Evidence from both functional and neuropathological studies suggests that the frontal lobes are affected in schizophrenia (Reference Goldman-Rakic and SelemonGoldman-Rakic & Selemon, 1997), raising the issue of the potential for differences in the volume of frontal lobe tissue between individuals with and without schizophrenia. There is some evidence in the existing literature for either a reduction in frontal lobe grey matter volume or a global cortical reduction that is more marked in the frontal lobes (Reference Breier, Buchanan and ElkashefBreier et al, 1992). There is additional evidence for lateralised alterations in the frontal lobe volume in schizophrenia (Reference Bilder, Houwei and BogertsBilder et al, 1994). Early studies defined the posterior limit of the frontal lobes with some arbitrary landmark (e.g. the genu of the corpus callosum), as opposed to the central sulcus, which is anatomically a more valid boundary. A previous study of the present brain collection (Reference Highley, Esiri and McDonaldHighley et al, 1998a ) suggests that taking proper account of the central sulcus may be important. In that study, the length from the frontal pole to the central sulcus was measured dorsally over the external surface of both frontal lobes on post-mortem brains and a striking gender × diagnosis interaction was found (see Discussion). The present study was performed to elucidate the nature of volumetric differences (if present) in the brain in schizophrenia, when the frontal lobes are defined in a non-arbitrary manner. The following structures were measured: the pre-central gyrus, the superior frontal gyrus, the middle frontal gyrus, a composite of the inferior frontal gyrus and orbito-frontal cortex, the total frontal lobe cortex and white matter. In addition, the anterior cingulate volume was assessed.
METHOD
Tissue
Brains were fixed by suspension from the basilar artery in 10% formalin, collected from three centres (Oxford, Belfast and Wickford (in Essex)) in the UK and then assigned a randomised code by a third party so that measurements could be made blind to gender, diagnosis and age. Brains were stored in formalin for an average of 3.25 years prior to use in the study. It has been observed that any volume alterations related to fixation stabilise after a maximum of 3 weeks (Reference Quester and SchröderQuester & Schröder, 1997), and therefore, as all brains were fixed in excess of this duration, it can be assumed that they had reached their stable state.
The brains were screened to ensure that they were free of neuropathological disease. Brains from patients who had undergone leucotomy were excluded. Patients' clinical notes were assessed by a psychiatrist (T.J.C. or Steven J. Cooper (see Acknowledgements)) to ensure that diagnoses conformed to DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia or schizo-affective disorder. The control subjects' clinical notes were screened to ensure that they were free of psychopathology.
The mean age, cerebrum weight and post-mortem delay, as well as hospital of origin, cause of death and number of cases in the different groups, are given in Table 1. As can be seen from Table 1, the females were significantly older than the males. For this reason, age was entered into all ANOVAs as a covariate.
Table 1 The mean (s.d.) age, cerebrum weight and post-mortem delay, as well as hospital of origin and cause of death of cases
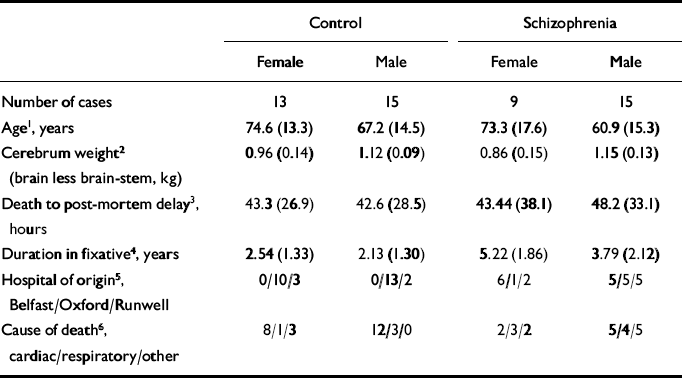
| Control | Schizophrenia | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Number of cases | 13 | 15 | 9 | 15 |
| Age1, years | 74.6 (13.3) | 67.2 (14.5) | 73.3 (17.6) | 60.9 (15.3) |
| Cerebrum weight2 (brain less brain-stem, kg) | 0.96 (0.14) | 1.12 (0.09) | 0.86 (0.15) | 1.15 (0.13) |
| Death to post-mortem delay3, hours | 43.3 (26.9) | 42.6 (28.5) | 43.44 (38.1) | 48.2 (33.1) |
| Duration in fixative4, years | 2.54 (1.33) | 2.13 (1.30) | 5.22 (1.86) | 3.79 (2.12) |
| Hospital of origin5, Belfast/Oxford/Runwell | 0/10/3 | 0/13/2 | 6/1/2 | 5/5/5 |
| Cause of death6, cardiac/respiratory/other | 8/1/3 | 12/3/0 | 2/3/2 | 5/4/5 |
Methods
The frontal lobes were defined as all cortex and white matter anterior to the central sulcus. The basal ganglia, insula, ventricles and internal capsule were not included.
All brains were stripped of the leptomeninges and disected in the median sagittal plane. The temporal and occipital lobes were dissected away by sectioning the white matter running between the temporal and frontal lobes and insula, and a cut through the parietal lobes.
Either side of the central sulcus, the pre- and post-central gyri were painted with permanent ink of different colours. The frontal lobes were then sectioned into serial, parallel coronal slices using 5-mm guides. After slicing, the slices were reasembled into a stack (excluding the first and last slices), the length of which was measured and divided by the number of slices, to generate the mean slice thickness. For all further measures, all slices (including first and last) were included.
The posterior aspect of each slice was patted dry of excess moisture and photographed using colour slide film, with a ruler in the field of view, ensuring that the planes of the camera lens and the brain slice were parallel. These photographs were then projected, in a random position, at an approximate magnification of × 1.7 onto a counting grid. The counting grid consisted of a series of crosses in a regular, uniform square array. The density of crosses was such that one cross represented an area of 2 cm2 on the counting grid. A subset of these crosses was circled such that one circled cross represented 4 cm2 on the counting grid. The total number of crosses (circled or otherwise) falling on each cortical structure of interest was counted for each slice and then summated. The number of circled crosses falling on white matter were counted and again summated. From these counts, the spacing of the grid, the magnification of the frontal lobe images and the mean slice thickness were calculated estimates of the volumes of the regions of interest, by application of the Cavalieri principle (Reference Gundersen, Bendtsen and KorboGundersen et al, 1988).
On slices on which the central sulcus was visible, a line from the central sulcus to the most supero-lateral point of the lateral ventricles defined the inferior boundary of the frontal lobe white matter. Between the most anterior extent of the central sulcus and the level at which the frontal and temporal lobes are no longer connected, the inferior boundary of the white matter was defined by a straight line from the superior insular circular sulcus to the most supero-lateral point of the lateral ventricles. Anterior to the join between frontal and temporal lobes, all white matter on the slice was counted, including white matter interposed between the basal ganglia and the orbito-frontal cortex. The internal capsule was not included.
The frontal lobe cortex was subdivided into the following regions: primary motor cortex/pre-central gyrus; infero-orbital cortex; middle frontal gyrus; superior frontal gyrus; and frontal pole. In addition, the cortex of the anterior cingulate gyrus was counted. In six cases the anterior cingulate gyrus had been damaged during bisection of the hemispheres, and in these cases the measure was excluded. The anterior cingulate was excluded from the total frontal cortical volume. The basal ganglia, thalamus and other subcortical grey matter structures were not counted. The subdivisions are depicted in Fig. 1.
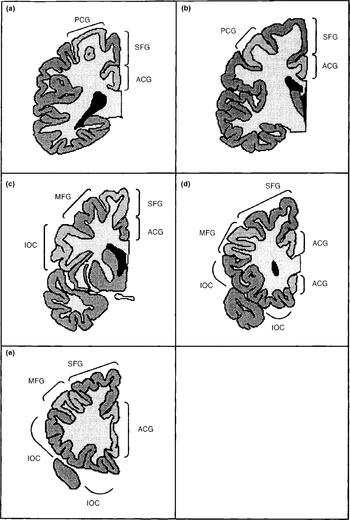
Fig. 1 The frontal lobe structures as defined in this study: PCG, pre-central gyrus; ACG, anterior cingulate gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; IOC, infero-orbital cortex (a most posterior to e most anterior).
The motor cortex was defined as the pre-central gyrus.
The superior frontal gyrus abuts the pre-central gyrus, separated from it by the pre-central sulcus. On the medial surface of the hemisphere, it lies superior to the cingulate. Anteriorly it wraps around the front of the cingulate to meet up with the orbito-frontal cortex. The superior frontal gyrus lies superior to the middle frontal gyrus, separated from it by the superior frontal sulcus.
The middle frontal gyrus lies between the superior and inferior frontal gyri and is bounded by the superior and inferior frontal sulci. Posteriorly it abuts the pre-central gyrus, and is separated from it by the pre-central sulcus.
The infero-orbital cortex is a composite of the inferior frontal gyrus and the orbito-frontal cortex. These two were compounded because it proved to be impossible to reliably separate one from the other. The inferior frontal gyrus is inferior to the middle frontal gyrus, separated from it by the inferior frontal sulcus. Medially, the orbito-frontal cortex is bounded by that portion of the anterior cingulate that lies underneath the rostrum of the corpus callosum and anterior to the genu. All grey matter on the inferior surface of the frontal lobes was included in this measure.
The frontal pole was defined as all the grey matter on the most anterior one or two slices in the situation where the different gyri were indistinguishable. The sole use of this measure was to be summated with the other grey matter measures to give the total frontal lobe cortical volume.
The anterior cingulate gyrus lies superior to the corpus callosum and curves anteriorly and inferiorly around the genu. The posterior boundary of the anterior cingulate was at the level one-fifth along the maximal anterior-posterior length of the corpus callosum. The cingulate was identified superior, anterior and inferior to the corpus callosum.
RESULTS
Analysis of measurement protocol
The observed coefficients of error (OCE) for estimates of volume were calculated as described by Gundersen & Jensen (Reference Gundersen and Jensen1987). The mean value of OCE was calculated for each measure for each group (female control, male control, female schizophrenia and male schizophrenia). The highest mean value of OCE for any grey matter component measure for any group was 0.087. For the total grey and white matter volumes, the maximum mean OCE values observed were 0.033 and 0.055, respectively. These values are all acceptable, being less than the target average value of 0.1 that was chosen when planning the investigation.
From the mean values of OCE, one can estimate the percentage of observed relative variance of each measure for each subject group accounted for by true inter-individual variance, as opposed to inaccuracy in the stereological volume estimate. For each comparison group, true inter-individual variation accounted for a minimum of 91.0% of the observed relative variance for the grey matter component measures. For the total grey matter and white matter volumes, true inter-individual variation accounted for a minimum of 97.6% of the observed relative variance for the different comparison groups.
For the cingulate gyrus, the largest mean OCE was 0.079. True inter-individual variation accounted for a minimum of 90.1% of the observed relative variance for any group.
Comparison with previous studies
The volume of total frontal lobes has been measured in a number of other studies, the most comparable being those of Pakkenberg (Reference Pakkenberg1993) and Pakkenberg & Gundersen (Reference Pakkenberg and Gundersen1997). In the former, the total volume (left+right) of cortex in the frontal lobe was measured in males both with and without schizophrenia. Although not explicitly stated, it appears that this measure included the cingulate. For the control males, the mean volume (211.6 cm3) was significantly larger (t=3.400, d.f.=29, P=0.002) than the equivalent volume in the present study (174.4 cm3). For the males with schizophrenia there was no significant difference (t=0.928, d.f.=18, P=0.366) between the measures of Pakkenberg (182.2 cm3) and the present study (195.8 cm3). Pakkenberg & Gundersen also measured the same volume in males unaffected by schizophrenia, and again recorded a significantly higher volume (213 cm3) than the present study (t=3.585, d.f.=75, P=0.0006). These workers also measured the volumes of females without schizophrenia, finding the mean volume to be 184 cm3, which did not differ significantly (t=1.195, d.f.=41, P=0.239) from the equivalent volume in the present study (169.4 cm3). The reason for the smaller values in the present study for control males is unclear, but there may possibly be slight differences in the working definition of the frontal lobes. However, given that two out of three of the groups for which a meaningful comparison can be made show no such difference, it can be concluded that the measures achieved in the present study reasonably estimate the volumes of frontal lobe structures.
Effects of diagnosis and gender
The mean values of each measure are shown in Table 2. For each frontal lobe grey matter component (pre-central gyrus, superior frontal gyrus, middle frontal gyrus, infero-orbital cortex), an ANCOVA was performed with diagnosis and gender as between-subject factors, side as a with-in-subject factor and age as a covariate. The main effects of gender and diagnosis were tested for, as were diagnosis × gender, diagnosis × side and diagnosis × gender × side interactions. The results of these analyses are given in Table 3. For analysis of the frontal cortical components, α was set to 0.0125 prior to the analyses because four such regions were studied. The frontal lobe total grey and white matter and cingulate cortex were analysed in a similar manner but with α set to 0.05.
Table 2 The mean (s.d.) volume measures (in cm3) for each group
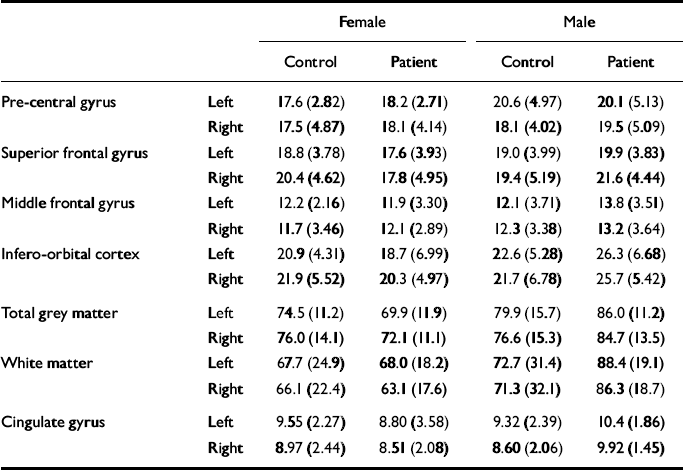
| Female | Male | ||||
|---|---|---|---|---|---|
| Control | Patient | Control | Patient | ||
| Pre-central gyrus | Left | 17.6(2.82) | 18.2(2.71) | 20.6(4.97) | 20.1(5.13) |
| Right | 17.5(4.87) | 18.1(4.14) | 18.1(4.02) | 19.5(5.09) | |
| Superior frontal gyrus | Left | 18.8(3.78) | 17.6(3.93) | 19.0(3.99) | 19.9(3.83) |
| Right | 20.4(4.62) | 17.8(4.95) | 19.4(5.19) | 21.6(4.44) | |
| Middle frontal gyrus | Left | 12.2(2.16) | 11.9(3.30) | 12.1(3.71) | 13.8(3.51) |
| Right | 11.7(3.46) | 12.1(2.89) | 12.3(3.38) | 13.2(3.64) | |
| Infero-orbital cortex | Left | 20.9(4.31) | 18.7(6.99) | 22.6(5.28) | 26.3(6.68) |
| Right | 21.9(5.52) | 20.3(4.97) | 21.7(6.78) | 25.7(5.42) | |
| Total grey matter | Left | 74.5(11.2) | 69.9(11.9) | 79.9(15.7) | 86.0(11.2) |
| Right | 76.0(14.1) | 72.1(11.1) | 76.6(15.3) | 84.7(13.5) | |
| White matter | Left | 67.7(24.9) | 68.0(18.2) | 72.7(31.4) | 88.4(19.1) |
| Right | 66.1(22.4) | 63.1(17.6) | 71.3(32.1) | 86.3(18.7) | |
| Cingulate gyrus | Left | 9.55(2.27) | 8.80(3.58) | 9.32(2.39) | 10.4(1.86) |
| Right | 8.97(2.44) | 8.51(2.08) | 8.60(2.06) | 9.92(1.45) | |
Table 3 Results of the ANCOVAs of frontal lobe volume data
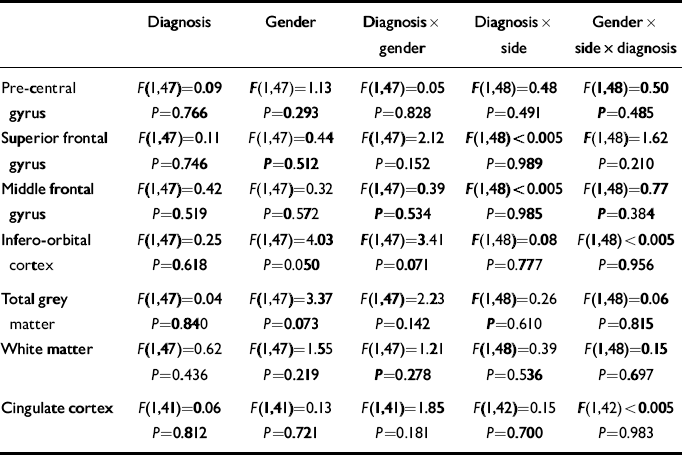
| Diagnosis | Gender | Diagnosis × gender | Diagnosis × side | Gender × side × diagnosis | |
|---|---|---|---|---|---|
| Pre-central | F(1,47)=0.09 | F(1,47)=1.13 | F(1,47)=0.05 | F(1,48)=0.48 | F(1,48)=0.50 |
| gyrus | P=0.766 | P=0.293 | P=0.828 | P=0.491 | P=0.485 |
| Superior frontal | F(1,47)=0.11 | F(1,47)=0.44 | F(1,47)=2.12 | F(1,48)<0.005 | F(1,48)=1.62 |
| gyrus | P=0.746 | P=0.512 | P=0.152 | P=0.989 | P=0.210 |
| Middle frontal | F(1,47)=0.42 | F(1,47)=0.32 | F(1,47)=0.39 | F(1,48)<0.005 | F(1,48)=0.77 |
| gyrus | P=0.519 | P=0.572 | P=0.534 | P=0.985 | P=0.384 |
| Infero-orbital | F(1,47)=0.25 | F(1,47)=4.03 | F(1,47)=3.41 | F(1,48)=0.08 | F(1,48)<0.005 |
| cortex | P=0.618 | P=0.050 | P=0.071 | P=0.777 | P=0.956 |
| Total grey | F(1,47)=0.04 | F(1,47)=3.37 | F(1,47)=2.23 | F(1,48)=0.26 | F(1,48)=0.06 |
| matter | P=0.840 | P=0.073 | P=0.142 | P=0.610 | P=0.815 |
| White matter | F(1,47)=0.62 | F(1,47)=1.55 | F(1,47)=1.21 | F(1,48)=0.39 | F(1,48)=0.15 |
| P=0.436 | P=0.219 | P=0.278 | P=0.536 | P=0.697 | |
| Cingulate cortex | F(1,41)=0.06 | F(1,41)=0.13 | F(1,41)=1.85 | F(1,42)=0.15 | F(1,42)<0.005 |
| P=0.812 | P=0.721 | P=0.181 | P=0.700 | P=0.983 |
These analyses yielded no significant effects. There were non-significant trends towards a gender effect and a diagnosis × gender interaction effect on the volume of the infero-orbital cortex. The gender effect reflected smaller volumes in the female brains compared with the male brains. The interaction corresponded to a smaller volume in female patients and an elevated volume in male patients compared with controls. However, given the large number of effects examined for, this is likely to be a chance occurrence.
Relationship between measures obtained from this and other studies
This study was designed in part to investigate whether a gender × diagnosis interaction in the asymmetry of the length of the frontal lobes over their superior surface as found in a prior report (Reference Highley, Esiri and McDonaldHighley et al, 1998a ) was due to alterations in the volume of the superior frontal gyrus. To investigate the relationship between the two measures, the correlations between the superior frontal length and the volume of the superior frontal gyrus as measures were assessed. As it was also feasible that the middle frontal gyrus may be related to this length measure, the correlation with this volume was also assessed. The results of these tests are given in Table 4.
Table 4 Correlation between the superior frontal length and the volume of the ipsilateral superior and middle frontal gyri

| Superior frontal gyrus volume | Middle frontal gyrus volume | |
|---|---|---|
| Left length | r=0.3297, d.f.=47, P=0.021 | r=0.4743, d.f.=47, P=0.001 |
| Right length | r=0.3130, d.f.=47, P=0.029 | r=0.3026, d.f.=47, P=0.035 |
All of these correlations were significant but modest: the greatest correlation coefficient is r=0.4743 (for the left middle frontal gyrus), suggesting that variations in the left middle frontal gyrus volume can account for only 22.5% of the variance of the length measure. All other correlation coefficients were smaller than this.
Potential artefacts
It may be argued that some aspects of brain extraction and fixation in different hospitals may have differentially affected the tissue studied in this paper. This was analysed for both grey and white matter using the data from the males with schizophrenia (five cases came from each of the three centres). Two repeated-measures ANOVAs were performed to investigate the effect of hospital of origin on the total grey and white matter volumes. There was no effect of origin on either the total grey volume (F(2,12)=0.88, P=0.440) or the white matter volume (F(2,12)=0.16, P=0.853).
Goldstein et al (Reference Goldstein, Goodman and Seidman1999) assessed the volumes of grey matter components while controlling for overall cerebrum volume. The inclusion of cerebrum weight as well as age as a covariate in the ANCOVA analysis of data in this study did not alter the outcome of the analyses.
The possible effect of death to fixation delay and duration in fixative was investigated by repeating all the ANCOVAs for cortical components, total grey and white matter and anterior cingulate, with fixation delay and duration as covariates. These analyses revealed no effect of either fixation delay (all—0.227<z<1.869, all P≥0.069) of duration in fixative (all—1.683<z<0.511, all P≥0.100). The outcomes of the analyses of the effects of diagnosis and gender were not altered.
DISCUSSION
This study finds no evidence for any alteration, lateralised or otherwise, in the volume of the cortex or white matter of the frontal lobe or of the anterior cingulate gyrus.
Previous studies of the frontal lobes
When this study was commenced, only one study assessing any aspect of frontal lobe size had used the anatomically correct central or pre-central sulcus as the boundary (Reference PakkenbergPakkenberg, 1993). With this exception, prior studies used arbitrary landmarks to select coronal slices to define the posterior boundary, a procedure that is unsatisfactory on two counts: first, if the position of the central sulcus (the true boundary of the frontal lobes) should alter in schizophrenia, such that more or less cortex lies within the frontal lobes, this will not be detected; and second, if the position of a landmark used to delineate the frontal lobe boundary is altered in schizophrenia, this may adversely affect the volumes estimates. Thus, for example, both Bilder et al (Reference Bilder, Houwei and Bogerts1994) and Breier et al (Reference Breier, Buchanan and Elkashef1992) used the genu of the corpus callosum as the boundary between the pre-frontal and pre-motor cortices. However, there is evidence for alterations in callosal anatomy in schizophrenia (Reference Woodruff, McManus and DavidWoodruff et al, 1995; Reference Highley, Esiri and McDonaldHighley et al, 1999a ).
Since the current study was commenced, four magnetic resonance imaging (MRI) studies have examined frontal lobe cortical volumes in schizophrenia using either the pre-central or central sulci as the boundary of frontal lobe components (Reference Buchanan, Vladar and BartaBuchanan et al, 1998; Reference Baaré, Hulshoff Pol and HijmanBaaré et al, 1999; Reference Goldstein, Goodman and SeidmanGoldstein et al, 1999; Reference Crespo-Facorro, Kim and AndreasenCrespo-Facorro et al, 2000). The findings of these studies, and the post- mortem study mentioned above (Reference PakkenbergPakkenberg, 1993), are summarised in Table 5. In addition, Szeszko et al (Reference Szeszko, Bilder and Lencz1999) have performed an MRI analysis of frontal lobe components with measures including white as well as grey matter.
Table 5 Summary of results of this and previous studies
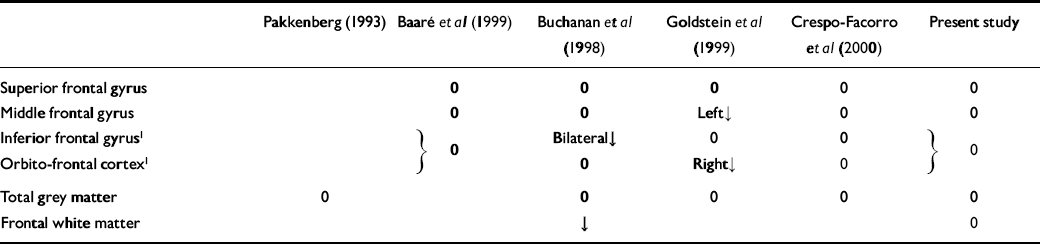
| Pakkenberg (Reference Pakkenberg1993) | Baaré et al (Reference Baaré, Hulshoff Pol and Hijman1999) | Buchanan et al (Reference Buchanan, Vladar and Barta1998) | Goldstein et al (Reference Goldstein, Goodman and Seidman1999) | Crespo-Facorro et al (Reference Crespo-Facorro, Kim and Andreasen2000) | Present study | |
|---|---|---|---|---|---|---|
| Superior frontal gyrus | 0 | 0 | 0 | 0 | 0 | |
| Middle frontal gyrus | 0 | 0 | Left↓ | 0 | 0 | |
| Inferior frontal gyrus1 | [UNK] 0 | Bilateral↓ | 0 | 0 | [UNK] 0 | |
| Orbito-frontal cortex1 | 0 | Right↓ | 0 | |||
| Total grey matter | 0 | 0 | 0 | 0 | 0 | |
| Frontal white matter | ↓ | 0 |
As can be seen from Table 5, the majority of measures made in these studies find no effect of schizophrenia. Those effects that are found are inconsistent between studies. For example, Szeszko et al's measure of orbito-frontal tissue (which included underlying white matter) found a right-sided increase in male but not female patients with schizophrenia. This was not replicated in any other study.
The reduction in prefrontal white matter volume (excluding white matter underlying the pre-central sulcus) reported by Buchanan et al (Reference Buchanan, Vladar and Barta1998) was not confirmed in the present study (which included all white matter). No other study has measured white matter using appropriate landmarks, leaving this disagreement unresolved.
Is the primary focus in the temporal lobes?
Taking these studies (Reference PakkenbergPakkenberg, 1993; Reference Buchanan, Vladar and BartaBuchanan et al, 1998; Reference Baaré, Hulshoff Pol and HijmanBaaré et al, 1999; Reference Goldstein, Goodman and SeidmanGoldstein et al, 1999; Reference Crespo-Facorro, Kim and AndreasenCrespo-Facorro et al, 2000) together with the present study, it would appear that the frontal lobe grey matter volumes are unaltered in schizophrenia. The view that the structural changes in schizophrenia are selective to the frontal lobes as suggested by Shelton et al (Reference Shelton, Karson and Doran1988) is unsupported. By contrast, as noted by Wible et al (Reference Wible, Shenton and Hokama1995), the literature contains many reports of reductions in the volume of temporal lobe structures that are either selective to or greater on the left (Reference Highley, McDonald and WalkerHighley et al, 1999b ; Reference McDonald, Highley and WalkerMcDonald et al, 2000). In addition, many studies observe a non-lateralised reduction in the volumes of temporal lobe structures (Reference Zipursky, Marsh and LimZipursky et al, 1994). Other temporal lobe pathology includes a relatively greater enlargement of the temporal horn than the rest of the lateral ventricle (Reference Crow, Ball and BloomCrow et al, 1989), and shortening of the temporal lobes (Reference Highley, Esiri and McDonaldHighley et al, 1998b ). Such findings raise the possibility of a qualitative difference between frontal and temporal lobes as the site of a pathophysiology of schizophrenia.
For the anterior cingulate gyrus, consistent with the current report, three MRI studies have found no alteration in the volume of this structure (Reference Noga, Aylward and BartaNoga et al, 1995; Reference Woodruff, Wright and ShuriquieWoodruff et al, 1997; Reference Szeszko, Bilder and LenczSzeszko et al, 1999). Three studies that report differences lack a consensus as to what the differences are. Albanese et al (Reference Albanese, Merlo and Mascitti1995) measured the weight and surface area of the anterior cingulate from dissected post-mortem brain. They found controls to show a right-greater-than-left asymmetry, whereas patients showed the opposite or absence of asymmetry. Examination of their data reveals this to be largely due to right-sided reductions in anterior cingulate volume and surface area. In contrast, Goldstein et al (Reference Goldstein, Goodman and Seidman1999) claim a reduction in total (presumably left+right) anterior cingulate volume. Finally, Hirayasu et al (Reference Hirayasu, Shenton and Salisbury1999) describe a non-significant left-sided volume reduction in that portion of the cingulate that lies inferior to the corpus callosum, a measure in which Goldstein et al (Reference Goldstein, Goodman and Seidman1999) found no change.
Surface asymmetries and sulco-gyral structure
A previous study on this set of brains (Reference Highley, Esiri and McDonaldHighley et al, 1998a ) reported a striking and unexpected gender × diagnosis interaction in the length from the frontal pole to the central sulcus when measured over the superior surface of the brain. Female controls had a left-greater-than-right asymmetry and male controls had a right-greater-than-left asymmetry; the pattern was reversed in schizophrenia. No significant effect was apparent when the tape measure was passed around the lateral aspect of the brain, suggesting a regional specificity. We predicted that this asymmetry would reflect a gender × diagnosis × side interaction in the volume of the superior temporal lobe. No such volume changes were observed.
The minimal relationship between the frontal lobe length measures of our prior study and the volume measures of the present study may explain the failure to demonstrate the hypothesised gender × diagnosis × side interaction in superior frontal gyrus volume: some aspect of frontal lobe anatomy other than volume must be responsible for the interaction effect on the length measure. An obvious alternative is the shape and sulco-gyral structure of the lobe. Gyral folding can be viewed as an index of cortico-cortical connectivity (Reference GriffinGriffin, 1994; Reference Van EssenVan Essen, 1997). There is evidence for alterations in gyral folding in both the frontal (Reference Vogeley, Schneider-Axman and TepestVogeley et al, 1998) and temporal (Reference Highley, Esiri and McDonaldHighley et al, 1998b ) lobes in schizophrenia.
In a recent MRI study (further details available from the author upon request) with volume assessments similar to those used in the present investigation, we find effects of diagnosis that interact with gender and hemisphere when age of onset is taken into account. No such effects are seen when age of onset is omitted from analysis. Age of onset may therefore be a variable that is critical to understanding the morphological changes in psychosis.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ The volumes of the frontal lobes are unchanged in schizophrenia, in contrast with decreased volumes and altered asymmetries in the temporal lobes.
-
▪ This combination of findings constrains theories of the origin of the structural brain changes in schizophrenia.
-
▪ Schizophrenia cannot be considered as a simple disorder of the frontal lobes.
LIMITATIONS
-
▪ Although large for a post-mortem study, the sample size does not exclude small differences in frontal lobe volume in schizophrenia.
-
▪ There may be alterations in other morphological aspects of the frontal lobes (e.g. sulco-gyral structure and cellular composition).
-
▪ The study was limited to frontal lobe structure.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Steven J. Cooper and Dr Brain M. Herron for their assistance with acquiring tissue and analysing clinical notes. This project was funded by an MRC project grant to M.M.E. and T.J.C. and a Wellcome Trust project grant to M.M.E. and P.J.H.









eLetters
No eLetters have been published for this article.