quantifiable, concentrations in the statistical analyses.
Long-term reproducibility of quality-control plasma samples (10 % of the unknowns) was assessed by their analysis in parallel with the unknowns. Intra-assay precisions (CV) of the quality-control plasma samples containing three concentrations of phylloquinone (means 0·4, 1·4 and 3·4 nmol/l) were 5·2 % (n 6), 8·2 % (n 6) and 3·0 % (n 12) respectively. Inter-assay precisions were 16·0 % (n 22), 12·0 % (n 21) and 8·1 % (n 15) respectively.
Dietary assessment and phylloquinone intake
Details of the dietary assessment methodology and phylloquinone intake data are reported elsewhere (Thane et al. Reference Thane, Bolton-Smith and Coward2006). Briefly, participants kept a weighed record of all food and drink consumed over 7 d. Dietary phylloquinone intake and the relative contribution of different food groups were estimated using both published (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer2000; Food Standards Agency, 2002) and unpublished (MJ Shearer and C Bolton-Smith, unpublished results) food content data. For each participant, UK-specific food phylloquinone contents were assigned to every consumption occurrence of each of over 4500 foods consumed during the assessment period. All individual intakes of phylloquinone were then aggregated, in order to provide estimates of daily intake and food sources.
Data analysis
Data were analysed for men and women separately. Associations were examined between plasma phylloquinone concentration and season, smoking status and several socio-demographic factors (age group, occupational social class, region and adiposity). Occupational social class was dichotomised into ‘non-manual’ and ‘manual’ (Thane et al. Reference Thane, Bolton-Smith and Coward2006). Adiposity was indicated by BMI and considered as a continuous factor when assessing its association with plasma phylloquinone concentration. BMI was also categorised ( < 25, ≥ 25 to < 30, and ≥ 30 kg/m2) when examining the variation in plasma phylloquinone concentration. The likelihood of having ‘low’ plasma phylloquinone concentration (defined as values in the lowest fifth of the distribution for men and women separately; < 0·51 and < 0·38 nmol/l respectively) was also assessed by these non-dietary factors. The prevalence and distribution of plasma phylloquinone concentrations below 0·333 nmol/l (0·15 ng/ml) have also been examined since such low concentrations for healthy normolipaemic adults in a non-fasting state have been regarded as sub-optimal in the clinical setting (O'Shaughnessy et al. Reference O'Shaughnessy, Allen, Woodcock, Pearce, Harvey and Shearer2003).
Associations were examined between plasma phylloquinone concentration and dietary phylloquinone intake. These were examined for all participants and, owing to the variable separation of days between dietary assessment and blood sampling, a subset of participants (n 576) for whom plasma phylloquinone concentration was related to phylloquinone intake on the day before blood sampling. Associations were also examined by food sources (i.e. percentage of phylloquinone intake derived from vegetables (range 0–98 %) and fat spreads (0–62 %) respectively, categorised as fifths). Since phylloquinone is fat-soluble, the association between phylloquinone intake and plasma concentration might be expected to be stronger if more of its intake was derived from fats and oils. However, conflicting findings exist in the literature; some report higher absorption of dietary phylloquinone intake when derived from fat sources or when phylloquinone-rich vegetables are consumed with fat or edible oils (Gijsbers et al. Reference Gijsbers, Jie and Vermeer1996; Schurgers & Vermeer, Reference Schurgers and Vermeer2000; Booth et al. Reference Booth, Lichtenstein and Dallal2002), whereas others found no difference in percentage absorption by food source of the whole diet or fat content of meals (Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995, Reference Booth, O'Brien-Morse, Dallal, Davidson and Gundberg1999; Garber et al. Reference Garber, Binkley, Krueger and Suttie1999).
Associations of plasma phylloquinone concentration with consumption of vegetables and the sub-group of cooked leafy green vegetables (as main sources of phylloquinone intake; Thane et al. Reference Thane, Bolton-Smith and Coward2006), other biochemical indices of nutritional status and blood analytes were also examined. The latter two groups included plasma concentrations of total, HDL and non-HDL (mainly LDL) cholesterol, retinol, retinyl palmitate, carotenoids, 25-hydroxyvitamin D, α- and γ-tocopherols, erythrocyte glutathione reductase activation coefficient (as an index of riboflavin status), serum and erythrocyte folate, and haematological indices (including blood Hb, serum ferritin, plasma total Fe-binding capacity, percentage transferrin saturation and serum Fe concentration).
In the analyses, plasma cholesterol and nutritional status indices were further adjusted for intakes of total fat, saturated fatty acids, and their corresponding vitamins, as appropriate. Correlation analyses between plasma phylloquinone concentration and phylloquinone intake only included participants who provided a complete 7 d weighed dietary record. Forward stepwise multiple regression analysis was used to assess the percentage of the variation in plasma phylloquinone concentration associated with the variation in season, smoking status and socio-demographic factors, phylloquinone intake and concentrations of blood analytes and biochemical nutritional status indices. The impact of oral anticoagulants (for example, warfarin) on plasma phylloquinone concentration was discounted since blood samples were not taken if participants were taking these types of drugs, or if they suffered from a clotting or bleeding disorder.
Due to the skewed distributions of both plasma phylloquinone concentrations and estimated intakes, geometric means (with 95 % CI) are given throughout. These were obtained by back-transformation of loge-transformed values and represent better averages for such non-normally distributed data. Other summary statistics for plasma phylloquinone concentration are also provided for comparison. ANOVA (restricted to main effects with no interaction terms) with Scheffé tests, multiple linear and logistic regressions, χ2 tests and two-tailed Pearson's correlation coefficients were performed, with P < 0·01 indicating statistical significance. Data reduction and analyses were carried out using Excel (Microsoft Corp., Redmond, WA, USA) and SPSS (SPSS Inc., Chicago, IL, USA) respectively.
Results
Plasma phylloquinone concentrations showed a positively skewed distribution (geometric mean 0·94, inner 95 % of values 0·10–8·72 nmol/l), with a significant difference by sex (Table 1). Table 1 also shows other summary statistics for the entire sample and for men and women separately. Plasma phylloquinone concentration ranged from ≤ 0·04 to 18·61 nmol/l, with a lower maximum of 13·25 nmol/l in men. Although blood samples were taken at different times of the day, with the consequent differential influence of previous timings and amounts of food consumption, plasma phylloquinone concentration did not vary significantly by time of day in men or women. ‘Sub-optimal’ plasma phylloquinone concentrations were found in 13 % (147 out of 1154) of participants, with a higher prevalence among women than men (17 v. 8 %; P < 0·001). Of the participants, 2 % (22 out of 1154) had concentrations below the lower limit of quantification and were assigned a value of 0·02 nmol/l.
Table 1 Plasma phylloquinone concentration of British adults aged 19–64 years
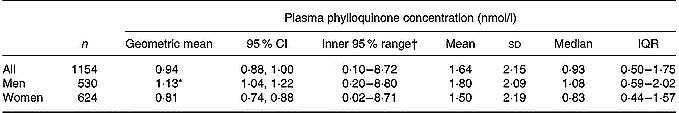
IQR, interquartile range (25th–75th percentile).
* Geometric mean was significantly different from that for women (P < 0·001; ANOVA, after adjusting for age group, region, season, occupational social class, smoking status and adiposity).
† 2·5–97·5 Percentile range. The value of 0·02 nmol/l indicates ≤ 0·04 nmol/l (lower limit of quantification for HPLC).
Variation by socio-demographic factors, smoking status and season
In women (Table 2), differences in plasma phylloquinone concentration by age and season reached statistical significance (P < 0·01). Plasma phylloquinone concentration was directly associated with age, independent of other factors. Blood taken from women in summer also contained lower plasma phylloquinone concentrations compared with winter and spring (January to June). Women aged 19–34 years had lower plasma phylloquinone concentrations than those of men of the same age (P < 0·001), independent of other factors. To a lesser extent, this also applied to participants aged 35–44 years (P = 0·02). Differences in plasma phylloquinone concentration by sex were greatest among participants with non-manual occupations and among non-smokers (each P < 0·001), independent of other factors.
Table 2 Plasma phylloquinone concentration (nmol/l) of British adults aged 19–64 years, by season and selected socio-demographic and lifestyle factors (Geometric means (GM) and 95 % CI)
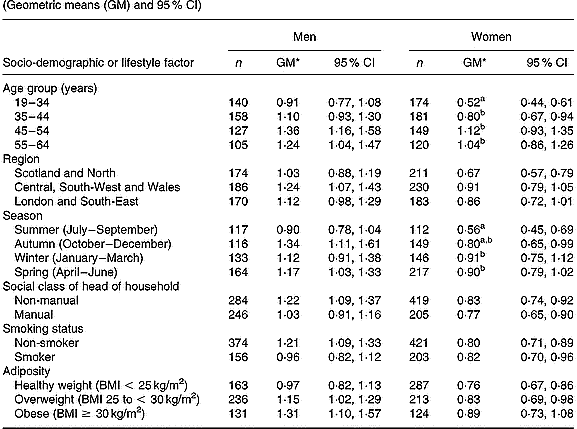
a,b Mean values, within each sex, for categories within respective socio-demographic or lifestyle factors, with unlike superscript letters were significantly different (Scheffé test, P < 0·01; following P < 0·01 in ANOVA after adjusting for age group, region, season, occupational social class, smoking status and adiposity).
* Back-transformed from loge(plasma phylloquinone concentration).
Plasma phylloquinone concentration did not vary significantly by any of the assessed factors in men, although it tended to be directly associated with age and was lowest in summer (July to September) compared with the other seasons. Although plasma phylloquinone concentration showed a clear tendency to increase with adiposity in men (P = 0·08 for trend, with BMI as a continuous variable), differences by BMI categories did not achieve statistical significance (P = 0·13).
In terms of percentages of adults with ‘low’ plasma phylloquinone concentration, those for men did not vary significantly by socio-demographic factor, smoking status or season. However, a higher percentage of participants aged < 45 years had ‘low’ plasma phylloquinone concentration compared with their older counterparts (25 v. 13 %; P = 0·01). In women, the likelihood of having ‘low’ plasma phylloquinone concentration fell independently with age (P < 0·001; trend), and again ‘low’ concentrations were more likely in participants aged < 45 years (19–34 years, 32 %; 35–44 years, 22 %, 45–64 years, 11 %). For both sexes, the prevalence of sub-optimal plasma phylloquinone concentrations fell significantly with age (men, P = 0·007; women, P < 0·001; trend). The percentage of men and women with plasma phylloquinone concentrations below the lower limit of quantification (1 and 3 % respectively) was not associated with socio-demographic factors, smoking status or season (each P>0·01; multiple logistic regression).
Plasma phylloquinone concentration and phylloquinone intake
Although the dispersion of both variables was large, plasma phylloquinone concentration was significantly correlated with phylloquinone intake in men and women (Fig. 1). After adjustment for age, correlation coefficients became 0·24 and 0·27 respectively (P < 0·001). When the data were examined for a subset of participants for whom plasma phylloquinone concentration could be related to phylloquinone intake on the previous day, the association was not altered significantly in men (r 0·30; P < 0·001; n 270), although in women it became weaker (r 0·13; P = 0·02; n 306).
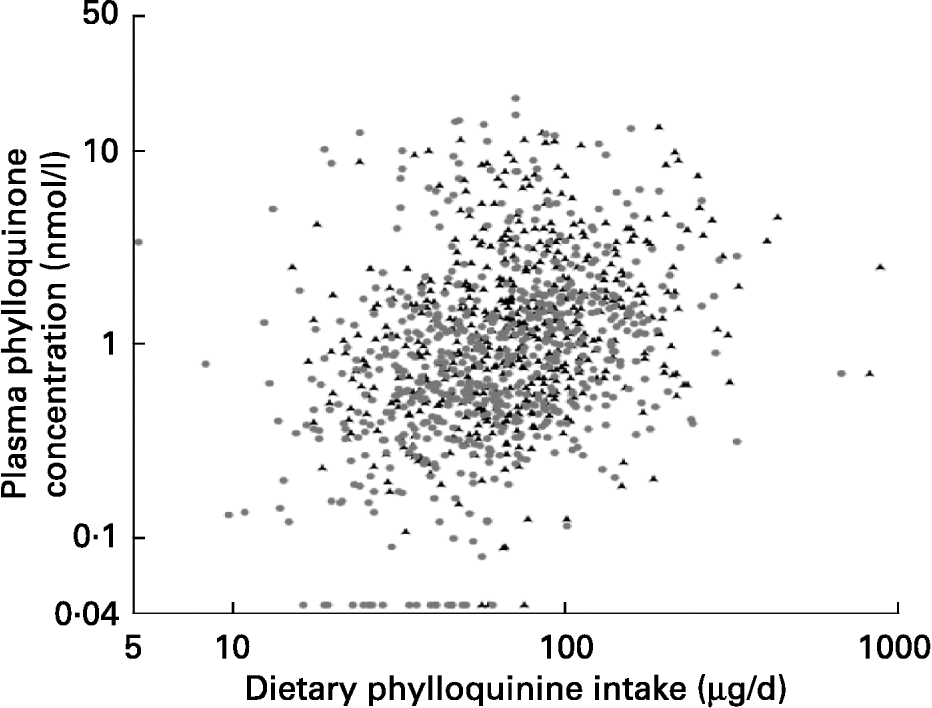
Fig. 1 Association between dietary phylloquinone intake and plasma phylloquinone concentration in 530 men (▲) and 624 women (●) aged 19–64 years. After natural logarithmic transformation, Pearson's correlation coefficients were 0·26 and 0·32 (each P < 0·001) for men and women respectively.
For the entire sample, the association between plasma phylloquinone concentration and intake did not vary significantly according to percentage contribution to phylloquinone intake from vegetables and fat spreads (each categorised as fifths) in the whole diet. For both sexes, plasma phylloquinone concentration was directly associated with daily consumption of vegetables and the sub-group of cooked leafy green vegetables, even after adjusting for age (with Pearson's simple and partial correlation coefficients ranging from 0·14 to 0·28; each P ≤ 0·001).
Plasma phylloquinone concentration and other blood analytes
After adjusting for age and BMI, plasma phylloquinone concentration was directly associated with plasma concentrations of total and non-HDL (mainly LDL) cholesterol, retinyl palmitate, several carotenoids (β-carotene, lycopene, lutein plus zeaxanthin) and α- and γ-tocopherols (Table 3). In men, plasma phylloquinone concentration was also associated with erythrocyte and, of borderline significance, serum folate concentrations, while a borderline inverse association was observed with biochemical status of riboflavin (assessed by erythrocyte glutathione reductase activation coefficient). Associations were not altered significantly after further adjustment for the corresponding nutrient intakes, and saturated fats in the case of plasma cholesterol.
Table 3 Association between plasma concentrations of phylloquinone and those of selected blood analytes in British adults aged 19–64 years
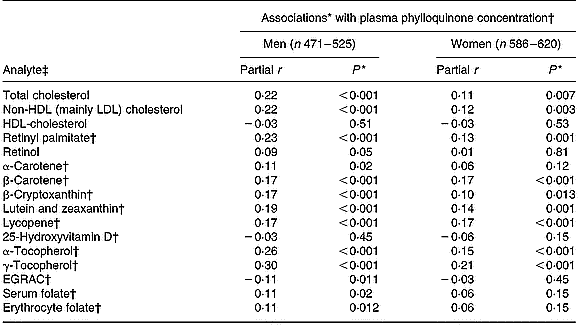
EGRAC, erythrocyte glutathione reductase activation coefficient (index of riboflavin status).
* Adjusted for age and BMI.
† Loge-transformed values.
‡ Measured in plasma, unless marked otherwise.
Determinants of plasma phylloquinone concentration
Phylloquinone intake explained 8 % of the variation in plasma phylloquinone concentration. A further 6 and 4 % of the variation were explained by plasma concentrations of γ-tocopherol and retinyl palmitate respectively. Over 9 % of plasma phylloquinone concentration was explained by phylloquinone intake in women, compared with only 3 % in men. Overall, 22 % of plasma phylloquinone concentration was explained by variation in five factors among men (plasma γ-tocopherol > plasma retinyl palmitate > dietary phylloquinone intake > season > plasma α-tocopherol). In women, 21 % of plasma phylloquinone concentration was explained by nine factors (dietary phylloquinone intake > plasma γ-tocopherol > plasma retinyl palmitate > plasma total cholesterol > smoking habit > plasma α1-antichymotrypsin > age > plasma total Fe-binding capacity > plasma β-carotene). Other socio-demographic and lifestyle factors and energy intake were not selected for entry into the final multiple regression equation as significant independent determinants of plasma phylloquinone concentration.
Discussion
The purpose of the present study was to provide information on plasma phylloquinone concentration in non-fasted blood samples from a representative sample of British adults aged 19–64 years in 2000–1, in the context of data available from other studies. Comparison of plasma phylloquinone concentrations between studies is not necessarily straightforward since, although an international quality assurance scheme is now operating, data may be reported as arithmetic or geometric means, be obtained from fasted or non-fasted blood samples, and be presented with or without adjustment for plasma lipid concentrations. In the National Diet and Nutrition Survey from which the present study has been derived, it was decided, primarily for logistical reasons, not to require fasted blood samples. Plasma phylloquinone concentrations may therefore have been influenced by postprandial lipidaemia (Shearer, Reference Shearer1992), to different extents according to types of meals consumed and length of time before blood sampling. While the collection of samples of unspecified fasting status may not be ideal, it represents circumstances that cannot always be avoided in large surveys.
A non-fasted state could not be confirmed by plasma triacylglycerol concentrations since they were not measured. However, plasma retinyl palmitate concentrations for the entire sample of adults were very low, and significantly lower than those from a representative sample of free-living older British adults (Finch et al. Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke1998). As a fatty acid ester of retinol, circulating concentrations are reported to peak at 4–6 h after a meal (Ruston et al. Reference Ruston, Hoare, Henderson, Gregory, Bates, Prentice, Birch, Swan and Farron2004). In addition, one large American study found that serum phylloquinone was not associated with fasting status when the blood sample was provided (Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999), and the lower limits of a normal adult reference range used in the UK are almost identical in fasting and non-fasting subjects (O'Shaughnessy et al. Reference O'Shaughnessy, Allen, Woodcock, Pearce, Harvey and Shearer2003). Although plasma phylloquinone concentration has been suggested to exhibit circadian rhythmicity – typically being at its lowest in the morning and highest late in the day (Kamali et al. Reference Kamali, Edwards, Wood, Wynne and Kesteven2001), no such variation was found in the present study. In this instance, the variable times of blood sampling may therefore be disregarded as a confounder of the association between plasma concentration and intake of phylloquinone.
Average plasma phylloquinone concentrations in the present study are somewhat higher than those reported in fasted blood samples from older adults of mainland Britain (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a), but are comparable with those reported from fasted blood samples in the USA (Sadowski et al. Reference Sadowski, Hood, Dallal and Garry1989; Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995; Sokoll & Sadowski, Reference Sokoll and Sadowski1996; Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999) and from non-fasted blood samples in the Netherlands (Schurgers et al. Reference Schurgers, Geleijnse, Grobbee, Pols, Hofman, Witteman and Vermeer1999).
Plasma phylloquinone concentration varied widely, was lower in women than men, lower in women aged 19–34 years compared with their older counterparts, and lower among women during summer compared with winter and spring. Plasma phylloquinone concentration was directly, although weakly, correlated with phylloquinone intake, and with plasma concentrations of vitamin E and carotenoids. Only 2 % (22 out of 1154) of participants had plasma phylloquinone concentrations below the lower limit of quantification (0·04 nmol/l), although 13 % had concentrations indicative of sub-optimal status – at least in the clinical setting in the UK (O'Shaughnessy et al. Reference O'Shaughnessy, Allen, Woodcock, Pearce, Harvey and Shearer2003). Although vitamin K status (as indicated by plasma phylloquinone concentration) would seem to be adequate in the majority of British adults of this age range, and higher than that found in a representative sample of older British adults (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a), it may be less than optimal in a not insignificant proportion.
Overall, geometric mean plasma phylloquinone concentration was 28 % lower in women than men; the difference being greatest among those aged < 45 years. In a cohort of over 1000 adults in the USA, serum phylloquinone concentrations of women were typically 14 % less than those observed in men (Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999). Conversely, most studies involving adults have not reported a significant difference in plasma phylloquinone concentration by sex (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer1998; Binkley et al. Reference Binkley, Krueger, Engelke, Foley and Suttie2000; McKeown et al. Reference McKeown, Jacques, Gundberg, Peterson, Tucker, Kiel, Wilson and Booth2002; Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a).
The direct association observed between plasma phylloquinone concentration and age has been reported elsewhere (Sadowski et al. Reference Sadowski, Hood, Dallal and Garry1989; Booth et al. Reference Booth, Tucker, McKeown, Davidson, Dallal and Sadowski1997; Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999; Binkley et al. Reference Binkley, Krueger, Engelke, Foley and Suttie2000), although not universally (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer1998; McKeown et al. Reference McKeown, Jacques, Gundberg, Peterson, Tucker, Kiel, Wilson and Booth2002; Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a). In one study that found lower plasma phylloquinone concentrations in younger (20–40 years) v. older (60–80 years) adults, the difference was attributed to the younger adults having lower blood lipid concentrations, particularly plasma triacylglycerols, with which phylloquinone was associated (Sadowski et al. Reference Sadowski, Hood, Dallal and Garry1989). This possible explanation for lower plasma phylloquinone concentration in younger adults, and women v. men, could not be examined since plasma triacylglycerol concentration was not measured in the present study.
Seasonal variation in plasma phylloquinone concentration has also been reported in British elderly individuals (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a), although its nature was different from that reported previously when concentrations were lower in autumn and winter (October to March) compared with other times of the year. Plasma phylloquinone concentrations from fasted blood samples were also lower during winter (than autumn) in a cohort of adults living in New Mexico, USA (Sadowski et al. Reference Sadowski, Hood, Dallal and Garry1989), and during winter compared with summer and autumn, independent of phylloquinone intake, among adults from the Framingham Offspring Study (McKeown et al. Reference McKeown, Jacques, Gundberg, Peterson, Tucker, Kiel, Wilson and Booth2002). In contrast, seasonal differences were not observed by other researchers in Scotland (Fenton et al. Reference Fenton, Bolton-Smith, Harrington and Shearer2000) and the USA (Sokoll & Sadowski, Reference Sokoll and Sadowski1996). Both the existence and measurement of seasonality may depend on the population being studied, a likely location-dependent relationship between the availability of specific foods and season, and the ratio of intra- to inter-individual variation in plasma phylloquinone concentration. This latter characteristic is reported to be high (Booth et al. Reference Booth, Tucker, McKeown, Davidson, Dallal and Sadowski1997; Fenton et al. Reference Fenton, Bolton-Smith, Harrington and Shearer2000), and so may obscure or attenuate any association even if present.
The seemingly paradoxical differences in plasma phylloquinone concentration but not phylloquinone intake by season and vice versa by region in the present study (Thane et al. Reference Thane, Bolton-Smith and Coward2006) have also been reported in British elderly individuals (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a,Reference Thane, Paul, Bates, Bolton-Smith, Prentice and Shearerb). In the present study, it may be a reflection of variable separation in the timings of dietary assessment and blood sampling. However, it may also reflect the relatively weak association between plasma phylloquinone concentration and intake. For example, in a cohort of healthy postmenopausal women, plasma concentrations were also reported to be lower in May than February despite a lack of seasonal variation in phylloquinone intake (Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995).
Although the correlations between plasma phylloquinone concentration and intake were highly significant in both sexes (each P < 0·001), associations were weak (r 0·26 and 0·32 for men and women respectively). Most other studies have shown significant (P < 0·05), albeit fairly weak, correlations between serum or plasma phylloquinone concentrations and dietary phylloquinone intakes (Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995, Reference Booth, Tucker, McKeown, Davidson, Dallal and Sadowski1997; Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer1998; Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999; McKeown et al. Reference McKeown, Jacques, Gundberg, Peterson, Tucker, Kiel, Wilson and Booth2002; Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a; Yan et al. Reference Yan, Zhou, Greenberg, Wang, Nigdikar, Prynne and Prentice2004), with some exceptions (P = 0·22, Schurgers et al. Reference Schurgers, Geleijnse, Grobbee, Pols, Hofman, Witteman and Vermeer1999; P = 0·11, Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004). Weak or non-existent relationships may result from a lack of juxtaposition of dietary and plasma assessments, since foods with a very high phylloquinone content tend to be consumed irregularly and plasma phylloquinone concentration tends to reflect only the previous few days' intake or an even shorter period of intake – owing to rapid clearance from plasma, high turnover and low body stores relative to other fat-soluble vitamins (Usui et al. Reference Usui, Tanimura, Nishimura, Kobayashi, Okanoue and Ozawa1990; Sokoll et al. Reference Sokoll, Booth, O'Brien, Davidson, Tsaioun and Sadowski1997; Dolnikowski et al. Reference Dolnikowski, Sun, Grusak, Peterson and Booth2002; Olson et al. Reference Olson, Chao, Graham, Bates and Lewis2002; Kurilich et al. Reference Kurilich, Britz, Clevidence and Novotny2003; Erkkilä et al. Reference Erkkilä, Lichtenstein, Dolnikowski, Grusak, Jalbert, Aquino, Peterson and Booth2004).
Owing to the reported influence of recent phylloquinone intake on plasma phylloquinone concentration (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer1998; Booth & Suttie, Reference Booth and Suttie1998), blood sampling would have been desirable at about the time of dietary assessment or shortly afterwards. In practice, this occurred with many participants but a separation of variable numbers of days also occurred for a sizeable minority of participants. At the extremes, one adult had blood taken 8 d before day 1 of the 7 d period of dietary assessment while another had blood taken 103 d after the end of dietary assessment. However, for most participants (>80 %), blood samples either coincided with dietary assessment or were taken within a fortnight afterwards. However, when data were examined for a subset of participants for whom plasma phylloquinone concentration could be related to phylloquinone intake the day before, the association was not altered significantly in men and paradoxically became weaker in women. This suggests that the 7 d weighed dietary record provided a good estimate of habitual phylloquinone intake that, for the entire sample, was associated more strongly with plasma phylloquinone concentration than intake from only 1 d, even when it immediately preceded blood sampling.
The relatively weak association between plasma concentration and intake is not unique to phylloquinone. Correspondingly weak correlations have also been reported for retinol (which is regulated physiologically within narrow limits), several carotenoids and vitamin E – both before and after its expression as a ratio to total cholesterol concentration (Ruston et al. Reference Ruston, Hoare, Henderson, Gregory, Bates, Prentice, Birch, Swan and Farron2004).
Direct linear associations reported in the present study between plasma phylloquinone concentration and consumption of all vegetables and the sub-group of cooked leafy green vegetables has also been reported in the USA with regard to green vegetable consumption up to twelve servings per week, above which plasma phylloquinone concentration reached a plateau (McKeown et al. Reference McKeown, Jacques, Gundberg, Peterson, Tucker, Kiel, Wilson and Booth2002). Despite significant correlations between plasma phylloquinone concentration and vegetable consumption, variation in food sources of phylloquinone intake did not influence plasma concentration or its association with intake. This was also found in a study involving whole diets given to healthy postmenopausal women (Booth et al. Reference Booth, Sokoll, O'Brien, Tucker, Dawson-Hughes and Sadowski1995), but contrasts with some well-controlled feeding studies that examined phylloquinone absorption from single foods or meals (Gijsbers et al. Reference Gijsbers, Jie and Vermeer1996; Schurgers & Vermeer, Reference Schurgers and Vermeer2000; Booth et al. Reference Booth, Lichtenstein and Dallal2002).
Plasma phylloquinone concentration was directly associated with plasma total cholesterol concentration. This finding tallies with that of some (Cham et al. Reference Cham, Smith and Colquhoun1999; Olson et al. Reference Olson, Chao, Graham, Bates and Lewis2002) but not all researchers (Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999), while Thane et al. (Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a) found a significant linear association in older women but not in men. In contrast to a lack of association reported between plasma concentrations of phylloquinone and LDL-cholesterol (Kamali et al. Reference Kamali, Edwards, Wood, Wynne and Kesteven2001), the present findings agree with those of Cham et al. (Reference Cham, Smith and Colquhoun1999) who, similar to the expression of vitamin E status, have advocated that circulating phylloquinone, as a biochemical index of vitamin K status, should always be adjusted for concurrent lipid concentrations. However, at present, there is no consensus with regard to the reporting of lipid-adjusted in preference to absolute plasma phylloquinone concentrations.
Direct associations observed in the present study between plasma concentrations of phylloquinone and fat-soluble vitamins may partly be explained by their co-existence in foods (green vegetables for lutein and β-carotene, and vegetable oils for α- and γ-tocopherols). Associations may also be attributed to their absorption and co-transport on lipoprotein particles in circulation (Kohlmeier et al. Reference Kohlmeier, Salomon, Saupe and Shearer1996; Lamon-Fava et al. Reference Lamon-Fava, Sadowski, Davidson, O'Brien, McNamara and Schaefer1998; Schurgers & Vermeer, Reference Schurgers and Vermeer2002). Since fasted blood samples were not required in the present study, associations of plasma phylloquinone concentration with those of fat-soluble vitamins may have been confounded by postprandial lipidaemia. However, similar associations were observed among older British adults who did provide fasted blood samples (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a).
Plasma phylloquinone concentration has been correlated with plasma α-tocopherol concentration elsewhere (Sadowski et al. Reference Sadowski, Hood, Dallal and Garry1989; Cham et al. Reference Cham, Smith and Colquhoun1999). However, in the latter case, when the variation in plasma phylloquinone concentration was examined by multiple linear regression, circulating concentrations of α-tocopherol explained only an additional 1 % above the 69 % of its variation already explained by plasma concentrations of apo A1 and B. As noted by Sadowski et al. (Reference Sadowski, Hood, Dallal and Garry1989), the direct association between circulating phylloquinone and tocopherols may be related more to plasma lipid concentrations (triacylglycerols and cholesterol), as main transporters of fat-soluble vitamins in circulation, than to common dietary sources or correlated intakes. Most phylloquinone intake in the present study was derived from vegetables and particularly cooked leafy green vegetables (Thane et al. Reference Thane, Bolton-Smith and Coward2006), whereas most vitamin E intake is derived from edible oils that, compared with vegetables, contribute relatively little phylloquinone intake.
The percentage of plasma phylloquinone concentration attributable to dietary and non-dietary factors in the present study (men, 23 %; women 21 %) was considerably higher than the 11 % obtained, by the same statistical procedure, in free-living older British adults (Thane et al. Reference Thane, Bates, Shearer, Unadkat, Harrington, Paul, Prentice and Bolton-Smith2002a). In contrast, 44 % of the variation in plasma phylloquinone concentration was explained by three factors (phylloquinone intake, energy intake and plasma triacylglycerol concentration) in a small sample of adults in Scotland (Bolton-Smith et al. Reference Bolton-Smith, Price, Fenton, Harrington and Shearer1998), and 40 % by dietary, socio-demographic and lifestyle factors among over 1000 adults living in the USA (Rock et al. Reference Rock, Thornquist, Kristal, Patterson, Cooper, Neuhouser, Neumark-Sztainer and Cheskin1999). A fuller understanding of the association between phylloquinone intake and plasma concentration, and dietary and non-dietary determinants of the latter, will only emerge when more data become available on factors affecting its absorption and turnover.
Acknowledgements
The present study was funded by the Food Standards Agency (project number N05050). We also thank The UK Data Archive (University of Essex, Colchester CO4 3SQ, UK) for providing an electronic copy of the dataset for the National Diet and Nutrition Survey of adults aged 19–64 years, and Dr Caroline Bolton-Smith and Dr Martin Shearer for allowing us to use the comprehensive unpublished database of food phylloquinone contents in order to facilitate our estimation of phylloquinone intake.






