Introduction
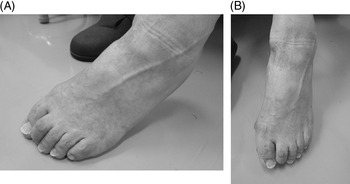
Local injection of botulinum toxin (or BoNT) is widely used for treatment of lower limb spasticity in post-stroke patients. Reference Kaji, Osako and Suyama1–Reference Dunne, Heye and Dunne4 Claw foot deformity (abbreviated here as “claw toe”) due to lower limb spasticity is often observed in such patients, and treatment with injections of BoNT into the flexor hallucis longus (FHL), flexor digitorum longus (FDL), flexor digitorum brevis, or quadratus plantae muscles has been reported previously. Reference Lim, Ong and Seet5–Reference Rousseaux, Compere, Launay and Kozlowski12 However, injection of BoNT into the FDL often does not produce any noticeable improvement in patients with foot claw deformity involving mainly the second and third toes (Figure 1A and B). In this regard, movements of the first (large) toe are considered to be controlled by the FHL muscle while those of the 2nd to 5th toes by the FDL muscle.
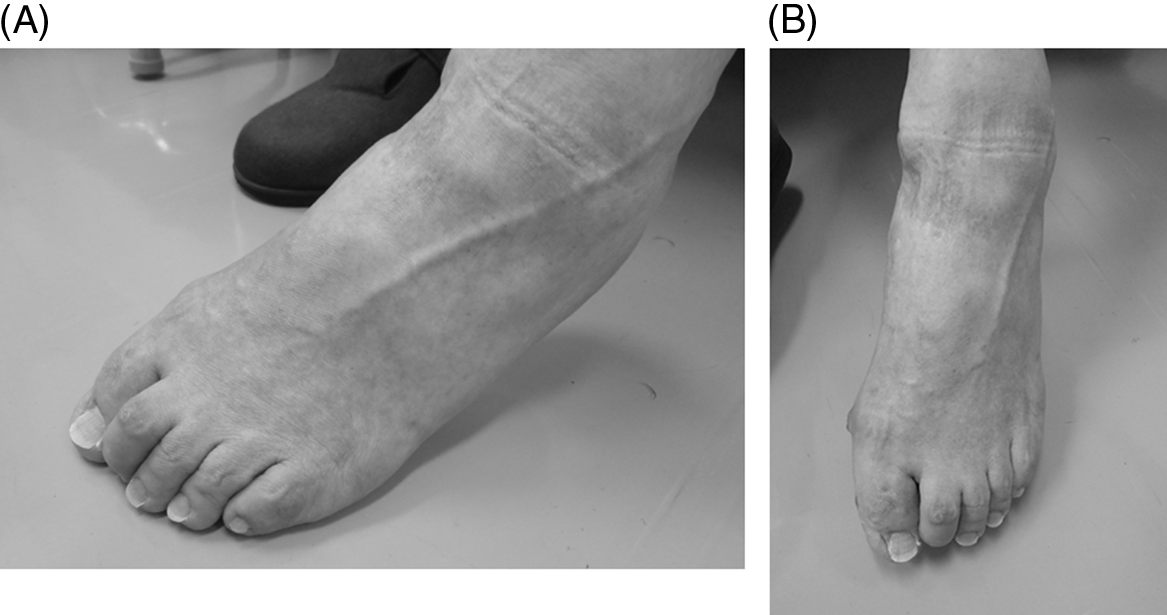
Figure 1: (A and B) The foot of a 74-year-old man with left post-stroke hemiplegia. The time period since onset was 7 years and 2 months. In this patient, the claw toe is dominant, mainly affecting the second toe.
We previously advocated the injection of BoNT into the FHL especially for treatment of claw toe affecting the second toe. Reference Takekawa, Mochio and Sato10 We also reported previously the anatomical correlations between the FHL and FDL in six limbs of three cadavers. Reference Takekawa, Mochio and Sato10 In all the six limbs, the FHL tendon was not only inserted into the distal phalanx of the big toe but also sent branches to the FDL tendon (Figure 2A and B), i.e., “Junctura Tendinum,” Reference Matsui, Kinoshita and Taguchi13 and it was also inserted in the second toe. Furthermore, previous studies also demonstrated that the FDL tendon attaches to the phalanxes of the 2nd, 3rd, 4th, and 5th toes. Reference Takekawa, Mochio and Sato10

Figure 2: (A) The left foot of an 86-year-old man. Left: cephalic direction, right: caudal direction, top: FHL tendon. The FDL tendon has been cut, and it is swept down with forceps. Note the tendon branching from the FHL tendon to the FDL tendon. The flexor digitorum brevis muscle was cut and inverted to the right. (B) The left foot of an 89-year-old woman. Left: cephalic direction, right: caudal direction, top: FHL tendon, bottom: FDL tendon. Images from reference #10. Note the thick main tendon of the flexor hallucis longus, which inserts into the first toe, and the other relatively thin branches, which join the tendon of the flexor digitorum longus, ending toward the second toe.
The present retrospective study is an extension to our previous investigation Reference Takekawa, Mochio and Sato10 and was designed to determine the anatomical and functional relationships between the FHL and FDL muscles. In patients with history of stroke and toe spastic deformity, we studied the toe movements during electrical stimulation of these two muscles at the time of BoNT administration. In the second part of the study, we conducted human autopsy examination to determine the pattern of insertion and’ “Junctura Tendinum” of FHL and FDL tendons into the phalanxes of the five toes.
Subjects and Methods
The study protocol was approved by the Human Ethics Review Committee of The Jikei University School of Medicine for Biomedical Research [approval number 26–377(7883)], and a signed consent form was obtained from each subject before the injection. The study was also conducted in compliance with the Helsinki Declaration.
The study participants satisfied the following criteria: (1) history of stroke-related leg paralysis with the degree of leg spasticity of ≥1 on the modified Ashworth scale (mAS) Reference Bohannon and Smith14 ; (2) latency between stroke onset and study enrolment ≥1 year; (3) previous visitation to the Outpatients Department seeking treatment for lower limb spasticity, and recognition of “claw toe due to spasticity” at the time of clinical examination; (4) no contraindications to BoNT administration, as indicated previously. 15,16,Reference Mori, Ogawa, Ouchi and Mori17 ; and (5) previous treatment with BoNT injection into the FHL or FDL, together with electrical stimulation for claw toe during the period of August 1, 2013, to April 30, 2016.
Each subject received injection of BoNT (onabotulinumtoxin A injection) into certain spastic muscles of the paralyzed upper and lower limbs, especially the FHL and FDL muscles. BoNT was diluted with saline to a concentration of 1.25 units/0.1 ml and administered under guidance of neuromuscular electrical stimulation. For injection, 25G sterile pole anesthesia needles (Top Co., Tokyo) were used. Electrical stimulation was applied using New Tracer NT-11 (Top Co.). Figure 3A and B illustrates the site of injection and the direction of needle insertion into the FHL and FDL muscles. Preliminary studies in each subject confirmed that the stimulation needle was inserted into the FHL or FDL muscles through the observation of muscle contraction/toe movement during the application of electrical stimulation. These observations also served to determine the dominance of FHL and FDL with regard to the movement of each toe. In this regard, the tibialis nerve is in close contact with the FHL muscle, and its stimulation induces strong and painful muscle contractions. Contraction of the entire foot confirmed stimulation of the nerve and distinguished it from focal stimulation of the muscles themselves.
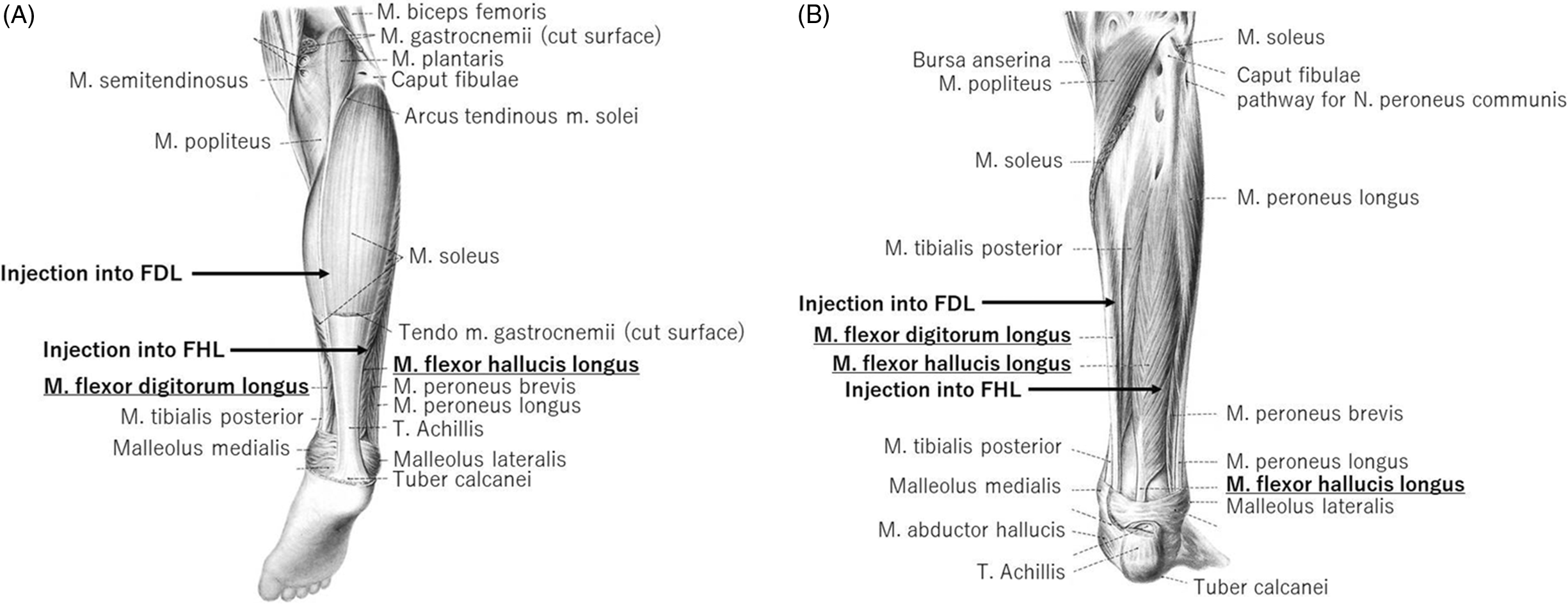
Figure 3: The location and direction of insertion of the needle into the FHL and FDL muscles (illustrations from reference #17, with minor modifications). The needle was inserted directing from the tibial side of the lower leg to the fibula side along the posterior side of the tibia. Its point of entry was the distal part of the lower leg for the FHL muscle and the middle of the lower leg for the FDL muscle. (A) Posterior aspect of the right lower leg, excluding the gastrocnemius muscle. (B) Posterior aspect of the right lower leg, excluding the gastrocnemius and soleus muscles.
Based on the difference in the response to electrical stimulation between the FHL and FDL muscles in terms of the strength of muscle contraction, we retrospectively classified the latter into + (marked muscle contraction), ± (weak contraction), and − (no contraction). Furthermore, we also classified the FHL muscle according to the number of toes that contracted in response to electrical stimulation of the muscle; type I represented contraction of the first toe only upon FHL stimulation, type II for the first and second toes, type III for the first to third toes, and type IV for the first to fourth toes. Also, strong contraction on the fibular side of the stimulated toes was tagged with no mark, while that of weak contraction was marked with ”>” (more than sign) mark. Similarly, for the FDL muscle, type I represented contraction of the first to fifth toes, type II of the second to fifth toes, type III of the third to fifth toes, and type IV of the fourth and fifth toes only. In addition, strong contraction on the most tibial side of the toes and fifth toe was marked with no mark, while that of weak contraction in the most tibial side of the toes was marked as “<” (less than sign), while weak contraction of the fifth toe, i.e., the most fibular side, was marked as “>” (more than sign).
Muscle contraction in response to electrical stimulation was confirmed by two physicians or a physician and a nurse. Changes in clinical signs and symptoms related to the claw toe at 14–42 d after BoNT injection, including any improvement or worsening, were collected from the medical records. The reported changes were based on clinical assessment by the attending physician and face-to-face interview with the patients/relatives.
We also studied six limbs of three cadavers in whom stroke was not the cause of death as well as the six limbs of three cadavers on which we reported previously on the “Junctura Tendinum” between the FHL and FDL muscles. Reference Takekawa, Mochio and Sato10
In addition, we compared the anatomical findings in the 12 limbs of six Japanese cadavers with those from the UK, Reference O’Sullivan, Carare-Nnadi, Greenslade and Bowyer18 Canada, Reference LaRue and Anctil19 and Turkey, Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20 with regard to the proportion of tendon divisions/tendon branches from FHL to FDL. With regard to the “Junctura Tendinum” between the FHL and FDL muscles, the tendon branched in one direction only from FHL to FDL in the majority of cases, whereas tendon branching at one-way direction from the FDL to FHL, two-ways, no branching, and other forms were relatively uncommon. Reference Takekawa, Mochio and Sato10,Reference O’Sullivan, Carare-Nnadi, Greenslade and Bowyer18–Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20 Pearson’s chi-square test was used to compare the two types of one-way branches from FHL to FDL and other types. All data are expressed as mean ± SD. A p value of <0.05 denoted the presence of a statistically significant difference.
Results
The study subjects were 31 patients (age, 59.7 ± 8.9 years, males=25, females=6) with time since stroke of 6.0 ± 4.2 years. The stroke type was intracerebral hemorrhage (n = 22, 71%) and cerebral infarction (n = 9, 29.0%). The cause of cerebral hemorrhage was unknown.
BoNT injection into the FHL was scheduled in 28 patients and into the FDL in 29 patients. Not all patients who underwent electrical stimulation received BoNT injection. One of the 28 patients who underwent FHL stimulation did not receive BoNT injection because the muscle did not induce movement of the target toe on the fibular side probably due to disruptions in tension transmission from the FHL muscle. Furthermore, 2 of the 29 patients who underwent electrical stimulation of the FDL were also not treated with BoNT due to the lack of movement of the targeted second toe probably due to disruptions in tension transmission from the FDL muscles. Thus, BoNT was injected into the FHL in 27 subjects and into FDL in 27 subjects. The mean total dose of BoNT was 26.5 ± 10.9 units and 25.3 ± 7.2 units, respectively.
Clinical examination and patients’ self-reports confirmed improvement of the claw toe-related symptoms and signs after administration in 17 of 25 patients (68%). Among them, 16 showed improvement in symptoms, such as reduction of claw toe. However, no improvement was observed in 8 out of 25 (32%) patients. Unfortunately, no mention of such follow-up was found in the medical records of 6 patients.
Several patterns of FHL and FDL muscle contractions were noted in response to electrical stimulation (Tables 1 and 2). With regard to stimulation of the FHL muscle, flexion of the first toe was observed in response to electrical stimulation in all the 28 patients (types I–IV). However, flexion of the first toe (type I) only was observed in 3 (11%) patients. Flexion of the second toe (type II to IV) was observed in 25 of 28 (89%) subjects, that of the third toe (types III and IV) in 12 (43%), and of the fourth toe in 1 (4%) (type IV). In none of the subject did electrical stimulation of the FHL muscle elicit movement of the fifth toe (Table 1).
Table 1: Classification of patterns of contracted toes in response to electrical stimulation of the flexor hallucis longus muscle (FHL) and number and proportion of patients with each pattern

+: Electrical stimulation elicited clear muscle contraction or toe flexion.
±: Electrical stimulation elicited weak muscle contraction.
– : Electrical stimulation did not elicit muscle contraction.
For type of electrical stimulation applied to the FHL, the roman numerals are relative to toes on the most fibular side that showed flexion.
>: For toe plantar flexion, the toe on the most peroneal with weak contraction.
Table 2: Classification of patterns of toe flexion elicited by electrical stimulation of the flexor digitorum longus muscle and the number and proportion of patients with each pattern

+: Electrical stimulation elicited clear muscle contraction or toe flexion.
±: Electrical stimulation elicited weak muscle contraction.
– : Electrical stimulation did not elicit muscle contraction.
For type of electrical stimulation applied to the FDL, the roman numerals are relative to toes on the most tibial side that showed flexion.
<: For toe flexion, the toe on the most tibial with weak contraction.
>: The 5th toe showed the weak flexion movement.
Stimulation of the FDL muscle produced mild flexion of the first toe in 1 out of 29 (3.4%) subjects (type I <), flexion of 4 toes (second to fifth toes, types I and II) in only 16 (55%), of 3 toes (third to fifth toes, type III) in 12 (41.4%), and of 2 toes (fourth and fifth toes, type IV) in 1 (3.4%) (Table 2).
The cause of death of the three cadavers conducted in this study was uremia, chronic renal failure, and diabetic nephropathy for the 61-year-old male, ruptured dissecting aortic aneurysm for the 81-year-old female, and congestive heart failure for the 96-year-old male. The right first toe had been amputated in one of the cadavers. The anatomical positions of the FHL and FDL muscles were identified in the three cadavers. We confirmed the presence of branches from the FHL tendon to the FDL tendon. The FHL tendon was inserted into the phalanx of the first toe and branches from this merged with those of the FDL tendon in all the six feet examined. On the other hand, no branches emerged from the FDL tendon into the FHL tendon (Table 3).
Table 3: The mode of branching and fusion of the FHL and FDL tendons and the tension transmission mode determined in five limbs of three cadavers

For the tension transmission pattern to each toe, data on the left are for tension from the FHL tendon and those on the right are for tension from the FDL tendon.
Type classification conforms to the classification of moving toes during electrical stimulation.
Analysis of transmission of mechanical tension (pulling the tendon) in five limbs of three cadavers (excluding one limb of one cadaver with the missing right first toe) showed that tension in FHL tendon moved the first to third toes in four limbs (type III>) and the first to fourth toes in one limb (type IV>). In each case, the transmission of force to the toe was weak on the most fibular side. Tension in the FDL tendon moved the second to fifth toes in all five limbs (type II), though that of the fifth toe was weak in three limbs (type II>) (Table 3). (The classification within the parenthesis matched that of the pattern of toe movements during electrical stimulation).
Comparison of the differences in the pattern of “Junctura Tendinum” for the FHL and FDL muscles observed in our studies compared with previous studies from UK, Canada, and Turkey Reference O’Sullivan, Carare-Nnadi, Greenslade and Bowyer18–Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20 showed significant association between each study (race) and the mode of “Junctura Tendinum” (p < 0.01, for each side). In other words, the proportion of tendon branches in one direction from the FHL to the FDL within the “Junctura Tendinum” was different among the respective studies. However, the expected frequency in one cell (12.5%) was less than 5, and the minimum expected frequency was 4.0. Table 4 shows cross-tabulation, and Figure 4 shows graphic presentation of the results of each study and mode of tendon insertion.
Table 4: Mode of FHL and FDL tendon insertion reported in the previous studies and the present study


Figure 4: Effect of race on the mode of Junctura Tendinum between flexor hallucis longus and flexor digitorum longus. The proportion of subjects with tendon branches passing in one direction from the flexor hallucis longus (FHL) to the flexor digitorum longus (FDL) is relatively low in the Canadian study of Caucasians (reference #19), compared to other racial groups. On the other hand, the proportion of subjects with tendon branches passing in one direction from the FHL to the FDL is relatively high in Japanese subjects.
Discussion
We often experience patients with claw toe deformity mainly affecting the second toe in whom administration of BoNT into the FDL does not produce satisfactory therapeutic results. In general, the FHL muscle is inserted into the distal side of the plantar surface of the first distal phalanx, Reference Mori, Ogawa, Ouchi and Mori17 and its function is flexion of the interphalangeal joint of the big toe. Reference Aldo21 On the other hand, the tendons of the FDL muscle are inserted on the plantar surface of the distal phalanxes of the second to fifth toes. Reference Mori, Ogawa, Ouchi and Mori17 The function of the FDL muscle is considered to be flexion of the proximal interphalangeal joints of the second to fifth toes. Reference Aldo21
In most cases, ultrasonography was not used during the injection to check the position of the needle tip, though it was applied in cases with difficult to explore muscles. In some cases, it was difficult to probe the muscle using only electrical stimulation because the target muscle was thin compared to the size of the lower leg. Nevertheless, ultrasound is often used at present, but electrical stimulation of the muscles can also be used in patients with spasticity of the lower extremity and claw toe, such as post-stroke patients, in order to identify the target muscles before the administration of BoNT. However, electrical stimulation of the FHL is not expected to result in flexion of the first toe only, and that of the FDL is not expected to result in flexion of the second to fifth toes only. We previously examined toe movements during electrical stimulation of the FHL and FDL muscles, especially those of the second toe. Reference Takekawa, Mochio and Sato10 The results showed that caution should be exercised when interpreting the results of such procedure since the control of the FDL muscle is insufficient. Reference Takekawa, Mochio and Sato10 We also reported that BoNT should be administered not only into the FDL but also into the FHL, especially when the target toe is the second or third toe. Reference Takekawa, Mochio and Sato10 In this study, we used electrical stimulation as a guide for BoNT injection after confirming the actions of the FHL and FDL muscles on each toe. However, we believe it is easier to administer the drug under ultrasonography. In the future, and based on the findings of this study, we plan to develop a simpler and less invasive method for the treatment of claw toe under ultrasonographic guidance.
In the present study, FHL muscle contraction and flexion of the first toe were observed in all cases upon electrical stimulation. In addition, electrical stimulation of the FHL resulted in muscle contraction and flexion of the second toe in 25 of the patients (89%) and of the third toe in 43% of the cases. On the other hand, FDL stimulation resulted in muscle contraction and flexion of the second to fifth toes in 55% of the patients, lack of the first and second toes in 45%, and total lack of such response in the first to third toes in one case (3.4%).
Although the second and third toes are connected to the FDL tendon, in a certain number of patients, the primary tension is probably related to the FHL tendon rather than the FDL tendon. In addition, strong contraction was observed in some toes in response to electrical stimulation, while others showed weak contraction. In the present study, we assigned the “more than” (>) sign to weak contraction of the toe on the peroneal side and the “less than” (<) sign to weak contraction of the toe on the tibial side. Thus, the FHL tendon exerts tension not only on the first toe but also on the more peroneal side, though the transmission of tension may be inadequate in the most peroneal toe. In addition, tension of the FDL tendon is not uniformly transmitted from the second to the fifth toe, and our findings showed that, at least in some cases, the transmission of tension was inadequate, or did not reach one or more toes.
Our data showed a tendency for improvement of claw toe-related symptoms and signs in 68% of the subjects. However, no significant change in the appearance of the claw toes was noted in some patients, which could reflect the limitation of the BoNT treatment. We believe that injection of BoNT into both “extrinsic muscles,” i.e., the FHL and FDL muscles, should improve claw toe deformity in at least about two-thirds of patients. In this study, BoNT was not used in three of the patients (BoNT was not injected into the FHL muscle in one patient and into the FDL muscle in 2 patients). In the former case, the results of electrical stimulation of the FHL, which was the target muscle, showed that the toe on the peroneal side was not controlled by the FHL muscle. In the latter case, the results of electrical stimulation of the FDL, which was the target muscle, showed that the second toe was largely controlled by the FHL muscle and less by the FDL muscle. It is not clear whether we would have had achieved therapeutic effect in these three patients if BoNT was injected under ultrasonographic guidance.
Since the main purpose of this study was to investigate the anatomical and functional relationship between the FHL and FDL muscles, none of the patients received adjunct therapy, such as orthoses, even after the intervention. Furthermore, treatment of claw toe is not limited to BoNT injection into FHL and FDL muscles. One should also consider other treatment options such as injection therapy into the flexor digitorum brevis muscle or the quadratus plantae muscle, orthotics, such as metatarsal bar, sack for toes, and oral medications. These options may have the potential to enhance the therapeutic efficacy of BoNT injection.
Even when flexion of the first toe cannot be achieved at rest, the FHL muscle may contract when the subject tries to move the lower limb. Many of our patients with leg spasticity and low mAS suffered spastic dystonia, which in some may have required tenotomy or surgical neurectomy or at least a higher dose of BoNT, in addition to injection of BoNT into other intrinsic muscles. In this regard, the claw foot in dystonic patients is often associated with extension of the big toe. Though rare, BoNT injection may result in hyperextension of the first toe. Therefore, care should be exercised during BoNT injection. In this study, the average dose of BoNT injected directly into the FHL and FDL muscles was about 25 units, the usual dose commonly used in our patients with claw toe treated with BoNT for the first time. When the injection is repeated in the same patient, we often use a larger doses of BoNT to amplify the previous effect. We believe repeated injections of BoNT in the same muscle are feasible as required.
The FHL tendon divides into smaller tendons that fuse with those of the FDL. The tendinous relation between FHL and FDL in the foot was examined in the past and the two are known as “the Knot of Henry” Reference O’Sullivan, Carare-Nnadi, Greenslade and Bowyer18 or “Junctura Tendinum.” Reference Matsui, Kinoshita and Taguchi13 The Knot of Henry is located 1.8 cm below the tuberositas ossis navicularis and 5.9 cm distal to the medial malleolus. Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20 Several patterns of the tendinous coupling have been described, in addition to various combinations of modes of transmission of tension of the two muscles to each toe. Reference Mori, Ogawa, Ouchi and Mori17–Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20,Reference Ali, Griffin, Ellis and Meyr22 Dissection of the six cadavers in the present and our previous study Reference Takekawa, Mochio and Sato10 showed tendon coupling from the FHL to the FDL in all the 12 feet, but lack of such relation from the FDL to FHL (Figure 2A and B); the FHL tendon divided and the branches fused with those of the FDL in all cadavers. In this regard, one previous study examined the characteristics of the Knot of Henry in 16 limbs of cadavers. Reference O’Sullivan, Carare-Nnadi, Greenslade and Bowyer18 In 11 limbs, contraction of the FHL was transmitted not only to the first toe but also to the second toe and toes on the fibular side, whereas contraction of the FDL resulted in flexion of the second toe or other toes, but not the first toe. The authors also indicated that the pattern of transmission of tension was unidirectional and specific; from the FHL to FDL. A similar tension transmission pattern from the FDL to FHL was noted in two limbs, and from the FHL to FDL and from FDL to FHL in both directions in three limbs. Reference O’Sullivan, Carare-Nnadi, Greenslade and Bowyer18 In a similar study involving 24 limbs of 24 cadavers, Reference LaRue and Anctil19 tendon branches from the FHL to FDL were recognized in 10 limbs (42%), both FHL to FDL and FDL to FHL in 10 limbs (42%), but no such tendinous fusion was found in 4 limbs (17%). Analysis of dissection of 20 limbs of 10 cadavers in another study allowed the classification of Knot of Henry into seven types (20); FHL to FDL tendon in a single direction (15 limbs, 75%), and in both directions from FHL to FDL and FDL to FHL (2 limbs, 10%), two tendons in one direction from FHL to FDL in one case (5%), two tendons from FHL to FDL and one tendon from FDL to FHL in a single case (5%), one tendon from FHL to FDL and two tendons from FDL to FHL in one case (5%). Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20 Considered together, the above studies showed the prevalence of FHL extensions to the FDL. Furthermore, it seems that FHL has a stronger influence on the second or third toe. Reference Ali, Griffin, Ellis and Meyr22
In the present study, tension of the FHL was transmitted to the first to third toes in all five limbs examined in three cadavers and weakly transmitted to the fourth toe in one cadaver. This result is somewhat similar to that reported previously, Reference Beger, Elvan, Keskinbora, Ün, Uzmansel and Kurtoğlu20 which concluded that the contribution of FHL was to the second toe alone in 25% of the examined samples, the second to third toes in 60%, and to the second to fourth toes in the remaining 15%. However, it should be noted that the results of transmission of tension at the time of dissection in the cadavers are not identical to the results of electrical stimulation in the living body.
Although Canada is a multiracial country, the differences between our results and those from Quebec, Canada, Reference LaRue and Anctil19 highlighted some racial differences in the proportion of each type of “Junctura Tendinum” between FHL and FDL. As shown in Figure 4, the proportion of Canadian subjects in the study of LaRue and Anctil Reference LaRue and Anctil19 with tendon branching limited to only one direction from FHL to FDL was relatively low compared to other racial groups, while the same rate was relatively high among the Japanese subjects in our studies. Further studies are needed to determine the true differences in the type of “Junctura Tendinum” between different races.
The present study has several limitations. First, the claw toe-related clinical symptoms and signs tended to improve in 68% of subjects. It should be noted, however, the study subjects included those who received BoNT injection into the flexor digitorum brevis muscle and did not include patients who received orthosis therapy after BoNT treatment for claw toe. Second, when examining the effects of treatment on the claw toe, we need to take into account that tenodesis from the ankle position also contributes to the claw toe. Many patients exhibited plantar flexion and inversion position, for whom further dosage of BoNT was injected simultaneously into the triceps surae and tibialis posterior. Third, the time of assessment of the effects of BoNT injection varied from 2 to 6 weeks, and thus some effects may have been missed in some patients. Fourth, in the analysis of transmission of mechanical tension (pulling the tendon) in the limbs of cadavers, we extrapolated the findings from the cadaver to the living and tried to match the classification of toe movements patterns to those during electrical stimulation. While such extrapolation might be approximate, it ignores the effects of the embalming process on the mechanical properties of the tissues.
In conclusion, the above data and our findings indicate coupling of the FHL and FDL tendons in most subjects to achieve plantar flexion of the second and third toes. We recommend injection of BoNT into both these muscles because in many cases injection into FDL alone does not produce satisfactory clinical results, especially when targeting the second or third toe, in patients with claw toe deformity associated with lower limb spasticity.
Funding
The study was conducted without financial support from any public, private, or non-for-profit organizations.
Conflicts of Interest
All authors declare no conflict of interest.
Statement of Authorship
Each author contributed to the study concept and design. Material was prepared by TT and KK. Data were collected by TT, ST, TK, TS, and KK. Data were analyzed by TT. MA supervised the study. The first draft of the manuscript was written by TT, and all authors commented on the initial versions of the manuscript. All authors read and approved the final manuscript.