Dietary n-3 long-chain PUFA (LC-PUFA) supplied through marine oils such as fish oil are considered to be anti-inflammatory. The anti-inflammatory properties are associated with their cellular incorporation, which may change membrane structures, signal transduction pathways, gene transcription, and production of n-3 and n-6 LC-PUFA-derived compounds including resolvins, protectins and eicosanoids(Reference Calder1, Reference Chapkin, Kim and Lupton2).
In human subjects, the incorporation of 20 : 5n-3 (EPA) and 22 : 6n-3 (DHA) into inflammatory cell phospholipids depends on the dosage and duration of supplementation(Reference Arterburn, Hall and Oken3). The n-3 LC-PUFA incorporation is partly at the expense of n-6 LC-PUFA such as 20 : 4n-6 (arachidonic acid, AA)(Reference Arterburn, Hall and Oken3). During an infection, AA and EPA are converted through the cyclo-oxygenase-2 pathway to PGE2 and PGE3, respectively, and consequently increasing cellular EPA abundance competitively inhibits PGE2 formation(Reference Schmitz and Ecker4, Reference Wada, DeLong and Hong5). PGE2 was originally classified as being pro-inflammatory, because of its involvement in the pathogenesis of the cardinal signs of inflammation: heat, redness, swelling, pain and loss of function, all of which affect clinical outcome during inflammatory processes. Lately, PGE2 has also shown anti-inflammatory properties as it inhibits the leukotriene pathway and some pro-inflammatory cytokines(Reference Calder6). PGE2 is a local-acting para- or autocrine inflammatory mediator, and it is metabolised in plasma by a single pulmonary passage, therefore measuring the PGE2 metabolite rather than PGE2 in plasma is a better estimate of the general PGE2 level in the animal model(Reference Eling and Ally7, Reference Granstrom and Kindahl8).
The n-3 LC-PUFA reduce growth suppression during infection by reducing pro-inflammatory cytokines centrally and in peripheral tissues(Reference Fossum9). Growth suppression is induced through several pathways as reviewed by Borghetti et al. (Reference Borghetti, Saleri and Mocchegiani10). In brief, pro-inflammatory cytokines (IL-1, IL-6 and TNF-α) induce the production of glucocorticoids by acting on each level of the hypothalamic–pituitary–adrenal axis, and glucocorticoids along with locally produced cytokines induce catabolic changes in most tissues, in addition the pro-inflammatory cytokines act centrally causing anorexia, and, finally, they reduce metabolism in peripheral tissues by inducing insulin resistance and by inhibiting insulin-like growth factor 1 directly and through the somatotropic axis. It is generally known that glucose, TAG, cholesterol and NEFA are metabolic biomarkers, but in patients who are critically ill, hypocholesterolaemia and hypotriacylglycerolaemia also indicate the severity of disease(Reference Chiarla, Giovannini and Giuliante11).
Early aortic vascular prosthetic graft infection (AVPGI) is a seldom but severe post-operative complication with high morbidity and mortality rates(Reference Swain, Calligaro and Dougherty12); the most prevalent pathogen reported is Staphylococcus aureus (Reference Mussa, Hedayati and Zhou13). Powerful randomised human trials concerning this post-operative complication are difficult to conduct due to the limited number of patients, and consequently a porcine model was set up to study early AVPGI caused by S. aureus (Reference Gao, Sandermann and Prag14). The porcine model on early AVPGI was thought to provide a reproducible inflammatory insult for studying the effect of n-3 and n-6 LC-PUFA on post-operative clinical outcome, and due to the similarities in morphology and physiology in the gastrointestinal system, the porcine model is considered an acceptable model for human lipid nutrition(Reference Miller and Ullrey15–Reference Innis17).
The aim of the present study was to evaluate the effect of a dietary intervention with fish oil rich in n-3 LC-PUFA, sunflower oil rich in n-6 LC-PUFA or animal fat on post-operative clinical outcome using the porcine model of early AVPGI described earlier.
The hypotheses were that 3 weeks of 10 % (w/w) dietary treatment with fish oil or sunflower oil as opposed to animal fat would change n-3 and n-6 LC-PUFA concentrations in porcine plasma and erythrocytes, and that plasma PGE2 metabolite concentration would decrease in the fish oil treatment as a consequence of increased EPA intake. The post-operative clinical outcome, i.e. the number of days with fever, weakness in the hindquarters and impaired general appearance, as well as feed intake and body-weight gain, was expected to improve in the fish oil treatment due to increased cellular incorporation of anti-inflammatory n-3 LC-PUFA, particularly EPA and DHA.
Experimental methods
Animals
In total, eighty-four pigs were included in the study. These animals (fourteen litters with six female littermates of similar genetic background; Landrace/Yorkshire sows mated with Duroc boars) were obtained from the specific-pathogen-free herd at the Faculty of Agricultural Sciences, Aarhus University, Tjele, Denmark. Mean body weight at the time of study admission was 55·9 (sd 10·5) kg. The pigs were housed individually in pens with saw-dusted solid floors and drinking nipples. To ensure adjustment to intensive care housing facilities, the pigs were moved 5 d before surgery, and they stayed in the intensive care unit throughout the remaining study period. The National Guidelines for the care and use of animals were followed and the Danish Ministry of Justice, Animal Research Inspection, approved all experimental procedures involving animals.
Dietary intervention
Pigs were weaned at 28 d of age, and continued on growers feed until the time of study admission. Despite the high fat content (10 %, w/w), the dietary treatments were optimised to meet all nutritional recommendations used for slaughter swine in Danish pig production. The dietary treatments were mixed at the feed plant of the faculty research centre, and consisted of a ground feed, into which the three fat sources, fish oil, sunflower oil or animal fat, were added. Feed components, chemical analysis and fatty acid composition of each dietary treatment are given in Tables 1–3, respectively. Within each litter, two female littermates were randomised for each of the dietary treatments. The pigs were fed restrictively throughout the entire study period. In the 3-week preoperative period, they were fed 1 kg twice a day, on the morning of surgery, pigs were fasted, and in the 14 d post-operative period, they were gradually brought back to the preoperative feeding regimen. Total feed intake was determined for each pig by weighing back any remaining feed in the feeding bin before every morning feed out.
Table 1 Feed components in the dietary treatments (%, w/w)
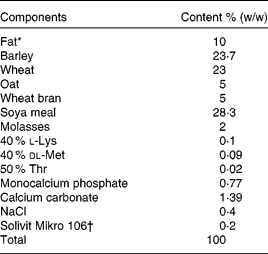
* Fish oil, provided by FF of Denmark (Skagen, Denmark); Trisun 80 RBWD high-oleic sunflower oil (ACH Food Companies, Inc., Memphis, TN, USA), provided by Frede J Damm (Ølstykke, Denmark); animal fat, provided by the research centre at the Faculty of Agricultural Sciences, Aarhus University (Foulum, Denmark).
† Containing (per kg pre-mix): 2 500 000 IU (750 mg) vitamin A; 500 000 IU (12·5 mg) vitamin D3; 30 000 mg vitamin E; 1100 mg vitamin K3; 1100 mg vitamin B1; 2000 mg vitamin B2; 1650 mg vitamin B6; 5500 mg d-pantothenic acid; 11 000 mg niacin; 27·5 mg biotin; 11 mg vitamin B12; 25 000 mg Fe; 40 000 mg Zn; 13 860 mg Mn; 10 000 mg Cu; 99 mg I and 150 mg Se, provided by Løvens Kemiske Fabrik (Vejen, Denmark).
Table 2 Chemical analysis of the dietary treatments
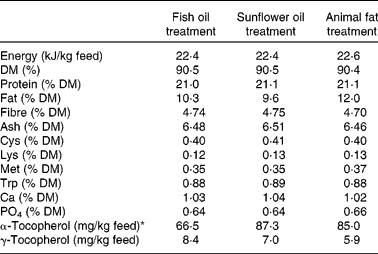
* The vitamin E content in the three fat sources was measured to be 57, 553 and 315 mg/kg fat source for fish oil, sunflower oil and animal fat, respectively. The differences in vitamin E content between the fat sources were adjusted by mixing vitamin E (d-α-tocopherol; Pharmalett A/S, Kolding, Denmark) with the fat source before adding to the ground feed.
Table 3 Fatty acid composition of the dietary treatments*

FA, fatty acid; LA, linoleic acid; DGLA, dihomo-γ-linolenic acid; ALA, α-linolenic acid; AA, arachidonic acid; DPA, docosapentaenoic acid; LC-PUFA, long-chain PUFA.
* The n-6:n-3 fatty acid ratio was 1·8, 33·4 and 10·8 for the fish oil, sunflower oil and animal fat treatments, respectively.
† Sum of 10 : 0, 12 : 0, 15 : 0, 17 : 1, 20 : 0, 22 : 0 and 24 : 1.
Surgical intervention
The two littermates that received the same dietary treatment were assigned different prosthetic graft materials: polyester or Ag-impregnated polyester. Gao et al. (Reference Gao, Sandermann and Prag14) have described the porcine model of early AVPGI with respect to anaesthetic, surgical and infection procedures. In brief, for induction of anaesthesia, 1 ml of mixture containing zolazepam (5 mg/ml), butorphanol (1 mg/ml), ketamine (10 mg/ml) and xylazine (2 mg/ml) per 15 kg of body weight was given intramuscularly. This was followed by an intravenous infusion of 10 mg/kg propofol per h and 0·025 mg/kg fentanyl per h throughout the surgery. For the post-operative pain treatment, all pigs were given a combination of flunixin (150 mg) intramuscularly with 24 h intervals and buprenorphine (0·6 mg) intramuscularly with 8 h intervals the first 3 d post-operatively.
The surgery was performed by abdominal laparotomy to expose the retroperitoneal part of the aorta. A 50 mm-long and 8 mm-wide prosthetic graft was inserted in the infra renal part of the aorta above the aortic bifurcation. For graft infection, S. aureus strain ATCC 29 213 (1 × 106 colony-forming units) was inoculated onto the graft, before the retroperitoneum was closed. The ATCC 29 213 strain was previously used successfully as a pathogen in a porcine model of vascular graft infection(Reference Hernandez-Richter, Schardey and Lohlein18). S. aureus growth on graft material and in peri-graft tissues was confirmed for all pigs at the end of the study as described by Gao et al. (Reference Gao, Sandermann and Prag14).
Blood sampling
Blood samples were drawn before morning feed out at the following time points: before dietary intervention (day − 21), before surgical intervention (day 0) and on day 12 post-operatively. Blood was obtained by puncture of the external jugular vein. Na-heparin and K-EDTA-stabilised plasma were isolated by centrifugation (2000 g, 10 min, 20°C), and stored at − 20 and − 80°C, respectively.
Plasma and erythrocyte fatty acid composition
Lipids were extracted from thawed K-EDTA plasma and erythrocyte samples obtained on day − 21 and day 0 by the method of Bligh & Dyer(Reference Bligh and Dyer19); 17 : 0 (heptadecanoic acid) was used as the internal standard. The extracted lipids were transesterified into fatty acid methyl esters and separated by GLC as described by Rotenberg & Andersen(Reference Rotenberg and Andersen20).
Plasma PGE2 metabolite
PGE2 metabolite (13,14-dihydro-15-keto metabolite) concentration was determined in thawed K-EDTA plasma samples obtained on day − 21 and day 0 using a commercial PGE metabolite EIA kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer's guidelines.
Clinical outcome
Pigs were weighed before the dietary intervention (day − 21), before the surgical intervention (day 0) and at the end of the study period (day 14). Rectal temperature, hindquarter function, general appearance and feed intake were monitored daily in the 14 d post-operative period. Fever was noted, when rectal temperature was above 39·5°C. Weakness in the hindquarters was observed in pigs as a preference for the sitting position. General appearance was considered impaired, when pigs showed lack of interest in surroundings, staff or feed, and had a general preference for lying down. Daily feed intake was measured by weighing back any feed present in the feeding bin before every morning feed out. In addition, changes in metabolic biomarkers (glucose, TAG, cholesterol and NEFA) were evaluated in plasma post-operatively. The fasting plasma concentrations of cholesterol, TAG and glucose were determined in thawed Na-heparin plasma samples according to standard procedures (Siemens Diagnostics® Clinical Methods for ADVIA 1650; Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Plasma NEFA concentration was determined using the acyl-CoA synthase–acyl-CoA oxidase method as prepared by Wako Chemicals USA, Inc. (Richmond, VA, USA). Analyses were performed using an auto analyser (ADVIA® 1650 Chemistry System; Bayer Corporation, Tarrytown, NY, USA).
Statistical analysis
A linear mixed model was fitted to the data, concerning the preoperative changes in fatty acid concentrations, preoperative changes in PGE2 metabolite concentration, and post-operative feed intake, body-weight gain and changes in metabolic biomarkers. Dietary treatment was included in the model as a fixed effect, whereas the effect of graft material was excluded from the model after initial analysis. The random effects of litter, paired littermates on the same dietary treatment and pig within paired littermates were hierarchically included in the model, and all random effects were considered normally distributed. Results are expressed as model-based means with their standard errors.
Remaining clinical signs of infection, monitored post-operatively, were analysed as quasi-binomial distributed data in a generalised linear model, with dietary treatment as a fixed effect.
Pearson's correlations between the plasma PGE2 metabolite concentration and erythrocyte AA, EPA and DHA concentrations were determined with corresponding 95 % CI.
For all statistical analyses performed, significance was stated at the 5 % level. Data were analysed using the software R (A Language and Environment for Statistical Computing, version 2.10.1 R Development Core Team (2009), Vienna, Austria; http://www.R-project.org).
Results
In total, seven out of the eighty-four pigs were excluded from the study. The post-operative complications leading to exclusion were not considered to be related to the dietary treatments (Table 4). Graft infection with S. aureus was confirmed at the end of the study in all pigs completing the study.
Table 4 Animals excluded

Plasma and erythrocyte fatty acid composition
In the fish oil treatment, n-3 LC-PUFA concentration including EPA and DHA concentrations increased, and n-6 LC-PUFA concentrations including linoleic acid (LA) and AA concentrations decreased, in both plasma and erythrocytes (Table 5). In the sunflower oil treatment, all selected n-3 LC-PUFA concentrations decreased in both plasma and erythrocytes, but among the n-6 LC-PUFA concentrations, only 18 : 2n-6 (LA) increased, whereas AA concentration remained unchanged and 20 : 3n-6 (dihomo-γ-linolenic acid) declined in both plasma and erythrocytes (Table 5). In the animal fat treatment, LA, 18 : 3n-3 (α-linolenic acid) and 22 : 5n-3 (docosapentaenoic acid) concentrations decreased in plasma, whereas only docosapentaenoic acid concentration decreased in erythrocytes (Table 5).
Table 5 Preoperative changes in selected fatty acid (FA) concentrations from day −21 to day 0 for both plasma and erythrocytes given for the fish oil (FO), sunflower oil (SO) and animal fat (AF) treatments†
(Mean values with their standard errors)

LC-PUFA, long-chain PUFA; LA, linoleic acid; DGLA, dihomo-γ-linolenic acid; AA, arachidonic acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid.
a,b,c Mean plasma values with unlike superscript letters within the same row were significantly different (P < 0·05).
A,B,C Mean erythrocyte values with unlike superscript letters within the same row were significantly different (P < 0·05).
* Observed change in fatty acid concentration was significant (P < 0·05).
† Litters 2–4 and 6–12 were analysed with respect to FA composition.
‡ The number of animals was reduced in the FO and SO treatments because some animals were excluded during the study period due to complications that were unrelated to the dietary treatments.
Plasma PGE2 metabolite
Plasma PGE2 metabolite concentration decreased preoperatively in the fish oil treatment, whereas no decrease was observed in the sunflower oil and animal fat treatments (Fig. 1). A positive correlation between plasma PGE2 metabolite concentration and erythrocyte AA concentration, and a negative correlation between plasma PGE2 metabolite concentration and erythrocyte EPA and DHA concentrations were observed at the time of surgical intervention (day 0; Table 6).

Fig. 1 Individual (○) and mean (●) changes in plasma PGE2 metabolite (13,14-dihydro-15-keto metabolite) concentration in the preoperative period from day − 21 to day 0 in each dietary treatment: fish oil, sunflower oil and animal fat. Values are means, with 95 % CI represented by vertical bars. * The observed change in PGE2 metabolite concentration was significant (P < 0·05). a,b,c Mean values with unlike letters were significantly different among the dietary treatments (P < 0·05). Plasma from litter numbers 2–3, 6–12 and 14 were analysed for PGE2 metabolite concentration.
Table 6 Pearson's correlation coefficients between PGE2 metabolite concentration in plasma and arachidonic acid (AA), EPA and DHA concentrations in erythrocytes just before the surgical intervention (day 0)*

* Data from litters 2, 3 and 6–12 were included as they were analysed with respect to both fatty acids and the PGE2 metabolite.
Body-weight gain and clinical observations
Feed intake and body-weight gain in the post-operative period were larger in the fish oil treatment compared with the sunflower oil treatment, whereas the animal fat treatment was not different compared with the other dietary treatments (Table 7). Body weights at the time of dietary and surgical intervention and preoperative body-weight gain did not differ among the dietary treatments (Table 7). No significant difference in the number of days with fever, impaired hindquarter function or impaired general appearance was observed among the dietary treatments.
Table 7 Daily energy intake and body-weight gain observed pre- and post-operatively in each of the three dietary treatments; fish oil (FO), sunflower oil (SO) and animal fat (AF)*
(Mean values with their standard errors)
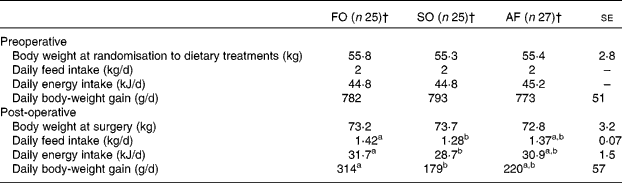
a,b Mean values within the same row with unlike superscript letters were significantly different (P < 0·05).
* Data from all litters were included.
† The number of animals per treatment was reduced from the twenty-eight included because some animals were excluded during the study period due to complications that were unrelated to the dietary treatments.
At the time of surgery, there was no difference in fasting plasma glucose or TAG among the dietary treatments, and in the post-operative period, fasting glucose and TAG decreased equally in all dietary treatments (Table 8). At time of surgery, fasting plasma cholesterol was higher in the fish oil treatment compared with the sunflower oil treatment. Whereas in the post-operative period, plasma cholesterol decreased in all treatments, and the decrease was larger in the fish oil treatment compared with the sunflower oil and animal fat treatments (Table 8). Fasting plasma NEFA concentration was higher in the sunflower oil and animal fat treatments compared with the fish oil treatment at the time of surgery. In the post-operative period, plasma NEFA concentration increased equally in all dietary treatments (Table 8).
Table 8 Fasting plasma concentration for glucose, TAG, cholesterol and NEFA before surgical intervention and the change in fasting plasma concentrations from surgical intervention (day 0) to day 12 post-operatively for the dietary treatments; fish oil (FO), sunflower oil (SO) and animal fat (AF)†
(Mean values with their standard errors)

μeq, Microequivalents.
a,b Mean values in the same row with unlike superscript letters were significantly different (P < 0·05).
* Observed change in metabolite concentration was significant (P < 0·05).
† Data from all litters were included.
‡ The number of animals was reduced in the FO, SO and AF treatments because some animals were excluded during the study period due to complications that were unrelated to the dietary treatments.
Discussion
Clinical trials evaluating parenteral infusions with n-3 LC-PUFA in surgical patients have recently been reviewed by Waitzberg & Torrinhas(Reference Waitzberg and Torrinhas21) and Calder(Reference Calder22). Infusions with lipid formulas containing n-3 LC-PUFA alter the formation of inflammatory mediators and immune function and, in some studies, reduce the length of intensive care unit and hospital admission. Furthermore, perioperative n-3 LC-PUFA infusions are considered superior compared with post-operative infusions(Reference Tsekos, Reuter and Stehle23). The preoperative dietary fish oil treatment was applied in the present porcine model in an attempt to mimic perioperative parenteral infusion with n-3 LC-PUFA in patients; both treatments are expected to increase the plasma and cellular n-3 LC-PUFA content before the surgical procedure. The advantage of using a porcine model, as opposed to conducting a clinical trial, was that variation according to age, sex, lifestyle and co-morbidity could be eliminated in the study design, and in addition, the surgical, infectious and analgesic procedures could be standardised.
The fish oil and sunflower oil treatments changed the n-3 and n-6 LC-PUFA concentrations in both plasma and erythrocytes, and the fish oil treatment decreased the PGE2 metabolite concentration in plasma, during the preoperative period. The fish oil treatment improved post-operative feed intake and reduced weight-gain suppression compared with the sunflower oil treatment. However, the fish oil treatment did not improve clinical signs of infection such as fever, impaired hindquarter function and impaired general appearance in pigs.
Pigs given the fish oil treatment received approximately 1 % (w/w) n-3 LC-PUFA each day, which was an enteral dose physiologically relevant for human clinical trials and also a dose recommended for animal feeding trials(Reference Kim, McMurray and Chapkin24). The daily EPA and DHA doses obtained in the fish oil treatment were 6·3 g EPA/d and 6·5 g DHA/d. The increase in EPA and DHA and the decrease in LA and AA observed in plasma and erythrocytes within the fish oil treatment are in agreement with previous porcine studies feeding 3·5–8 % (w/w) fish oil in a period of 14–40 d(Reference Fritsche, Huang and Cassity25, Reference Thies, Peterson and Powell26). The LC-PUFA turnover in erythrocytes results from de- and re-acylation of membrane phospholipids and phospholipid exchange with plasma(Reference Innis17), and this linked the changes observed in plasma LC-PUFA concentrations to the changes observed in erythrocytes.
The predominant fatty acid in the sunflower oil was LA, and consequently, mainly plasma and erythrocyte LA concentrations increased in sunflower oil-treated pigs. The unchanged plasma and erythrocyte AA concentration indicated a possible limited elongation and desaturation of LA to form AA. The total n-3 LC-PUFA dose was 3 g/d, which is a high dose, considering that the intake of a typical American consumer is 0·7–1·6 g n-3 LC-PUFA/d(Reference Kim, McMurray and Chapkin24); however, the contribution from EPA and DHA was only 0·12 and 0 g/d, respectively. The decrease observed for EPA, docosapentaenoic acid and DHA in plasma and erythrocytes is much in agreement with Møller & Lauridsen(Reference Møller and Lauridsen27), who found high LA concentration, unchanged AA and EPA concentration and reduced docosapentaenoic acid and DHA concentration in alveolar macrophages isolated from piglets given a 4-week, 5 % (w/w) dietary treatment with sunflower oil compared with animal fat.
The animal fat treatment was included in the present study as a control treatment. It had a high fat content (10 %, w/w), but it was not expected to affect plasma and erythrocyte n-6 and n-3 LC-PUFA concentrations. The last assumption was not entirely fulfilled, as the higher amount of MUFA in the animal fat treatment reduced total LC-PUFA content in the feed and this resulted in reduced total LC-PUFA concentration in plasma. The daily dose of n-3 LC-PUFA was 4·34 g/d, including 0·06 g EPA/d and 0 g DHA/d, which was very similar to the sunflower oil treatment.
The decrease in plasma PGE2 metabolite concentration observed in the preoperative period for the fish oil treatment was expected. In addition, the overall positive correlation between plasma PGE2 metabolite concentration and erythrocyte AA concentration, and the negative correlation between plasma PGE2 metabolite concentration and erythrocyte EPA and DHA concentration corresponds to the findings of Møller & Lauridsen(Reference Møller and Lauridsen27), who reported a negative correlation between PGE2 production and total n-3 LC-PUFA concentration and a positive correlation between PGE2 production and AA concentration in alveolar macrophages. The preoperative reduction in PGE2 metabolite concentration combined with negative Pearson's correlations between the PGE2 metabolite and EPA and DHA erythrocyte concentration could indicate reduced inflammatory activity in fish oil-treated pigs at surgical intervention.
The fish oil treatment improved feed intake and body-weight gain in the post-operative period compared with the sunflower oil treatment. The n-3 LC-PUFA are suggested to reduce anorexia during an inflammatory insult by reducing pro-inflammatory cytokines centrally. The exact mechanisms for inducing anorexia are not fully understood, but may well include cytokine-induced alterations in hypothalamic peptides and neurotransmitters such as serotonin, dopamine, neuropeptide Y and melanocortins(Reference Das, Ramos and Meguid28). The increase in feed intake and post-operative body-weight gain in the fish oil treatment might very well have been reinforced if pigs had been fed unrestrictedly in the post-operative period. In a porcine sepsis model, higher feed intake and average daily weight gain were observed in pigs given a 12 d, 5 % (w/w) menhaden fish oil treatment compared with a maize oil treatment(Reference Gaines, Carroll and Yi29). These observations were combined with reduced cortisol spikes explained by a fish oil-induced decrease in hypothalamic pro-inflammatory cytokines, which inhibited the hypothalamic–pituitary–adrenal axis(Reference Gaines, Carroll and Yi29). In a porcine abdominal sepsis model, higher feed intake and average daily weight gain were observed in piglets given a 14 d, 7 % (w/w) fish oil treatment compared with the maize oil treatment(Reference Liu, Li and Gong30). This observation was combined with decreased PGE2, IL-1β and cortisol levels, increased insulin-like growth factor 1 level and unchanged growth hormone levels(Reference Liu, Li and Gong30). The anti-inflammatory properties of fish oil were thought to cause the decrease in IL-1β and cortisol levels, as well as the alleviation in plasma insulin-like growth factor 1 reduction without affecting the growth hormone level, which suggested an uncoupling of the somatotropic axis during the immunological challenge(Reference Liu, Li and Gong30). Despite the numerous clinical trials with parenteral n-3 LC-PUFA infusions in surgical patients, little has been reported about the effects on patients' weight loss. However, in one clinical trial, which included post-surgical cancer patients, weight loss was prevented when patients were given parenteral infusion with fish oil compared with soyabean oil(Reference Heller, Rossel and Gottschlich31). Additionally, in relation to patients with cancer and cachexia, dietary fish oil supplementation is generally thought to improve weight loss and appetite(Reference Colomer, Moreno-Nogueira and Garcia-Luna32).
In the present study, post-operative NEFA concentration increased equally in all dietary treatments; however, due to the higher NEFA level at the time of surgical intervention in the sunflower oil and animal fat treatments, the NEFA level was highly displaced compared with the fish oil treatment during the post-operative period. The higher NEFA concentration in sunflower oil- and animal fat-treated pigs could have had an inhibitory effect on the somatotropic axis, as NEFA, and in particular LA, has been reported to act on the anterior pituitary cell membrane suppressing growth hormone-releasing hormone and growth hormone release in vitro and in vivo (Reference Barb, Kraeling and Rampacek33, Reference Barb, Kraeling and Barrett34). In addition to reduced feed intake, this might have contributed to the body-weight gain suppression observed in sunflower oil-treated pigs, as both NEFA and LA concentrations were elevated compared with the fish oil treatment. Cholesterol was higher at the time of surgical intervention in the fish oil treatment compared with the sunflower oil treatment; conversely, cholesterol decreased more in the fish oil treatment compared with both the sunflower oil and animal fat treatments in the post-operative period. A decrease in cholesterol was expected as surgery and sepsis are known to cumulatively decrease plasma cholesterol in patients; furthermore, cholesterol is considered to be a negative acute-phase reactant, with plasma levels being inversely correlated with C-reactive protein and leucocyte counts(Reference Chiarla, Giovannini and Giuliante11, Reference Chiarla, Giovannini and Siegel35).
The clinical signs of infection, i.e. the number of days with fever, impaired hindquarter function and impaired general appearance, were not affected by the dietary treatments. Accordingly, parenteral infusion with n-3 LC-PUFA was not observed to affect body temperature in post-operative cancer patients(Reference Heller, Rossel and Gottschlich31). However, a tendency towards lower increases in body temperature was observed after parenteral infusion with n-3 LC-PUFA in patients undergoing aneurism surgery(Reference Berger, Tappy and Revelly36). Unfortunately, dietary effects on clinical signs might have been concealed by the post-operative non-steroidal anti-inflammatory drug (NSAID) treatment. Compared with n-3 LC-PUFA, NSAID are much stronger inhibitors of the regulatory eicosanoids. Eicosanoid production affects clinical signs, as they induce heat, redness, swelling, pain and loss of function during inflammation. In the present setting, the use of NSAID was expected to reduce increases in body temperature, improve hindquarter function and general appearance, as well as prevent pain during the 3 d post-operative analgesic treatment. However, even though the total clearance time for flunixin has been reported to be 36–48 h for intramuscular and intravenous administration in pigs(Reference Buur, Baynes and Smith37, Reference Yu, Jiang and Guo38), plasma PGE2 metabolite concentrations were still below the preoperative level on day 12 (data not shown). This indicated that eicosanoid production was affected by flunixin during the full post-operative period. However, due to animal ethics, proper analgesia was warranted, and by using alternative analgesics such as fentanyl patches or buprenorphine injections, the same level of analgesia was not obtained. Emphasis was therefore directed towards providing a similar analgesic treatment to all pigs to ascertain that any difference in the clinical signs was not related to differences in analgesic treatment. However, since NSAID are widely used to improve clinical outcome in patients, any additional improvement in clinical outcome through increased incorporation of n-3 LC-PUFA was considered of clinical relevance.
In conclusion, during the 3-week preoperative period, n-3 and n-6 LC-PUFA concentrations in porcine plasma and erythrocytes changed according to the fatty acid composition of the dietary treatments, and a reduction in plasma PGE2 metabolite concentration was observed in the fish oil treatment. Post-operative feed intake and body-weight gain were improved in the fish oil treatment compared with the sunflower oil treatment. This improvement in clinical outcome was thought to result from the preoperative incorporation of n-3 LC-PUFA at the expense of n-6 LC-PUFA in the fish oil treatment compared with the sunflower oil treatment. If the preoperative treatment with dietary fish oil in the present porcine model is comparable with perioperative parenteral fish oil infusion in surgical patients, the present results suggest that lipid infusions containing fish oil rich in n-3 LC-PUFA, as opposed to traditional soyabean oil rich in n-6 LC-PUFA, should be considered as a nutritional strategy to improve post-operative anorexia and weight loss.
Acknowledgements
Funding was provided partly by the Danish Research Council (Health and Disease), and partly by a collaboration fund given by the Faculty of Agricultural Sciences, Aarhus University and the Regional Hospital in Viborg. The Vascular Research Unit, Department of Vascular Surgery and Department of Clinical Microbiology, at Viborg Hospital conducted the surgical part of the study. The authors wish to thank laboratory assistants Inger Marie Jepsen, Mette Würtz and Elsebeth Lyng Pedersen, and the group of co-workers in the porcine intensive care facility at the Faculty of Agricultural Sciences for their contribution during the course of the animal trial and the later laboratory analysis. S. N. L. supervised the animal trial, conducted the sample collection, did the laboratory analysis, performed the statistical data analysis and drafted the manuscript. E. K. T. reviewed the final drafts of the manuscript. B. M. D. and K. H. J. participated in the planning of the study design and commented on the drafting of the manuscript. U. H. supervised the statistical analysis, and C. L. participated in the planning of the study design, the overall coordination of the study and was helpful with advice throughout the drafting of the manuscript. There are no conflicts of interest with regard to the present study.











