Introduction
In addition to core symptoms of hyperactivity, inattention and impulsivity, adults with attention deficit hyperactivity disorder (ADHD) experience deficits in executive function, including inhibition of motor impulses, verbal fluency, working memory, planning, organization and cognitive flexibility (Barkley, Reference Barkley1998; Woods et al. Reference Woods, Lovejoy and Ball2002; Hervey et al. Reference Hervey, Epstein and Curry2004; Roth & Saykin, Reference Roth and Saykin2004; Boonstra et al. Reference Boonstra, Oosterlaan, Sergeant and Buitelaar2005b; Lijffijt et al. Reference Lijffijt, Kenemans, Verbaten and van Engeland2005). These, together with the core symptoms, affect daily functioning, leading to impairments in education, work, relationships and social activities (Kessler et al. Reference Kessler, Adler, Barkley, Biederman, Conners, Demler, Faraone, Greenhill, Howes, Secnik, Spencer, Ustun, Walters and Zaslavky2006; Barkley et al. Reference Barkley, Murphy and Fischer2008). It is, however, unclear to what extent the symptoms contribute to impairments in daily life.
Stimulant medications such as methylphenidate (MPH) are highly effective for treatment of the core ADHD symptoms (Faraone et al. Reference Faraone, Spencer, Aleardi, Pagano and Biederman2004; Medori et al. Reference Medori, Ramos-Quiroga, Casas, Kooij, Niemelä, Trott, Lee and Buitelaar2008; Peterson et al. Reference Peterson, McDonagh and Fu2008; Adler et al. Reference Adler, Zimmerman, Starr, Silber, Palumbo, Orman and Spencer2009; Buitelaar et al. Reference Buitelaar, Ramos-Quiroga, Casas, Kooij, Niemela, Konofal, Dejonckheere, Challis and Medori2009a). There is also evidence that MPH treatment can improve symptoms relating to executive functioning in parallel with improvements in core symptoms (Aron et al. Reference Aron, Dowson, Sahakian and Robbins2003; Boonstra et al. Reference Boonstra, Kooij, Oosterlaan, Sergeant and Buitelaar2005a; Fallu et al. Reference Fallu, Richard, Prinzo and Binder2006). Few studies, however, have investigated the relationship between symptomatic improvement and improvements in daily functioning in patients with ADHD. A meta-analysis of clinical trials of atomoxetine in children and adolescents with ADHD found that symptomatic improvements (ADHD Rating Scale-IV – Parent Version: Investigator-administered; ADHDRS-IV-Parent:Inv) showed moderate to strong correlations with improvements in daily functioning, measured on the Life Participation Scale (LPS) (Buitelaar et al. Reference Buitelaar, Wilens, Zhang, Ning and Feldman2009b).
The osmotic-controlled release oral delivery system (OROS) MPH formulation is designed to deliver MPH in a controlled manner providing extended control of symptoms during the day. Studies have shown that once-daily treatment with OROS MPH is effective and well tolerated for treatment of ADHD in children and adolescents (Pelham et al. Reference Pelham, Gnagy, Burrows-Maclean, Williams, Fabiano, Morrisey, Chronis, Forehand, Nguyen, Hoffman, Lock, Fielbelkorn, Coles, Panahon, Steiner, Meichenbaum, Onyango and Morse2001; Wolraich et al. Reference Wolraich, Greenhill, Pelham, Swanson, Wilens, Palumbo, Atkins, McBurnett, Bukstein and August2001; Swanson et al. Reference Swanson, Gupta, Lam, Shoulson, Lerner, Modi, Lindemulder and Wigal2003), and adults (Biederman et al. Reference Biederman, Mick, Surman, Doyle, Hammerness, Harpold, Dunkel, Dougherty, Aleardi and Spencer2006; Medori et al. Reference Medori, Ramos-Quiroga, Casas, Kooij, Niemelä, Trott, Lee and Buitelaar2008; Adler et al. Reference Adler, Zimmerman, Starr, Silber, Palumbo, Orman and Spencer2009; Buitelaar et al. Reference Buitelaar, Ramos-Quiroga, Casas, Kooij, Niemela, Konofal, Dejonckheere, Challis and Medori2009a). In a 5-week, randomized, double-blind, placebo-controlled trial [Long Acting MethylpheniDate in Adult ADHD (LAMDA)], all three doses of OROS MPH tested led to significant improvements in symptoms of ADHD in adults, assessed using the investigator-rated 18-item Conners' Adult ADHD Rating Scale – Observer: Screening Version (CAARS-O:SV) (Medori et al. Reference Medori, Ramos-Quiroga, Casas, Kooij, Niemelä, Trott, Lee and Buitelaar2008). OROS MPH was also associated with statistically significant functional and global improvements, as measured using the non-disease-specific Sheehan Disability Scale (SDS) and the Clinical Global Impressions – Severity Scale (CGI-S). Regression analyses of data from LAMDA showed correlations between scores on the CAARS Hyperactivity/Impulsivity subscale and both SDS and quality-of-life scores (Rösler et al. Reference Rösler, Ginsberg, Arngrim, Adamou, Niemelä, Dejonkheere, van Oene and Schäuble2011).
In a 7-week open-label extension to LAMDA, subjects who received placebo in the double-blind phase showed significant improvement in CAARS-O:SV total score after 1 week of treatment in the extension, with further improvements at weeks 3 and 7, and CAARS-O:SV total scores continued to improve in patients who had previously received OROS MPH, with significant changes from baseline at weeks 3 and 7 (Buitelaar et al. Reference Buitelaar, Ramos-Quiroga, Casas, Kooij, Niemela, Konofal, Dejonckheere, Challis and Medori2009a). Here, we present data on functional and other secondary endpoints from the LAMDA open-label extension, and also explore the relationship between symptomatic and functional outcomes during the cumulative (double-blind and open-label) 12 weeks of treatment in adults with ADHD.
Method
Study design and patients
Adults with ADHD (n=401) were randomized to receive OROS MPH (18, 36 or 72 mg/day) or placebo for 5 weeks. Those who completed the double-blind phase or discontinued study medication as a result of poor tolerability after ⩾7 days of treatment were eligible to participate in a 7-week open-label extension during which they received flexibly dosed OROS MPH in the range 18–90 mg/day. To maintain blinding from the double-blind phase, all patients started the open-label phase at a dose of 36 mg/day (18 mg/day in Germany), which could subsequently be adjusted to optimize efficacy and tolerability according to the clinical judgement of the investigator.
Patients eligible for the initial placebo-controlled trial were adults (aged 18–65 years) with ADHD according to the criteria of DSM-IV and confirmed by the Conners' Adult ADHD Diagnostic Interview for DSM-IV (CAADID). The designs of the initial study and the open-label extension, together with full inclusion and exclusion criteria, have been published previously (Medori et al. Reference Medori, Ramos-Quiroga, Casas, Kooij, Niemelä, Trott, Lee and Buitelaar2008; Buitelaar et al. Reference Buitelaar, Ramos-Quiroga, Casas, Kooij, Niemela, Konofal, Dejonckheere, Challis and Medori2009a).
The study was conducted according to Good Clinical Practice and the ‘Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects’ contained in the Declaration of Helsinki. The study protocol was approved by the ethics committee at each site. All patients provided written, informed consent before participating in the study.
Endpoints and analyses
The primary efficacy assessment in the double-blind and open-label phases was the 18-item CAARS-O:SV total score (Medori et al. Reference Medori, Ramos-Quiroga, Casas, Kooij, Niemelä, Trott, Lee and Buitelaar2008; Buitelaar et al. Reference Buitelaar, Ramos-Quiroga, Casas, Kooij, Niemela, Konofal, Dejonckheere, Challis and Medori2009a). ADHD symptoms were also assessed using the patient self-report short version of the CAARS (CAARS-S:S). During the open-label phase, assessments were made at weeks 1 and 7 (CAARS-O:SV was also evaluated at week 3) with a post-study visit 1 week after the last dose of OROS MPH (whether this occurred in the open-label or in the double-blind phase). Global condition was evaluated using the CGI-S, for which the investigator rates the patient's severity of illness on a seven-point scale ranging from 1 (not ill) to 7 (extremely severe). Change in global condition was evaluated at endpoint using the CGI Change (CGI-C), a seven-point scale ranging from 1 (very much improved) to 7 (very much worse) (NIMH, 1985). Daily functioning was assessed using the SDS, a generic instrument with three 10-point visual analogue scales designed to measure the extent to which (i) work, (ii) social life and leisure activities and (iii) home life and family responsibilities are impaired (Sheehan et al. Reference Sheehan, Harnett-Sheehan and Raj1996). Quality of life was measured using the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) Short Form, a 16-item self-administered questionnaire designed to assess the degree of enjoyment and satisfaction experienced by patients in various areas of daily functioning. Patients rated items concerning physical health, feelings, work, household duties, work and leisure-time activities and social relationships across five response categories, ranging from very poor to very good (Endicott et al. Reference Endicott, Nee, Harrison and Blumenthal1993). The Q-LES-Q has been validated in adults with ADHD (Mick et al. Reference Mick, Faraone, Spencer, Zhang and Biederman2008).
Subjects who were treated in the double-blind phase could continue into the open-label phase. Efficacy analyses were performed on the open-label intent-to-treat (ITT) population, defined as those subjects who received at least one dose of open-label study medication and had at least one post-baseline assessment during the open-label phase.
Changes in CAARS-O:SV, CAARS-S:S and Q-LES-Q were analysed using a paired-samples t test. A regression analysis was performed for each functional scale on the change from double-blind baseline at the end of the open-label period, including baseline score, age, country, double-blind OROS MPH dose (or placebo), sex, change in CAARS-O:SV Hyperactivity/Impulsivity subscale score, change in CAARS-O:SV Inattention subscale score, and change in CGI-S at end of double-blind. The CAARS-O:SV total score was found to correlate strongly with the two CAARS-O:SV subscale scores, and was therefore not included in the analysis.
Results
Patient disposition
In the double-blind phase, 401 patients received at least one dose of study medication and 365 patients (91%) completed the 5-week double-blind study period. Full details of patient disposition in the double-blind phase have been published previously (Medori et al. Reference Medori, Ramos-Quiroga, Casas, Kooij, Niemelä, Trott, Lee and Buitelaar2008).
Of the 377 eligible patients, 370 entered the 7-week open-label extension. The ITT population comprised 369 patients, of whom 93 had received placebo and 276 had received OROS MPH in the double-blind phase. The mean (±s.d.) daily dose of OROS MPH in the open-label phase was 46.5±14.2 mg (range 18–82 mg) and the mean maximum dose was 57.6±18.1 mg (range 18–108 mg). The overall mean daily dose in the open-label phase was similar regardless of the original treatment group (45.6–48.0 mg). The most frequent maximum doses of OROS MPH in the open-label phase were 54 mg (36%), 36 mg (26%) and 72 mg (25%). The most frequent final doses in the open-label extension were 54 mg (34%), 36 mg (29%) and 72 mg (20%).
In total, 337 patients completed the open-label extension. Of the 33 patients (8.9%) who withdrew from the study prematurely, 18 withdrew because of an adverse event, five were lost to follow-up, one withdrew consent, one withdrew because of lack of efficacy, and eight withdrew for other reasons. Baseline characteristics of the patients who entered the open-label phase are shown in Table 1.
Table 1. Baseline demographics of all patients who entered the open-label phase
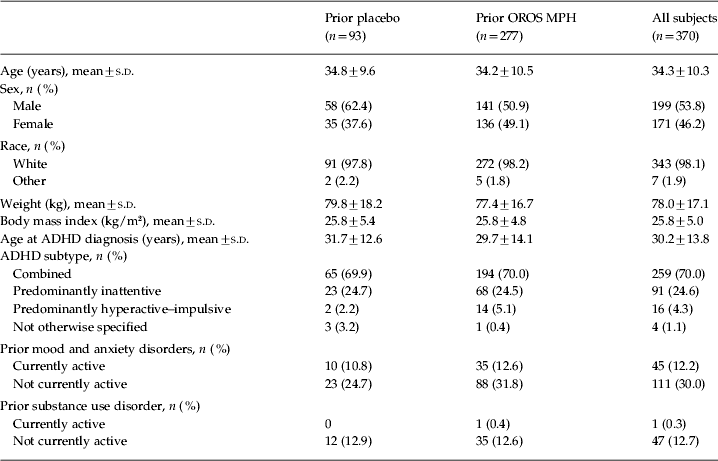
ADHD, Attention deficit hyperactivity disorder; OROS, osmotic-controlled release oral delivery system; MPH, methylphenidate; s.d., standard deviation.
Efficacy assessments
CAARS-O:SV scores at baseline, double-blind endpoint and open-label endpoint are shown in Table 2. Patients who switched from placebo to OROS MPH at the start of the open-label phase experienced improvement in total CAARS-O:SV score from double-blind endpoint after 1 week of treatment with OROS MPH [mean (±s.d.) change at week 1=–3.5±8.5, p<0.001 v. double-blind endpoint], whereas those who had previously received OROS MPH showed significant improvement relative to double-blind endpoint from week 3 of the open-label phase onwards (mean change at week 3=–4.3±8.2, p<0.001). At open-label endpoint, the mean changes from double-blind endpoint in the CAARS-O:SV score were −8.4±9.4 and −6.1±9.3 in the prior placebo and prior OROS MPH groups respectively (both p<0.001 v. double-blind endpoint; p=0.0073 for between-group comparison). Similarly, significant changes in CAARS-O:SV Hyperactivity/Impulsivity and Inattention subscale scores were seen from double-blind endpoint to open-label endpoint in both the prior placebo and prior OROS MPH arms (p<0.001 v. double-blind endpoint for both subscales) (Table 2). In the post-hoc subgroup analysis, changes in CAARS-O:SV score from double-blind endpoint to open-label endpoint were of similar magnitude regardless of patient subgroup (Table 3).
Table 2. Rating scale scores at baseline (week 0), double-blind endpoint (week 5) and open-label endpoint (week 12)

CAARS-O:SV, Conners' Adult ADHD Rating Scale – Observer: Screening Version; CAARS-S:S, CAARS – Self: Short Version; CGI-S, Clinical Global Impressions – Severity Scale; SDS, Sheehan Disability Scale; Q-LES-Q, Quality of Life Enjoyment and Satisfaction Questionnaire; OROS, osmotic-controlled release oral delivery system; MPH, methylphenidate.
Scores are mean±standard deviation or median (range).
* p⩽0.003 v. double-blind baseline.
Table 3. Change from double-blind endpoint to open-label endpoint in CAARS total scores in patient subgroups
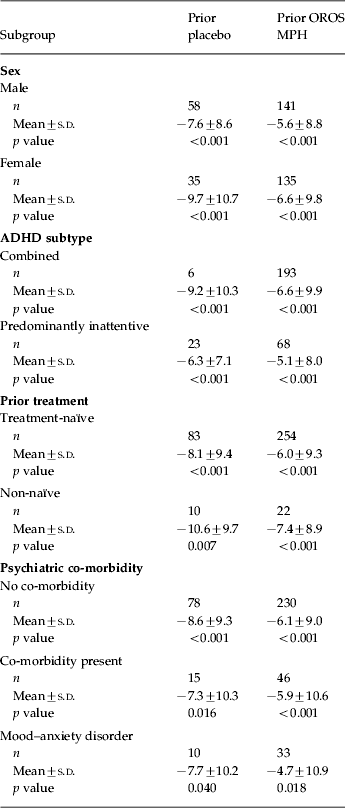
ADHD, Adult attention deficit hyperactivity disorder; CAARS, Conners' Adult ADHD Rating Scale; OROS, osmotic-controlled release oral delivery system; MPH, methylphenidate; s.d., standard deviation.
At week 7 of the open-label extension, significant improvement from double-blind endpoint was observed in the prior placebo and prior MPH groups for the CAARS-S:S, CGI-S and Q-LES-Q scales (Table 2). Significant improvement in SDS score was also seen (Table 2), and the results were similar for each of the three SDS subscales (Fig. 1). Clinically significant disability in terms of SDS subscale scores (score ⩾5) was present in most patients (80–89%) at double-blind baseline. At open-label endpoint, the percentage of patients with clinically significant disability was substantially reduced for each of the three SDS subscales (39–43%). At open-label endpoint, median (range) CGI-C scores were 2.0 (1–4) and 2.0 (1–5) in the prior placebo and prior OROS MPH groups respectively.

Fig. 1. Mean (±standard deviation) Sheehan Disability Scale (SDS) subscale scores at baseline, double-blind endpoint and open-label endpoint for patients previously treated with placebo (left panel, n=93) or osmotic-controlled release oral delivery system (OROS) methylphenidate (MPH) (right panel, n=269). ** p<0.01, *** p<0.001 versus baseline.
Post-study visit
One week after the last dose of OROS MPH (post-study visit), CAARS-O:SV scores worsened significantly compared with open-label endpoint in both the prior placebo [mean (±s.d.) increase=6.8±8.3] and prior OROS MPH (7.8±9.3) groups (both p<0.001). CGI-S scores had also worsened significantly from open-label endpoint at the post-study visit, with a median change of 0.0 and 1.0 in the prior placebo and prior OROS MPH groups respectively (both p<0.001 v. open-label endpoint).
Regression analysis
Regression analysis performed on the change in the functional scales from baseline to open-label endpoint showed that improvement in the CAARS-O:SV Hyperactivity/Impulsivity subscale at the end of the double-blind period was associated with functional improvement at the end of the open-label period for the SDS total and subscale scores, and the Q-LES-Q score (Table 4). Neither change in the CAARS-O:SV Inattention subscale nor change in CGI-S at the end of the double-blind period was significantly associated with functional outcome at the end of the open-label period. Overall, the total explained variances of the change in functional scale from baseline to open-label endpoint varied from 30% to 39% (Table 2).
Table 4. Regression coefficients of symptomatic improvement and other independent variables on functional improvement. The analysis was performed for each functional scale on the change from baseline to open-label endpoint including baseline score, age, country, treatment group, sex, and change in CAARS Hyperactivity/Impulsivity, CAARS Inattention and CGI-S at double-blind endpoint

CAARS, Conners' Adult ADHD Rating Scale; CGI-S, Clinical Global Impressions – Severity Scale; SDS, Sheehan Disability Scale; Q-LES-Q, Quality of Life Enjoyment and Satisfaction Questionnaire; OROS, osmotic-controlled release oral delivery system; MPH, methylphenidate; n.a., not available.
Values given are point estimates with p values (for difference from zero) in parentheses.
a Point estimates were calculated separately for each participating country.
Discussion
In this 7-week open-label extension following a 5-week double-blind, placebo-controlled trial, patients who received OROS MPH 18–90 mg/day experienced improvements in symptoms of ADHD, functioning and quality of life regardless of whether they were initially randomized to OROS MPH or placebo. These benefits were found to be similar when patients were categorized according to sex, ADHD subtype, prior treatment or the presence of psychiatric co-morbidities. Although the absolute benefits of OROS MPH in the open-label phase were generally greater in patients who were previously untreated, those who received placebo for 5 weeks in the double-blind phase had not ‘caught up’ in terms of CAARS-O:SV score at week 12 with those who had received 12 weeks of OROS MPH. This may be related to the flexible-dose design of the open-label phase, and may also be a result of patients who had the potential for further improvement receiving fixed, and therefore potentially suboptimal, doses in the double-blind phase. At a post-study visit 1 week after discontinuation, there was no evidence of rebound.
The post-hoc regression analysis showed that improvement in the CAARS-O:SV Hyperactivity/Impulsivity subscale was more closely associated with functional improvement (SDS) and improvement in quality of life (Q-LES-Q) than improvement in the CAARS-O:SV Inattention subscale. It is possible that this lack of correlation between observer-reported inattention and subject-reported functional disability reflects deficits in subjects' self-observation. After 12 weeks of treatment, no significant associations between change in CAARS-O:SV Inattention score or CGI-S at double-blind endpoint (week 5) and change in SDS or Q-LES-Q were observed, although the change in CAARS-O:SV Hyperactivity/Impulsivity subscale was significantly associated with functional improvement for all SDS subscales and Q-LES-Q. These results are consistent with a similar analysis carried out at week 5 (double-blind endpoint), when the change from double-blind baseline in the CAARS-O:SV Hyperactivity/Impulsivity subscale was significantly associated with both SDS total score and Q-LES-Q score (both p<0.001) (Rösler et al., in press).
In a meta-analysis of four studies of atomoxetine in children and adolescents with ADHD, a moderate to strong correlation (r=−0.68) was observed between changes in the LPS daily functioning scale and the ADHDRS-IV-Parent:Inv total, although no p value was provided for this correlation (Buitelaar et al. Reference Buitelaar, Wilens, Zhang, Ning and Feldman2009b). Of the LPS subscales, the Self-control subscale showed higher correlations than the Happy/Social subscale with the symptom measures. Regression analysis showed high sensitivity for functional measures to changes in symptom severity. Of note, impairments in daily functioning in children seemed to be driven by symptoms of inattention, suggesting that the effect of ADHD symptoms on daily living may change as individuals move from childhood to adulthood.
The magnitude of improvement in SDS score in the present study compares favourably with that demonstrated in a 4-year open-label study of atomoxetine, in which the change in SDS total score was −3.8 (Adler et al. Reference Adler, Spencer, Williams, Moore and Michelson2008b). Indeed, the improvement in SDS in the present study was almost as large as that seen in a 12-week open-label study of duloxetine in patients with major depressive disorder, in which the mean SDS total score was reduced from 18.7 to 9.5 (Hudson et al. Reference Hudson, Perahia, Gilaberte, Wang, Watkin and Detke2007). Although the SDS has not been validated formally in adults with ADHD, it has been validated in patients with bipolar disorder (Arbuckle et al. Reference Arbuckle, Frye, Brecher, Paulsson, Rajagopalan, Palmer and Degl'Innocenti2009), social anxiety disorder (Hambrick et al. Reference Hambrick, Turk, Heimberg, Schneier and Liebowitz2004) and panic disorder (Leon et al. Reference Leon, Shear, Portera and Klerman1992), and has been shown to be sensitive to treatment effects in patients with anxiety disorders, depression or premenstrual dysphoric disorder (Sheehan & Sheehan, Reference Sheehan and Sheehan2008).
In the present study, significant improvement from baseline in quality of life, as measured by the Q-LES-Q, was observed. These are consistent with the quality of life improvements reported in other studies in adults with ADHD receiving stimulant medication. In two 7-week studies in adults with ADHD treated with mixed amphetamine salts, improvements in health-related quality of life assessed using the disease-specific ADHD Impact Module (AIM-A) were significantly greater than those in subjects receiving placebo (Spencer et al. Reference Spencer, Adler, Weisler and Youcha2008a,b). Further analysis of data from these two studies showed that the improvements in quality of life, particularly the Performance and Function subscale of the AIM-A, were correlated with improvements in executive function (Brown & Landgraf, Reference Brown and Landgraf2010). Similarly, 8-week, 14-week and 6-month studies of adults with ADHD receiving atomoxetine have shown significant improvements versus placebo using the Adult ADHD Quality of Life Scale (AAQoL; Matza et al. Reference Matza, Johnston, Faries, Malley and Brod2007; Adler et al. Reference Adler, Spencer, Levine, Ramsey, Tamura, Kelsey, Ball, Allen and Biederman2008a, Reference Adler, Zimmerman, Starr, Silber, Palumbo, Orman and Spencer2009) or the 36-item Short Form questionnaire (SF-36; Adler et al. Reference Adler, Sutton, Moore, Dietrich, Reimherr, Sangal, Saylor, Secnik, Kelsey and Allen2006). In the latter study, the SF-36 score was shown to be significantly correlated with CAARS-O:SV.
Dosing in the open-label phase was flexible, based on clinical judgement, which more closely reflects clinical practice than fixed dosing. The patient cohort was, however, enrolled according to stringent inclusion and exclusion criteria, which may limit the generalizability of the data. In addition, it should be noted that the regression analysis was carried out on a post-hoc basis and the results remain to be confirmed in a prospective study.
In conclusion, the results of this analysis of data from a randomized, double-blind study and its open-label extension show that improvements in ADHD symptoms relating to hyperactivity and impulsivity in adults receiving OROS MPH are correlated with improvements in daily functioning and quality of life.
Acknowledgements
This study was supported by Janssen-Cilag EMEA (Europe, Middle East and Africa). Editorial support with the drafting and completion of the manuscript was provided by D. Booth (Bioscript Stirling Ltd, UK) and funded by Janssen-Cilag EMEA.
Declaration of Interest
J. K. Buitelaar has, in the past 3 years, been a consultant, a member of an advisory board and/or a speaker for Janssen-Cilag BV, Eli Lilly, Bristol-Myer Squibb, Organon/Shering Plough, UCB, Shire, Medice, and Servier. He is not an employee or a shareholder of any of these companies, and has no other financial or material support, including expert testimony, patents or royalties. M. Casas has, in the past 3 years, been a consultant, a member of an advisory board and/or a speaker for Janssen-Cilag, Eli Lilly, Shire and Rubió. He has received research grants from Janssen-Cilag, Rubió and Eli Lilly. He is not an employee or a shareholder of any of these companies, and has no other financial or material support, including expert testimony, patents or royalties. A. Philipsen has, in the past 3 years, been a consultant, a member of an advisory board and/or a speaker for Janssen-Cilag, Eli Lilly, Shire, Novartis. She has received research funding from Medice and Janssen-Cilag. She is not an employee or a shareholder of any of these companies, and has no other financial or material support, including expert testimony, patents or royalties. J. J. S. Kooij has been a speaker for Janssen-Cilag BV and Eli Lilly and has received research grants from Janssen-Cilag BV and Shire. She is not an employee or a shareholder of any of these companies, and has no other financial or material support, including expert testimony, patents or royalties. J. A. Ramos-Quiroga has, in the past 3 years, been a consultant, a member of an advisory board and/or a speaker for Janssen-Cilag, Eli Lilly, Shire and Rubió. He has received research grants from Janssen-Cilag, Rubió, Eli Lilly and Alicia Koplowitz Foundation. He is not an employee or a shareholder of any of these companies, and has no other financial or material support, including expert testimony, patents or royalties. J. Dejonckheere is a consultant working on behalf of SGS-Life Science Services, a company employed by Janssen-Cilag EMEA to provide statistical analysis. J. C. van Oene is a former employee of Janssen-Cilag EMEA. B. Schäuble is an employee of Janssen-Cilag EMEA.







