During moderate intensity exercise, the energy for ATP homeostasis in skeletal muscle is primarily obtained from the oxidation of glycogen and lipid. Glycogen plays a pivotal role in producing energy and preventing the development of muscle fatigue in submaximal exerciseReference Bergstrom1–Reference Coyle, Coggan, Hemmert and Ivy4. Intramuscular lipid levels are also readily oxidised during prolonged exerciseReference Romijn, Coyle, Sidossis, Gastaldelli, Horowitz and Endert5–Reference Johnson, Stannard, Mehalski, Trenell, Sachinwalla and Thompson7 and are elevated in endurance-trained individualsReference van Loon and Goodpaster8. The rate of substrate oxidation, whether glycogen or lipid, is influenced by both existing pre-exercise substrate within the muscleReference Stellingwerff, Boon, Jonkers, Senden, Spriet and Koopman9, and circulating substrate availability during exerciseReference Dyck, Putman, Heigenhauser, Hultman and Spriet10–Reference Odland, Heigenhauser and Spriet13. Although considerable work has been undertaken on optimising glycogen storage with diet, less is known about the relative influence of diet upon both glycogen and lipid oxidation.
High levels of carbohydrate intake have been shown to improve glycogen repletion after exerciseReference Costill, Sherman, Fink, Maresh, Witten and Miller14, Reference Ivy, Lee, Brozinick and Reed15. An early study also reported that consuming carbohydrates which are rapidly absorbed into the circulatory system, termed high glycaemic index (HGI) carbohydrates, optimised the storage of plasma glucose as skeletal muscle glycogen following glycogen-depleting exerciseReference Burke, Collier and Hargreaves16. It has also been reported that the postprandial hyperinsulinaemia accompanying high carbohydrate intake reduces the rate of fat oxidationReference Horowitz, Mora-Rodriguez, Byerley and Coyle17–Reference Stevenson, Williams, McComb and Oram19, increasing reliance upon glycogen oxidation during exercise. Although high postprandial levels of insulin may increase glycogen storage, the suppression of peripheral lypolysis may increase dependency upon intramuscular stores of glycogen and lipid during exerciseReference van Loon, Thomason-Hughes, Constantin-Teodosiu, Koopman, Greenhaff and Hardie20.
Plasma NEFA are esterified to form intramyocellular lipid (IMCL) in non-contracting muscle and are oxidised during exerciseReference Romijn, Coyle, Sidossis, Zhang and Wolfe21, Reference Sacchetti, Saltin, Osada and van Hall22. During moderate intensity exercise (40–65 % V˙O2max) fat oxidation contributes 40-60 % of the total energy expenditureReference Romijn, Coyle, Sidossis, Gastaldelli, Horowitz and Endert5, Reference van Loon, Koopman, Stegen, Wagenmakers, Keizer and Saris6, Reference Horowitz, Mora-Rodriguez, Byerley and Coyle17, Reference Coyle, Jeukendrup, Wagenmakers and Saris23. Of the total fat oxidised, 50–70 % is derived from plasma NEFA with the remaining proportion obtained from intramuscular and lipoprotein-derived TAGReference van Loon24. The postprandial rise in insulin suppresses lypolysis, thereby decreasing circulating NEFA available for storage as IMCL and oxidation in skeletal muscleReference Horowitz, Mora-Rodriguez, Byerley and Coyle17, Reference Montain, Hopper, Coggan and Coyle25. Artificially elevating circulating NEFA levels increases lipid oxidation and reduces glycogen use in exerciseReference Dyck, Putman, Heigenhauser, Hultman and Spriet10–Reference Odland, Heigenhauser and Spriet13, reinforcing the relationship between lipid and glycogen oxidation.
It is possible that optimising glycogen resynthesis with a HGI recovery diet occurs at the cost of compromised NEFA availabilityReference Horowitz, Mora-Rodriguez, Byerley and Coyle17–Reference Stevenson, Williams, McComb and Oram19. Maintaining lipid availability with a low glycaemic index (LGI) diet may therefore assist endurance exercise performance. We hypothesised that LGI meals consumed after prolonged endurance exercise improve fatty acid availability and reduce reliance on IMCL in subsequent bouts of exercise. To test this hypothesis, LGI or HGI meals were consumed during a 24 h recovery period following 90 min of moderate intensity cycling. After an overnight fast, IMCL was examined using 1H-magnetic resonance spectroscopy (MRS) before and after a second 90 min cycle. Venous blood samples were collected to observe changes in circulating lipids, glucose, insulin and lactate. Expired air samples were collected to evaluate changes in substrate use during exercise.
Subjects and methods
Subject information and initial testing
Seven endurance-trained male cyclists (30 (sem 6) years of age, 80 (sem 8) kg body weight) gave written informed consent. The study was approved by the Central Sydney Area Health, Human Ethics Research Committee, Sydney, Australia. Peak V˙O2 (V˙O2peak) was determined whilst cycling on a stationary cycle ergometer using four 5 min steady state stages (100, 150, 200 and 250 W) followed by a progressive increase in work of 10 W/min until voluntary exhaustion and averaged 4·6 (sem 0·2) l/min. All exercise tests were conducted on the same electronically braked cycle ergometer (Tunturi E85, Tunturi, Turku, Finland).
Pre-trial exercise
After a 12 h fast, participants undertook a 90 min cycle at 70 % V˙O2peak in the laboratory starting at 08.00 hours. Work rate (W) at 70 % V˙O2peak was calculated for each individual from the linear function of O2 uptake at the four steady-state work rates and maximal O2 uptake against the work rate (W) of the four steady-state work rates and maximal power output. Mean work rate at 70 % V˙O2peak was 216 (sem 8) W. During the cycle, water was provided ad libitum. During subsequent cycle trials, the same amount of water was consumed.
Dietary manipulation
At the end of the first 90 min cycle, participants were provided with food for the following 24 h containing either a HGI or LGI carbohydrate component. The treatment order of the interventions was randomised. Carbohydrate was provided at 8 g/kg body mass with protein and fat content constituting 11 and 17 % of energy respectively (see Table 1 for details). The glycaemic index (GI) values were calculated from international tablesReference Foster-Powell, Holt and Brand-Miller26. Breakfast was provided at the research facility. Packaged lunch, dinner and snacks were provided for the subject to consume at home. Water could be consumed ad libitum with no other drinks allowed. Participants were asked to only eat the food provided and to finish eating the food before 21.00 hours that night.
Table 1 Nutrient content of the high (H) and low (L) glycaemic index (GI) diets (for a 70 kg person)

* Cornflakes (Kellogg's, Melbourne, Australia); White bread (Sunblest, Tip Top bakeries, Sydney, Auystralia); Jam (Cottees strawberry conserve, Cottees, Melbourne, Australia); Margarine (Devondale extra soft spread, Murray Goulburn, Melbourne, Australia); Carbonated glucose drink (Lucozade, GlaxoSmithKleine, Boronia, Australia); Guardian Cereal (Kellogg's, Melbourne, Australia); Canned peaches (Goulburn Valley peach slices in natural juice (no added sugar); Yoghurt (Yoplait no fat fruit yoghurt, strawberry flavour if possible; National Foods, Sydney, Australia); Apple juice (Coles Farmland Apple Juice, no added sugar; Coles, Melbourne, Australia); Chicken (Castlemain shaved chicken breast, 97 % fat free; KR Castlemaine, Castlemaine, Australia); Cheese (Coles Farmland tasty cheddar cheese; Coles, Melbourne, Australia); Wholemeal pasta (San Remo wholemeal pasta spirals; San Remo, Sydney, Australia); Tomato sauce (Dolmio, Sydney, Australia); Grain snack bar (K-Time mixed berry bars; Kellogg's, Melbourne, Australia); Fruit snack (Arnott's Snack right fruit slice, sultana; Arnott's, Sydney, Australia).
Experimental trial protocol
Participants arrived in the laboratory 24 h after the first 90 min cycle participants arrived in the laboratory and were weighed in minimal clothing. An indwelling cannula was then inserted into the cephalic vein for blood sampling. Magnetic resonance examinations were completed 0·5 h before and 1 h after the end of the experimental trial cycle (see later). Following a 10 min warm- up at a self-selected pace, participants cycled for 90 min at 70 % of their V˙O2peak Expired air samples were collected for 2 min at 15, 30, 45, 60, 75 and 90 min time points and analysed online for expired fractions of O2, CO2 and ventilation (Cortex Biophysik, Leipzig, Germany). Substrate oxidation rates were calculated using non-protein stoichiometric equationsReference Frayn27. At rest and during each of the expired gas collections, 5 ml blood was collected into EDTA tubes and immediately centrifuged for 10 min at 4°C. Plasma was then extracted and stored at − 80°C for later analysis.
Intramyocellular lipid assessment
With the subject supine, a vitamin-E capsule was placed equidistant between the caudal tip of the patella and the caudal head of the femur over the vastus lateralis and marked with indelible ink. Image-guided (from the vitamin-E capsule), localised proton magnetic resonance spectra of the right vastus lateralis muscle were generated on a 1·5 Tesla Philips magnetic resonance scanner (Philips Medical Systems, Best, The Netherlands). The subject lay on his right-hand side with a 10 cm C3 flex coil (Philips Medical Systems, Best, The Netherlands) positioned over the vitamin-E capsule. The magnetic field was optimised using a fully automated shim routine to ensure magnetic field homogeneity. Water suppressed and unsuppressed spectra were collected from a 1·5 × 1·5 × 2·0 cm2 voxel using point resolved echo sequence spectroscopy (PRESS; echo time 32 ms, relaxation time 5 s).
Quantifictation of spectra
Calculation of metabolite (from water-suppressed spectra) and water (from water-unsupressed spectra) resonances was performed using the java-based magnetic resonance user interface (jMRUI version 2.0)Reference Naressi, Couturier, Castang, de Beer and Graveron-Demilly28, Reference Naressi, Couturier, Devos, Janssen, Mangeat and de Beer29. Following manual first and second order phase correction, spectra were analysed using a non-linear least squares algorithm (AMARES)Reference Vanhamme, Van Huffel, Van Hecke and van Ormondt30. With creatine CH-3 resonance selected as a reference at 3·02 ppm, eight other resonances were calculated: IMCL-CH3 (0·88 ppm), extra-myocellular lipid (EMCL)-CH3 (1·19 ppm), IMCL-CH2 (1·28 ppm), EMCL-CH2 (1·53), L-acetate 2·08, fatty acid-CH2 (2·24 ppm), trimethylacetate group (3·19 ppm), and taurine (3·37 ppm). From the non-water suppressed spectra, only the water peak was quantified. The signal amplitude IMCL-CH2 was corrected for T1Reference Bruhn, Frahm, Gyngell, Merboldt, Hanicke and Sauter31, Reference Schick, Eismann, Jung, Bongers, Bunse and Lutz32 and T2Reference Stannard, Thompson, Fairbairn, Huard, Sachinwalla and Thompson33 relaxation times reported in the literature under equivalent conditions. For absolute quantification of IMCL, metabolites were then corrected for proton content and the IMCL-CH2: water ratio (from the unsuppressed water spectra). Values were then converted into mm/kg wet weight, assuming a water concentration of 55 mm/kg wet weight and a tissue water fraction of 0·81, as previously describedReference Szczepaniak, Babcock, Schick, Dobbins, Garg and Burns34 (CV 0·71 mm/kg wet weight).
Blood assays
Plasma samples were analysed for glucose using a hexokinase enzymatic assay (Roche Diagnostic Systems, Sydney, Australia); insulin by a solid-phase, antibody-coated tube radioimmunoassay (Coat-A-Count Insulin RIA kit, Diagnostic Products Corporation, Los Angeles, CA, USA); lactate by enzymatic test (Roche Diagnostic Systems); glycerol using a colorimetric assay (RANDOX Laboratories Ltd, Crumlin, UK); free fatty acids (FFA) by a commercially available enzymatic colorimetric test kit (NEFA-HA; Wako Pure Chemical Industries Ltd, Osaka, Japan).
Statistical analysis
All statistical calculations were performed using SPSS version 11 (SPSS Inc., Chicago, IL, USA). A two-way analysis of variance (time and treatment) was used to assess metabolic and physiological differences between groups. A post-hoc Bonferroni step-wise correction was performed at the location of the variance. Statistical significance was accepted at P < 0·05. Data are presented as means with their standard error unless otherwise stated.
Results
Substrate oxidation
Whole-body fat oxidation increased and carbohydrate oxidation decreased over the 90 min of exercise (Fig. 1). During the HGI trial 197 (sem 23) g carbohydrate and 63 (sem 5) g fat were oxidised. During the LGI trial, 169 (sem 11) g carbohydrate and 76 (sem 9)g fat were oxidised. Lipid oxidation represented 37 (sem 6) % of the total substrate oxidation in the HGI trial and 47 (sem 8) % in the LGI trial. Despite trends in the predicted direction, none of the differences in substrate oxidation reached statistical significance (area under curve, P = 0·13).
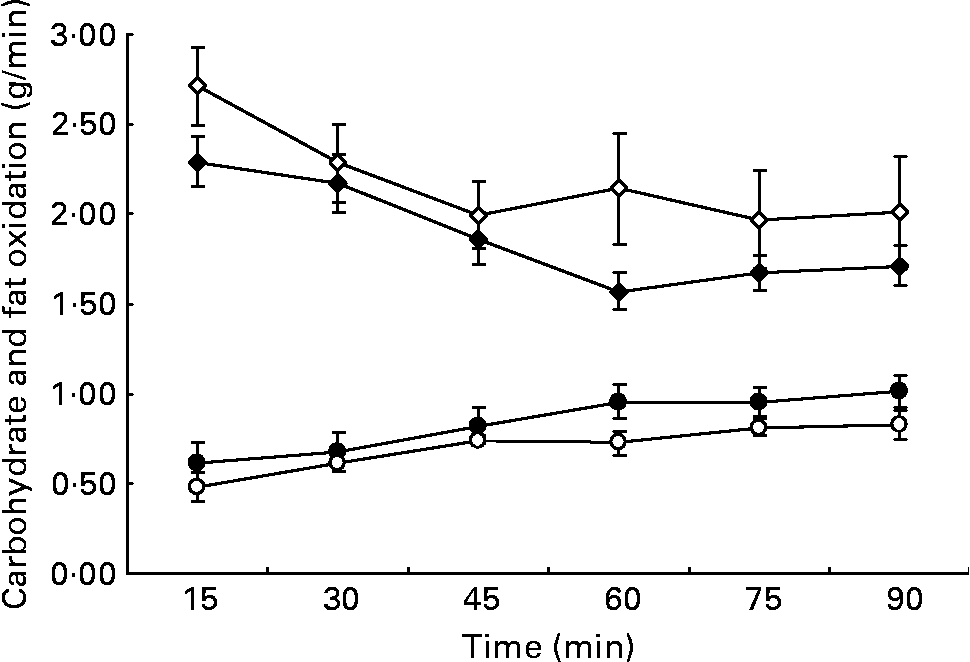
Fig. 1 Calculated carbohydrate (⋄, ♦) and fat oxidation (○, ●) every 15 min whilst cycling at 70 % V˙O2peak following a high glycaemic index (⋄, ○) or low glycaemic index (♦, ●) diet. Values are means with their standard errors shown by vertical bars.
Blood parameters
After an early decline, plasma FFA showed a progressive rise throughout exercise in both HGI (0·32 (sem 0·06) at baseline to 0·67 (sem 0·05) mm/l, P < 0·01) and LGI groups (0·57 (sem 0·14) at baseline to 0·81 (sem 0·11) m/l, P < 0·01) (Fig. 2 (A)). For the first 60 min of exercise, levels of circulatory FFA were higher in the LGI trial than in the HGI trial (P < 0·05; Fig. 2 (A)). Plasma glycerol concentrations showed a progressive rise throughout exercise in the LGI (84 (sem 17) at baseline to 483 (sem 48) μm/l, P < 0·01) and HGI trials (88 (sem 32) at baseline to 341 μm/l, P < 0·05), with higher concentrations shown in the LGI group at 15 and 90 min (P < 0·05; Fig. 2 (B)).
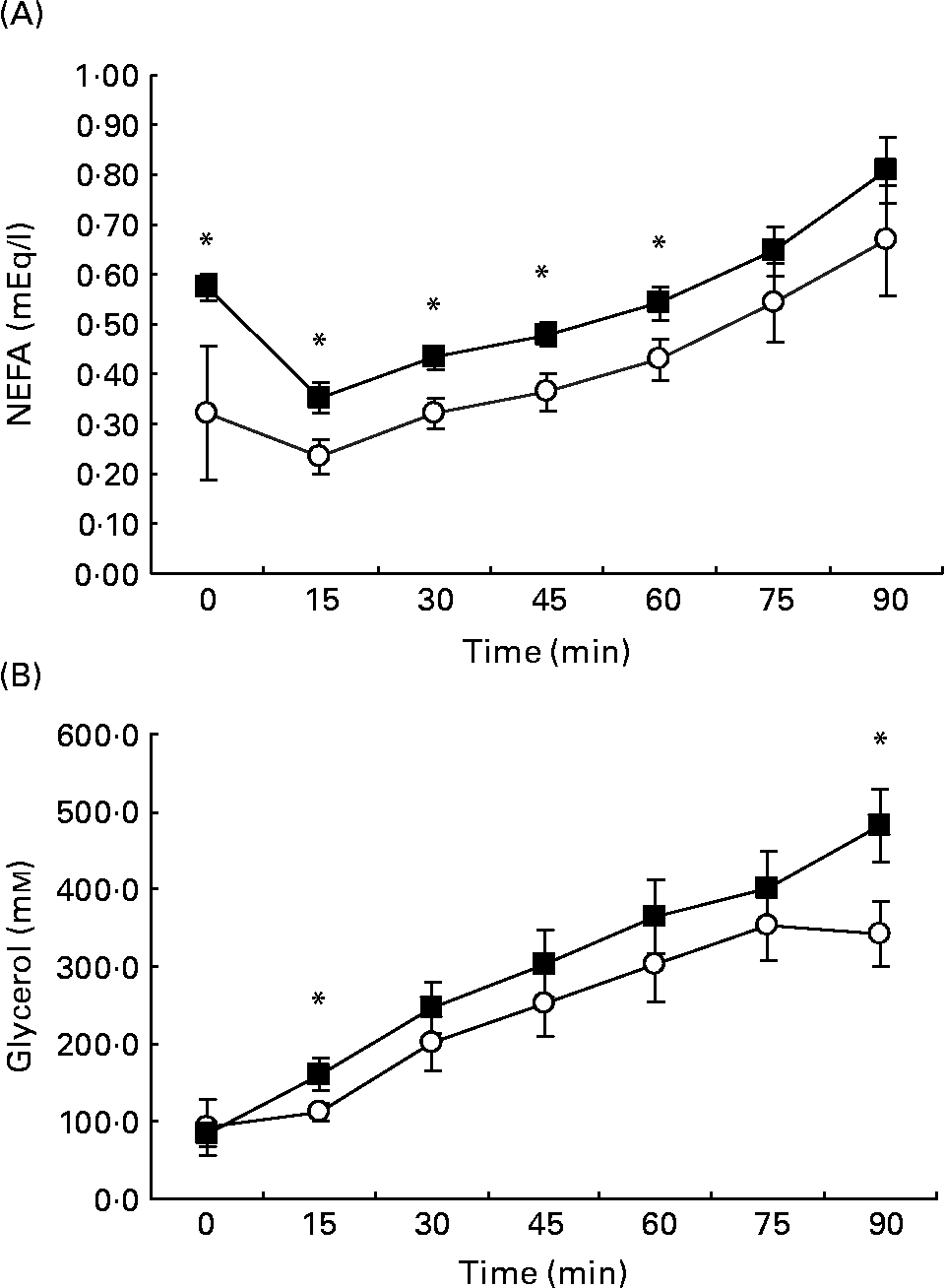
Fig. 2 Plasma NEFA (A) and glycerol (B) at rest and every 15 min whilst cycling at 70 % V˙O2peak following a high glycaemic index (HGI; ○) or low glycaemic index (LGI; ■) diet. Values are means with their standard errors shown by vertical bars. Mean values were significantly different from HGI relevant time point: *P < 0·05.
After an initial 45 min stability, there was a progressive decline in circulating glucose (P < 0·01; Fig. 3 (A)). Plasma glucose concentrations were higher in the LGI trial at 75 and 90 min (P < 0·05; Fig. 2 (A)). Insulin concentrations decreased progressively throughout exercise in both trials (P < 0·01; Fig. 3 (B)). After an initial rise from baseline, lactate concentrations remained stable throughout both trials (Fig. 3 (C)). Although mean lactate concentrations were consistently higher in the HGI trial, this did not achieve significance (P = 0·07). Plasma cortisol levels increased through exercise although there were no differences between the HGI and LGI trial (P>0·05; Fig. 3 (D)).

Fig. 3 Plasma glucose (A), insulin (B), lactate (C) and cortisol (D) at rest and every 15 min whilst cycling at 70 % V˙O2peak following a high glycaemic index (HGI; ○) or low glycaemic index (LGI; ■) diet. Values are means with their standard errors shown by vertical bars. Mean values were significantly different from HGI relevant time point: *P < 0·05.
Intramyocellular lipid
Exercise produced a significant reduction in IMCL in both the HGI (from 7·3 (sem 1·4) at baseline to 3·8 (sem 0·9) mm/kg wet weight, P < 0·05) and LGI (from 5·9 (sem 0·8) at baseline to 4·4 (sem 0·8) mm/kg wet weight, P < 0·05) groups over the 90 min of exercise (Fig. 4). The HGI group therefore showed a greater dependence on IMCL (3·5 (sem 1·0) mm/kg wet weight) than the LGI group (1·6 (sem 0·3) mm/kg wet weight; Fig. 4). There was no significant difference between baseline IMCL (P = 0·08) or end exercise IMCL (P = 0·06) between the LGI and HGI groups.
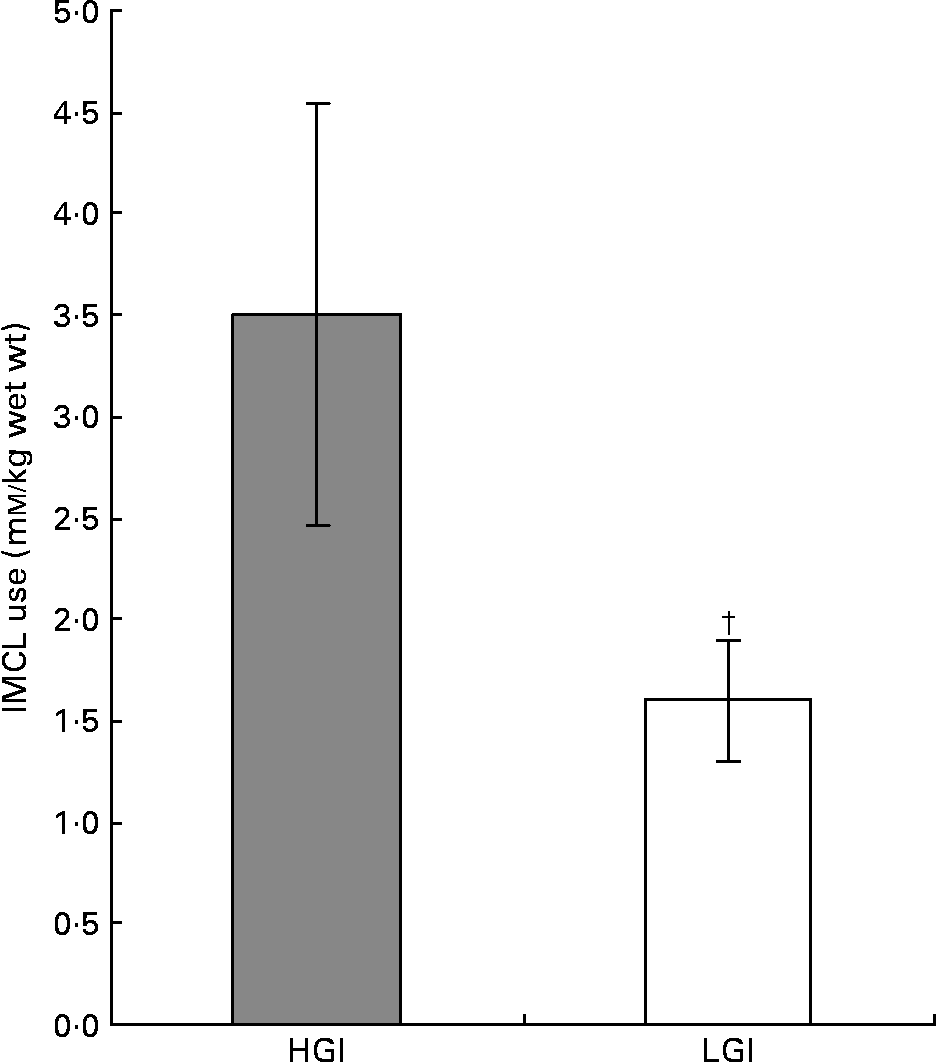
Fig. 4 Intramyocellular lipid (IMCL) use whilst cycling at 70 % V˙O2peak following a high (H) or low (L) glycaemic index (GI) diet. Values are means with their standard errors shown by vertical bars. Mean values were significantly different from HGI. †P < 0·05.
Discussion
This study shows that the GI of the diet consumed in the recovery period influences substrate availability and utilisation in subsequent prolonged exercise. Circulating fatty acid availability during exercise was about 34 % greater following a LGI diet compared with the HGI diet (quantified as area under the curve). The use of IMCL as a substrate source was >2-fold higher after the HGI meals than the LGI meals. Combined, these results suggest that consuming a LGI diet between bouts of prolonged strenuous exercise reduces dependence on intramuscular lipid stores and increases the use of circulating plasma FFA derived from other fat stores as a fuel source. In competitive sports and search-and-rescue operations, LGI meals might therefore improve final outcomes.
A novel aspect of the current study is the use of MRS to assess changes in IMCL following carbohydrate manipulation. The LGI diet was associated with a decreased IMCL oxidation and increased NEFA availability during exercise. In contracting skeletal muscle, the rate of NEFA uptake and oxidation is increased, preserving IMCL storesReference Sacchetti, Saltin, Osada and van Hall22. At rest, plasma NEFA are esterified in skeletal muscle to form IMCLReference Sacchetti, Saltin, Osada and van Hall22, Reference van Hall, Sacchetti, Radegran and Saltin35. Indeed, dietary intakes high in fat (40–60 % fat) promote IMCL storageReference Zderic, Davidson, Schenk, Byerley and Coyle36–Reference Starling, Trappe, Parcell, Kerr, Fink and Costill38 while diets high in carbohydrate (5–25 % fat) reduce IMCL storageReference Starling, Trappe, Parcell, Kerr, Fink and Costill38, Reference Coyle, Jeukendrup, Oseto, Hodgkinson and Zderic39.
Although the amount of IMCL oxidised during exercise was different between the treatment groups, the HGI group had a higher mean IMCL content before exercise and lower IMCL content after exercise, though these did not achieve statistical significance (P = 0·08 and P = 0·06 respectively). It is possible that the higher starting levels of IMCL in the HGI group could aid subsequent IMCL oxidationReference Stellingwerff, Boon, Jonkers, Senden, Spriet and Koopman9, theoretically, through an increase in the substrate–enzyme interfaceReference Prats, Donsmark, Qvortrup, Londos, Sztalryd and Holm40. The higher starting levels of IMCL in the HGI group would appear paradoxical, as a HGI diet should decrease NEFA availability to replenish IMCL in recovery from exerciseReference Stevenson, Williams, McComb and Oram19. However, the design of the present study cannot conclusively provide any explanation for these observations, as the study: (1) may be underpowered to detect changes in IMCL storage between groups, and (2) did not track postprandial lipid availability and oxidation over the recovery period. Irrespective of these comments, our data suggest that not only is the amount of carbohydrate and fat intake influential in determining substrate storage and use, but that the GI of the carbohydrate can change the availability and use of lipids during exercise. The observation of a trend towards differences in IMCL at the start and end of exercise between diets reinforces the need for further studies observing the influence of postprandial signalling and lipid availability upon IMCL storage.
Analysis of the indirect calorimetry data shows that there is a progressive increase in lipid oxidation and a decline in carbohydrate oxidation over the exercise period. The decline in carbohydrate utilisation has been attributed to a decrease in muscle glycogen utilisation and an increased uptake and use of plasma glucoseReference van Loon, Koopman, Stegen, Wagenmakers, Keizer and Saris6. Conversely, as plasma NEFA availability is reported to determine NEFA oxidationReference Romijn, Coyle, Sidossis, Zhang and Wolfe21, the progressive rise in plasma NEFA is likely to reflect an increase in NEFA oxidation. Studies using Intralipid infusion to elevate plasma NEFA concentrations show an increase in lipid oxidation and a reduced glycogen use in exerciseReference Dyck, Putman, Heigenhauser, Hultman and Spriet10–Reference Odland, Heigenhauser and Spriet13, suggesting that FFA availability can influence substrate use in skeletal muscle. In further support, use of the antilipolytic agent nicotinic acid reduces plasma fatty acid and increases dependence on glycogen during exerciseReference Stellingwerff, Watt, Heigenhauser and Spriet41. Despite this evidence, the pathways which control the relative contribution of carbohydrate and fat as substrate sources during metabolism are not completely understoodReference Wolfe42. However, the sustained suppression of circulating FFA during prolonged exercise may in part explain the increased reliance upon IMCL as a substrate source following a HGI diet.
Elevated insulin concentrations suppress lypolysis at rest and during subsequent exercise, reducing circulating levels of NEFAReference Horowitz, Mora-Rodriguez, Byerley and Coyle17. In line with this, acute intake of carbohydrate before exercise ( < 4 h) has been shown to decrease FFA availability and increase glycogen dependence during exerciseReference Montain, Hopper, Coggan and Coyle25. Altering the GI of carbohydrates consumed before exercise has been shown to influence substrate oxidation during exercise. Studies have reported that consuming a LGI breakfast 3 h before endurance exercise resulted in an increase in FFA availability and hence an increase in fat oxidation and decrease in carbohydrate oxidation compared to when a HGI breakfast is consumedReference Wu, Nicholas, Williams, Took and Hardy18, Reference Stevenson, Williams, Mash, Phillips and Nute43, Reference Wee, Williams, Tsintzas and Boobis44. In the current study, an increase in FFA availability was observed during exercise the day after consuming a LGI diet. Interestingly, the elevated circulatory NEFA observed at rest and during exercise in the LGI group occurred independent of any differences in circulating insulin between the treatment groups. This is suggestive of heightened or dampened sensitivity of the adipose tissue to insulin following exposure to high (HGI) or moderate (LGI) exposure to insulinReference Kallio, Kolehmainen, Laaksonen, Kekalainen, Salopuro and Sivenius45, respectively, the previous day.
Plasma glucose levels were stable over the first 45 min of exercise but then steadily declined. In the final 30 min, the decline was attenuated in the LGI group in comparison to the HGI group. It is likely that the difference represents increased dependence on glucose as the source of fuel in the HGI groupReference Stevenson, Williams and Nute46. It is possible that both liver and muscle glycogen stores are being depleted faster but little is known about the relative roles of each. Liver glycogen stores appear to be important. Using 13C magnetic resonance spectorscopyReference Casey, Mann, Banister, Fox, Morris and Macdonald47, glycogen content of the liver following exhaustive exercise was found to predict subsequent exercise capacity. Further 13C-MRS studies are needed to determine the relative importance of different fuel sources.
Our observation of a significant decrease in IMCL in both LGI and HGI groups with 90 min of exercise demonstrates that IMCL is a major substrate source during prolonged exercise. This observation is in line with previous studies using isotope-labelled FFAReference Romijn, Coyle, Sidossis, Gastaldelli, Horowitz and Endert5, Reference Stellingwerff, Boon, Jonkers, Senden, Spriet and Koopman9, muscle biopsyReference van Loon, Koopman, Stegen, Wagenmakers, Keizer and Saris6, Reference Stellingwerff, Boon, Jonkers, Senden, Spriet and Koopman9 and magnetic resonanceReference van Loon, Koopman, Stegen, Wagenmakers, Keizer and Saris6, Reference Johnson, Stannard, Mehalski, Trenell, Sachinwalla and Thompson7, Reference Stellingwerff, Boon, Jonkers, Senden, Spriet and Koopman9 analysis techniques. The use of IMCL as a substrate source during exercise has been the subject of much recent debate, with the inability of early studies to discriminate between intra- and extra-cellular lipids using biochemical analysis being central to this misunderstandingReference Watt, Heigenhauser and Spriet48. The problem is clearly demonstrated by Howald et al. Reference Howald, Boesch, Kreis, Matter, Billeter and Essen-Gustavsson49 who showed strong correlation between electron microscopic morphometry and MRS (r 0·93), demonstrating that both of these techniques are accurate in determining IMCL. However, the correlation between IMCL determined by biochemical analysis from muscle biopsy was weak with electron microscopic morphometry (r 0·41) and MRS (r 0·47). Our findings show that not only is IMCL (as assessed by 1H-MRS) an important substrate during exercise, but also that the origin of lipid oxidised during exercise, whether intra- or extra-muscular, can be influenced by the composition of diet during the previous 24 h. Future studies combining the use of 1H-MRS measurement of IMCL with 13C-MRS measurements of glycogen will add further to our understanding of this complex area.
In summary, the GI of carbohydrates consumed after prolonged exercise had a significant effect on substrate utilisation during subsequent exercise. The availability of plasma FFA was increased and IMCL use decreased when a LGI diet was consumed over a 24 h period compared to when a HGI diet was consumed. Further research is required to investigate whether the change in lipid oxidation with diets differing in GI alters glycogen usage and whether these changes in turn hold benefits for endurance-exercise performance.
Acknowledgements
The authors thank the volunteers for their participation and dedication. We also thank Helen Lackey, Jacquie Stewart, Dr John Magnusson and Dr Geoff Parker from Specialist Magnetic Resonance Imaging for assistance with magnetic resonance studies, The Woolcock Institute for Medical Research and Professor Carolyn Sue for access to exercise-testing facilities. This work was financially supported by the Sydney University Glycemic Index Research Service (SUGiRS).







