Ageing is not just a chronological process but also a life-long biological process referred to as senescence. Chronological age is measured in years while biological age is measured by assessing physical and mental function. Biological age can vary greatly between individuals with some 80 year olds having a similar physical and mental capacity as individuals in their 20s, whereas others may be frail and reliant on long-term care(1).
Older adults, defined as those aged 65 years and older, are the fastest growing population group, both globally(2), and in Ireland where there has been a 19 % increase in ‘over-65s’ between 2011 and 2016 according to the most recent Irish census(3).
The ageing process is associated with an increase in illnesses. While most older adults remain relatively healthy in old age, many are affected by chronic conditions such as CVD, type 2 diabetes mellitus (T2DM), cognitive disease, cancer and osteoporosis(Reference White, Holman and Boehm4–Reference Demontiero, Vidal and Duque8). A lifestyle incorporating healthy eating and physical activity can prevent or delay onset of these chronic conditions(1). The high prevalence of these chronic conditions of ageing leads to polypharmacy. Drug–nutrient interactions are critical to consider as nutritional status may be negatively affected by use of certain medications, particularly when polypharmacy is the case(Reference Maher, Hanlon and Hajjar9).
In addition, it is well-established that changes in body composition occur with increasing age, whereby muscle mass and lean tissue decline as fat mass increases(Reference van Asselt, de Groot, Raats, de Groot and van Asselt10,Reference St-Onge and Gallagher11) . There are also changes in body fat distribution with increasing age as the amount of intra-abdominal fat around the vital organs increases while the layer of subcutaneous fat declines, leading to abdominal obesity(Reference Hunter, Gower and Kane12,Reference Kuk, Saunders and Davidson13) . Such changes in muscle mass and body fat distribution add to the increased risks associated with ageing of developing conditions such as sarcopaenia, CVD and T2DM(Reference van Asselt, de Groot, Raats, de Groot and van Asselt10–Reference Kuk, Saunders and Davidson13). While sarcopaenia and frailty are conditions associated with ageing, neither of these conditions is inevitable and can be prevented, delayed or reversed with timely and appropriate interventions(Reference Reinders, Volkert and de Groot14–Reference Pennings, Koopman and Beelen18). Sarcopaenia, a progressive muscle disease characterised by a decline in muscle mass and strength, is common among older adults(Reference Cruz-Jentoft and Sayer19). Sarcopaenia is associated with an increased risk of negative health outcomes including frailty, falls, functional decline and mortality(Reference Cruz-Jentoft and Sayer19). Sarcopaenia accumulates over the lifetime. From the age of 40 years there is an 8 % decrease in muscle mass every decade which increases to 15 % per decade from the age of 70 years(Reference Flakoll, Sharp and Baier20). It is estimated that 10–40 % of community-dwelling older adults have sarcopaenia, depending on the definition used(Reference Mayhew, Amog and Phillips21). There is a high prevalence of obesity in older adults living in Ireland which can complicate the identification of sarcopaenia(Reference Leahy, Nolan and O'Connell22). Obesity can stimulate sarcopaenia by altering lipid metabolism, insulin resistance and inflammatory pathways as well as negatively impacting sarcopaenia by promoting deposition of fat into skeletal muscle(Reference Roh and Choi23). Therefore, individuals living with obesity and sarcopaenia, known as sarcopaenic obesity, will have poorer health status and functional capacity than that associated with either one of the conditions alone(Reference Baumgartner, Wayne and Waters24). Frailty, a distinct condition characterised by diminished strength and endurance and an increased vulnerability to stress caused by a decline in many physiological functions during ageing, is also common in this age group and is associated with poor health outcomes(Reference Volkert, Beck and Cederholm25). European data suggest that up to 76 % of this age group living in long-term care are frail(Reference O'Caoimh, Galluzzo and Rodríguez-Laso26). In Ireland, it is estimated that 15 % of community-dwelling older adults have frailty, with older age, female gender and lower socioeconomic status considered as predisposing factors(Reference O'Halloran, McGarrigle and Scarlett27).
Nutrition plays an important role in preventing or delaying the onset of both sarcopaenia and frailty, with nutritional interventions and physical activity considered to be the most effective interventions to delay or reverse these conditions(Reference Travers, Romero-Ortuno and Bailey15,Reference Pennings, Koopman and Beelen18,Reference Dent, Morley and Cruz-Jentoft28) . Nutritional factors associated with sarcopaenia include low protein and energy intakes, micronutrient deficiencies and malabsorption(Reference Cruz-Jentoft and Sayer19). Adequate energy intake and higher intakes of good quality protein (quality through amino acid profile and leucine content) are essential for maintaining muscle mass and preventing or delaying onset of these conditions(Reference Hector, Marcotte and Churchward-Venne16,Reference Murphy CH, Mitchell and Kolar17,Reference Isanejad, Sirola and Rikkonen29–Reference Cruz-Jentoft, Baeyens and Bauer32) . Preservation of lean body mass cannot be optimised through dietary protein intake alone, with research showing that a combination of high protein intake and exercise have a greater effect on lean body mass preservation(Reference Backx, Tieland and Borgonjen-van den Berg33–Reference Kim, Suzuki and Saito37). Specifically, resistance exercise is considered the most effective way of improving muscle mass in this age group(Reference Travers, Romero-Ortuno and Bailey15).
In Ireland, older adults are the population group most affected by overweight and obesity. While there are known benefits to losing weight, such as the potential prevention or delaying of T2DM(Reference Lean38), it is critically important that weight-loss diets induce gradual weight loss and include consumption of good quality protein combined with daily physical activity in order to prevent loss of muscle mass which can increase risk of sarcopaenia(Reference Volkert, Beck and Cederholm25).
Many other factors associated with ageing can impact nutritional requirements, such as poor food intake associated with loss of natural teeth, diminished sense of taste and dehydration(39).
For the last three decades in Ireland, healthy eating guidelines have been in place for the general population aged 5 years and older(Reference Flynn, O'Brien and Faulkner40,Reference Flynn, O'Brien and Ross41) . While these guidelines include older adults, they do not provide specific advice to cover nutritional issues associated with ageing. Therefore, the aims of the present paper were to (1) identify nutritional issues affecting older adults living independently in Ireland (i.e. not dependent on residential care) considering the evidence from dietary studies in Ireland and the scientific literature, (2) describe how national nutrient intake goals for older adults differ from the general population, requiring more specific guidelines on healthy eating and (3) summarise the key food-based dietary guidelines for independent living older adults in Ireland.
Methods
Two cohort studies and one population-based study have been completed or are currently on-going in Ireland, with a focus on older adults: the National Adult Nutrition Survey (NANS)(42), the Trinity-Ulster and Department of Agriculture (TUDA)(43) study and the Irish Longitudinal Study of Ageing (TILDA)(44). Dietary intakes and biomarker status of older adults in Ireland were explored using these three cohort studies to identify macro- and micronutrients of public health concern in this age group. This analysis identified protein, carbohydrate, fibre, fat, B vitamins (folate, vitamin B12, vitamin B6 and riboflavin), vitamin C, vitamin D, calcium, iron and zinc. These macro- and micronutrients were examined to explore where nutrient goals or food-based dietary advice differs for older adults compared with the general adult population.
Scientific literature on ageing and the nutrients of public health concern identified from the three cohort studies were reviewed. Key reports from international bodies, such as the European Food Safety Authority (EFSA)(45), the Institute of Medicine (IOM)(46,Reference Ross, Taylor, Yaktine and Del Valle47) and the Nordic Council of Ministers' Nordic Nutrition Recommendations(48), were examined to identify dietary intake reference values for these nutrients in older adults. Dietary intakes and biomarker status described in the three cohort studies of older adults in Ireland were examined in terms of these nutrient intake goals along with commonly-eaten food sources of key nutrients and patterns of consumption. Food sources of protein were examined in terms of protein quality using protein digestibility corrected amino acid score (PDCAAS), digestible indispensable amino acid score (DIAAS) and leucine content (an essential amino acid that appears to be of critical importance for the post-prandial stimulation of muscle protein syntheses)(Reference van Vliet, Burd and van Loon49). PDCAAS and DIAAS relate the essential amino acid content of a foodstuff to a reference amino acid profile, after applying a correction term for protein digestibility(50). Specific food-based dietary guideline recommendations for older adults were developed based on this examination.
Results and discussion
The main characteristics of the three studies of older adults in Ireland are outlined in Table 1 which describes the study design, population sample and dietary intake assessment methods. In addition, the weight status of the sample is described.
Table 1. Characteristics of the three national studies of older adults in Ireland

NANS, National Adult Nutrition Survey; TILDA, The Irish Longitudinal Study on Ageing; TUDA, Trinity-Ulster and Department of Agriculture study; TUDA 5+, resampling 5 years after initial investigation.
* NANS surveyed adults >18 and <90 years; 226 were aged >65 years.
† Blood samples only available for n 5356; total cohort n 8504.
Weight status
As shown in Table 1, up to 78 % of older adults in Ireland are living with overweight or obesity, with only approximately 2 % described as underweight(42,Reference Leahy, Donoghue and O'Connell51,Reference McCann, McNulty and Rigby52) . These surveys, however, do not include those living in residential care; therefore, the true prevalence of underweight among the total older adult population in Ireland may be underestimated.
There are known benefits to losing weight, such as the potential prevention or delaying of the onset of T2DM(Reference Lean38). However, as mentioned previously, for older adults living with overweight or obesity, rapid weight-loss diets are associated with loss of muscle mass which can lead to sarcopaenia(Reference Volkert, Beck and Cederholm25).
Prevention of further weight gain by combining a balanced, nutrient-rich diet with physical activity will help maintain lean muscle mass in older adults who are overweight(Reference Volkert, Beck and Cederholm25,39) and represents the best approach for overweight older adults unaffected by health conditions exacerbated by obesity. For those living with obesity and weight-related health problems where weight-loss interventions are required, weight loss should be slow, physical activity should be incorporated and rapid weight-loss diets avoided in order to preserve muscle mass(Reference Volkert, Beck and Cederholm25,39) .
Age-related nutritional issues
The nutritional issues affecting older adults in Ireland and the corresponding implications for diet-related advice are described next.
Drug–nutrient interactions
Many medications commonly used by older adults interact with nutrients, negatively impacting nutritional status. Specifically, opioid painkillers, calcium channel blockers, antidepressants and diuretics can interfere with the effects of dietary fibre, thus leading to constipation which can decrease quality of life(Reference Tvistholm, Munch and Danielsen53). The use of H2 receptor antagonists and proton pump inhibitors, which result in gastric acid suppression, have been associated with an increased risk of vitamin B12 deficiency(Reference Masclee, Sturkenboom and Kuipers54–Reference Lam, Schneider and Zhao56), while metformin (medication for the treatment of T2DM) has been linked to vitamin B12 and B6 deficiencies(Reference Aroda, Edelstein and Goldberg57).
In Ireland, studies from the TUDA cohort reported that metformin use was associated with a 45 % increased risk of vitamin B12 deficiency and a 48 % increased risk of vitamin B6 deficiency(Reference Porter, Ward and Hughes58), while those taking a proton pump inhibitor are more likely to have indicators of vitamin B12 deficiency(Reference Porter, Hoey and Hughes59).
The dietary recommendations in relation to drug–nutrient interactions are as follows:
(a) guidance to ensure adequate fibre intakes for the prevention of constipation and how this can vary among individuals due to drugs and/or immobility
(b) guidance on ensuring adequate intakes of iron, folate, vitamins B12 and B6 to prevent deficiencies due to medication use (see Table 2 for food sources of each nutrient).
Table 2. Nutrient goals from scientific literature and subsequent dietary recommendations for nutrients identified as of public health concern for this age group in Ireland(39)

bw, body weight; DFE, dietary folate equivalents; DIAAS, digestible indispensable amino acid scores; PDCAAS, protein digestibility-corrected amino acid scores; T2DM, type 2 diabetes mellitus.
* The nutrient goals outlined are for the general adult population, except for the additional protein goals (1–2 g/kg bw/d(Reference Dorrington, Fallaize and Hobbs73–76) and 1⋅1–1⋅3 g/kg bw/d(48)) which are specific to older adults.
Table 3. Commonly eaten protein-rich food sources (protein g/100 g), ranked according to protein and leucine content per g of typical food portion sizes with corresponding DIAAS and PDCAAS
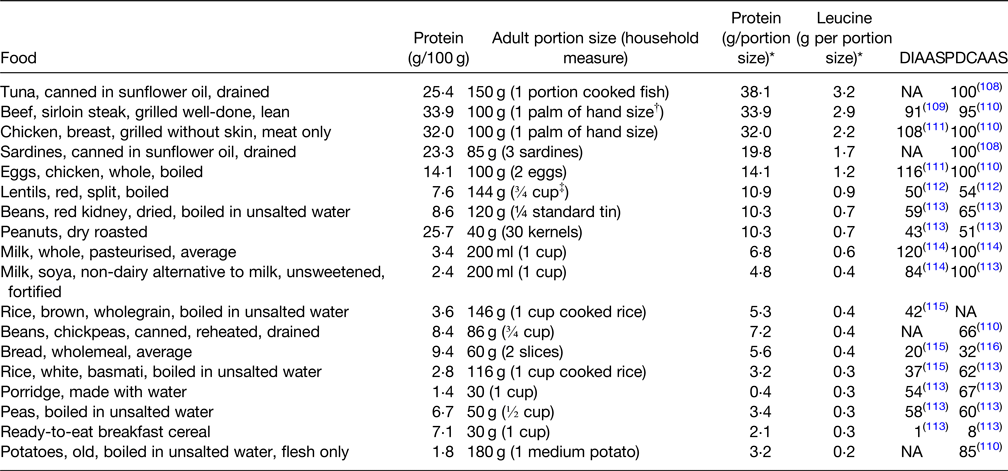
DIAAS, digestible indispensable amino acid score; NA, not available; PDCAAS, protein digestibility-corrected amino acid score.
A PDCAAS or DIAAS below 100 indicates that at least one amino acid is limiting in the food or diet, whereas a score of 100 indicates that there is no limiting amino acid in the food or diet.
* Derived from McCance and Widdowson's The Composition of Foods Integrated Dataset 2019(Reference McCance and Widdowson117).
† The width and depth of palm without fingers and thumb.
‡ 1 cup = 200 ml.
Dentition
Many older adults are edentate; thus, tend to choose softer and easier to chew foods, resulting in lower dietary intakes of nutrients such as n-3 fatty acids, non-starch polysaccharides, folate and vitamin C, compared with dentate older adults(Reference Watson, McGowan and McCrum60).
In Ireland, the NANS has reported that 87 % of older adults living independently either have all of their own teeth or are partially dentate with or without dentures(42). However, this age group is highly susceptible to chronic dental diseases caused mainly by reduced manual cleaning as well as high-dietary intakes of sugars and refined carbohydrates(Reference Hayes, Da Mata and Cole61).
Appropriate dietary advice, particularly in terms of free sugars and refined carbohydrate intakes, is important for this age group to prevent the onset of chronic dental diseases. This includes advice on dental hygiene, reduction in frequency of snacking and advice on reduction of cariogenic foods (sugars, refined carbohydrates and carbohydrate foods that are difficult to clear from mouth – sticky candies, crisps, biscuits, etc.). More specific dietary advice is required for edentate older adults to ensure adequate protein and nutrient intakes(62).
Sense of taste
Sense of taste diminishes with increasing age for a variety of reasons including physiological changes, disease and medication use(Reference Sergi, Bano and Pizzato63,Reference Imoscopi, Inelmen and Sergi64) . This can lead to increased use of salt at the table among older adults to increase food palatability(Reference Sergi, Bano and Pizzato63). Such high-dietary salt intake is associated with an increased risk of hypertension(65).
Currently in Ireland, >50 % of older adults have hypertension(Reference Murphy, Kearney and Shelley66). While the maximum salt limit for older adults is the same as that for the general population at 6 g salt/d(65), this cut off is of particular importance for the older adult population due to an increased risk of hypertension caused by excess salt intake, which is a major modifiable risk factor in the development of CVD(67).
Limiting consumption of salty foods (e.g. processed meats, anchovies and olives) and using alternatives to salt, such as herbs and spices, to flavour foods, can help to keep salt intakes below the 6 g/d limit. For those at risk of renal impairment (those with diabetes, heart failure or hypertension), minimal use of salt substitutes due to their high potassium and sodium content is important(65).
Hydration
The ageing process is associated with two changes in the physiological responses to inadequate fluid intake, which increase the risk of dehydration; the feeling of thirst is dampened, and primary urine concentration by the kidneys is impaired(Reference Volkert, Beck and Cederholm25). Older adults are also at an increased risk of dehydration for various reasons including use of medications resulting in fluid losses, memory problems, dysphagia and fear of incontinence(Reference Volkert, Beck and Cederholm25).
It is recommended that older women need at least 1⋅6 l of drinks daily, while older men need at least 2 l of drinks daily(Reference Volkert, Beck and Cederholm25). While water is mostly recommended, milk, tea, coffee and unsweetened fruit juice will all contribute to fluid intakes. Specific guidance is needed for those who are underweight or frail to have milky drinks which will also provide energy and protein as well as fluid. In addition, guidance to staff of the importance of offering drinks on a frequent basis to older adults in residential care to ensure adequate fluid intakes. Finally, consumption of strong tea at mealtimes is advised against to limit the effect of tannins interfering with iron absorption.
Nutrients of concern
The nutritional goals and recommendations developed for each nutrient of concern in older adults in Ireland are outlined in Table 2.
Protein
An adequate protein intake is one of the most important dietary factors for maintaining health during ageing due to its positive effects on body composition(Reference Volkert, Beck and Cederholm25). High-quality protein foods assessed using PDCAAS or DIAAS should be consumed in order to stimulate muscle protein synthesis(46). It is well established that dietary protein is essential for stimulating muscle protein synthesis(Reference van Vliet, Burd and van Loon49), and maintaining muscle mass (as mentioned previously) is critical for preventing or delaying the onset of sarcopaenia and frailty(Reference Cruz-Jentoft, Baeyens and Bauer32). Research has shown that spreading protein intake across different meals during the day and ensuring that each meal provides approximately 0⋅4 g protein/kg body weight (bw), will maximise muscle protein synthesis (Reference Loenneke, Loprinzi and Murphy68–Reference Symons, Sheffield-Moore and Wolfe71).
Both the EFSA population reference intake and the IOM recommended daily allowance for protein is the same for adults of all ages (0⋅8 g/kg bw/d)(45,72) . However, some international working groups have more recently recommended higher protein requirements for older adults, ranging from 1 g/kg bw/d up to 2 g/kg bw/d(Reference Dorrington, Fallaize and Hobbs73–76), with the Nordic countries subsequently increasing the protein requirements for older adults to 1⋅1–1⋅3 g/kg bw/d(48).
In Ireland, data from the NANS show that 33 % of older adults had protein intakes below the EFSA estimated average requirement of 0⋅66 g/kg bw/d(Reference Kehoe77), which is insufficient to maintain adequate muscle mass and function.
Older adults need a more protein-dense diet than the general adult population, with those at risk of frailty, sarcopaenia and undernutrition having even higher requirements of 1–1⋅2 g/kg bw/d(Reference Volkert, Beck and Cederholm25). Protein quality is important and is determined by the digestibility and quantity of essential amino acids necessary for growth, maintenance and repair, assessed using PDCAAS or DIAAS (Reference Symons, Sheffield-Moore and Wolfe71). As outlined in Table 3, the protein contents of commonly eaten foods in Ireland from animal sources, such as meat, poultry, fish and eggs, have higher PDCAAS and DIAAS quality scores and provide higher amounts of leucine. Consumption of high-quality protein foods (Table 3) providing approximately 0⋅4 g protein/kg bw in at least two meals daily will maximise muscle protein synthesis and thus reduce the likelihood or progression of sarcopaenia and frailty(Reference Bollwein, Diekmann and Kaiser30,Reference Paddon-Jones and Rasmussen31) . In addition, daily physical activity, resistance exercise, in particular, will greatly improve maintenance of muscle mass(Reference Travers, Romero-Ortuno and Bailey15,Reference Pennings, Koopman and Beelen18,Reference Dent, Morley and Cruz-Jentoft28) .
Carbohydrate
The EFSA reference intake range for carbohydrates is 45–60 % of total energy(45), while the IOM acceptable macronutrient distribution range is 45–65 % of total energy(72), with no recommendations specific for older adults. In terms of intake of free sugars, the WHO has established the most recent guidelines of <10 % total energy and <5 % if possible(78).
Average carbohydrate intakes among older adults in Ireland, while within the recommended range, are at the lower end and, according to NANS, almost one-third (31 %) exceed the 10 % limit for free sugars intake(42). Due to the high incidence of overweight and obesity and tendency for abdominal body fat distribution, older adults in Ireland are at an increased risk of T2DM.
Moderate intakes of fibre-rich carbohydrates and low free sugars consumption, eaten as mixed meals (with protein and minimal fat), will reduce the effect of carbohydrates on blood glucose levels(Reference Kim, Nam and Chung79,Reference Wee and Henry80) . This is due to the protein and fat promoting insulin secretion and delaying the absorption of carbohydrates by slowing the rate of gastric emptying, thus reducing the rate of glucose absorption(Reference Kim, Nam and Chung79,Reference Wee and Henry80) . Carbohydrate foods higher in fibre and lower in free sugars are also more slowly digested, absorbed and metabolised, resulting in a lower and slower rise in blood glucose; thus, representing an approach for protecting against the onset of T2DM, obesity and CVD(39,81,82) . Such foods include wholemeal breads, cereals, pasta and rice, as well as vegetables, salads and fruit.
Fibre
The current EFSA recommendation for fibre intake is 25 g/d(45). Fibre intakes are highly dependent on total energy (kcal) intake and it is well known that as age increases, energy requirements decrease due to changes in body composition along with a decrease in physical activity(Reference Roberts and Dallal83). The Nordic Nutrition Recommendations align fibre recommendations with energy intakes(48), allowing for the known variation in energy requirements.
Data from NANS indicate that up to 80 % of older adults do not meet the EFSA recommendation(42). Consideration of the variation in energy requirements due to difference in body size and activity levels among Irish adults(42) demonstrates the need for dietary fibre recommendations to be related to energy requirements; ≥3 g/MJ/d, as established by the Nordic Nutrition Recommendations(48).
Guidance to choose high-fibre versions of all carbohydrate foods eaten, such as wholegrain breads, pastas and cereals, fruit, salad and vegetables, will do much to ensure older adults achieve adequate fibre intakes.
Fat
Older adults are at greater risk of CVD than younger adults, with high saturated fat intakes representing one of the main risk factors for CVD. Strong evidence also exists supporting a beneficial role for the n-3 PUFAs, EPA and DHA, on cardiac health in older adults(45).
According to NANS(42), total dietary fat intakes among older adults, at 35 % energy, are at the top end of the EFSA reference intake range(45), with saturated fat contributing to 14 % of energy, exceeding the upper recommendation of 10 % energy(45).
While the dietary fat recommendations for older adults are the same as those for the general population, additional considerations for maintaining fat intakes within the recommended range in older adults need to be considered. In order to facilitate healthy ageing and help reduce disease risk in this age group, saturated fat intakes should be reduced by substituting with MUFAs and PUFAs, and increasing intakes of EPA and DHA(45,84) . Guidance to use minimal amounts of oils and reduced-fat margarines rich in MUFA and PUFA and to include foods, such as reduced-fat oily fish, nuts and seeds will contribute to healthier intake of fats among older adults in Ireland. In addition, total fat intakes should be maintained at current intake levels in order to avoid the glycaemic effects of high-carbohydrate low-fat diets, with PUFAs also being linked to beneficial effects on glycaemic control(84).
B vitamins
Deficient folate and related B vitamin intakes and status can be common in this age group(Reference Hopkins, Gibney and Nugent85–Reference Laird, O'Halloran and Carey88) and are associated with higher risk of diseases of ageing, including CVD, cognitive dysfunction and osteoporosis. Of note, lower biomarker status of folate, vitamin B6 and riboflavin are associated with an increased risk of depression in this age group, while deficient vitamin B6 status is associated with increased anxiety(Reference Moore, Hughes and Hoey86). The most common causes of folate and riboflavin deficiencies are low-dietary intakes, while deficient vitamin B12 status is mainly caused by food-bound malabsorption as a result of atrophic gastritis (affecting up to 20 % of older adults) and the widespread use of proton pump inhibitor drugs, and low vitamin B6 status is attributed to increased requirements in ageing(Reference Porter, Hoey and Hughes87).
In Ireland, data from NANS reported that 13 % of women have inadequate folate intakes(42), biomarker data from TUDA reported 2 % of participants having folate deficiency(Reference Moore, Hughes and Hoey86), while data from TILDA reported 15 % of participants had low or deficient folate status(Reference Laird, O'Halloran and Carey88). Studies from both TUDA(Reference Porter, Hoey and Hughes87) and TILDA(Reference Laird, O'Halloran and Carey88) reported that 12 % of participants had vitamin B12 deficiency. There was also a 12 % deficiency rate in vitamin B6 status reported from TUDA(Reference Hopkins, Gibney and Nugent85). Regarding riboflavin, NANS reported >50 % of older adults had suboptimal riboflavin status(42), while a similar level of 49 % was reported by TUDA(Reference Moore, Hughes and Hoey86).
Improving B vitamin status through improved diet requires separate consideration of each B vitamin, as the food sources differ for each. Natural food sources that should be included in the diet of this age group include lean meat (vitamins B12 and B6), reduced-fat (to minimise saturated fat intake) milk and dairy foods (riboflavin and vitamin B6) and green leafy vegetables, legumes and liver (folate). Fortified breakfast cereals are also key contributors to intakes of each of the B vitamins(Reference Hopkins, Gibney and Nugent85,Reference Moore, Hughes and Hoey86,Reference Kehoe, Walton and Hopkins89,Reference Hoey, McNulty and Askin90) , providing a practical and highly effective means of improving B vitamin status in this age group. In addition, consideration needs to be given to increasing the levels of fortification with vitamin B12 in order to optimise status of this nutrient.
Vitamin C
Vitamin C plays an important role in immune function(Reference Carr and Maggini91). Older adults are particularly vulnerable to infections due to their reduced immune function. Thus, low vitamin C status in this group represents a potentially correctable contributing factor to morbidity and mortality(Reference Carr and Maggini91). Older adults, particularly those from lower socioeconomic status groups and those dependent on long-term residential care, are at risk of low vitamin C status. This is caused mainly by low intakes of fresh fruit and vegetables, resulting in lower body stores, along with increased needs caused by smoking, infections and diseases, such as type 2 diabetes(Reference Carr and Maggini91).
In Ireland, data from NANS reported that 17 % of male older adults had inadequate intakes, while 1 % of older adults had intakes less than the UK lower reference nutrient intake of 10 mg/d(42,92) .
Including a small glass of unsweetened orange juice as one of the five daily servings of fruit and vegetables recommended for this age group will help in achieving adequate vitamin C intakes. A vitamin C supplement, as advised by a general practitioner, may be needed in some cases where diet is poor(39).
Vitamin D
The EFSA, IOM, Nordic Nutrition Recommendations and the Scientific Advisory Committee on Nutrition have all set dietary vitamin D requirements for older adults based on specified health outcomes and the associated serum 25-hydroxyvitamin D concentration, with requirements ranging from 10 to 20 μg/d(45,Reference Ross, Taylor, Yaktine and Del Valle47,48,93) . Adequate vitamin D intake is essential for bone health(45,Reference Ross, Taylor, Yaktine and Del Valle47,48,93) with low vitamin D status also strongly associated with frailty in this age group(Reference McKenna, Freaney and Meade94,Reference O'Halloran, Laird and Feeney95) .
In Ireland, vitamin D deficiency is common among older adults, particularly in those in long-term residential care(Reference Griffin, Wall and Blake96), and is more pronounced in winter months(Reference Laird, O'Halloran and Carey97–Reference McCarroll, Beirne and Casey99).
Vitamin D occurs naturally in few foods, such as oily fish and eggs. However, these foods do not provide adequate vitamin D for this age group. While vitamin D fortified foods (mostly breakfast cereals and milks) can significantly increase vitamin D intakes and improve status, due to the voluntary nature of food fortification practices in Ireland, these foods alone are insufficient for achieving adequate intakes. Thus, vitamin D supplementation is essential for this age group and it is recommended that all older adults take a daily 15 μg vitamin D supplement all year round(100,101) .
Calcium
An adequate intake of calcium is needed for optimal bone health in older adults. While the IOM recommends a higher calcium intake than that of younger adults(Reference Ross, Taylor, Yaktine and Del Valle47), EFSA does not recommend higher calcium intake for older adults because their modelling analysis excluded an effect of age or sex on calcium intake requirement(45).
Although calcium intakes among older adults in Ireland rank among the highest when comparing adults worldwide(Reference Balk, Adam and Langberg102), very few older adults consume the recommended three portions of dairy foods daily. TUDA reported that 96 % of older adults do not consume three portions of dairy foods daily(Reference Laird, Casey and Ward103), while TILDA reported 70 %(Reference O'Connor, Leahy and McGarrigle104). Additionally, NANS reported an average intake of 1⋅98 dairy portions daily(42).
Guidance to include four portions of calcium-rich dairy food sources (e.g. milk, yogurt and cheese) in the diet every day will not only help older adults achieve the calcium goal but will also contribute to their need for higher protein intake. While calcium is obtained from plant-based foods such as cereals, pulses, nuts, seeds and dark-green leaves, these sources are generally much less bioavailable. A daily calcium supplement (500 mg) may be needed for older adults who consume less than one portion of calcium-rich dairy food sources daily(39).
Iron
Prevalence of iron deficiency increases with age, particularly among older adults who are dependent on long-term residential care(Reference Bach, Schruckmayer and Sam105). Iron deficiency in this age group can result in increased ill health and mortality, and is caused mainly by occult blood loss, poor diet, renal insufficiency and malabsorption of iron in the gut(Reference Bach, Schruckmayer and Sam105). Iron status can be readily assessed by measuring serum iron, iron-binding capacity and ferritin.
In Ireland, most older adults meet the EFSA average requirement for iron (6 mg/d)(45) with NANS reporting average iron intakes of 15⋅8 mg/d from all sources and 10⋅8 mg/d from food alone(42).
The inclusion of iron-containing foods, such as meat, poultry, fish, eggs and beans, in the diet of this age group along with vitamin C-containing foods (potatoes, vegetables, salads and fruit) will enhance iron absorption, particularly from plant-based foods. Iron status should be regularly monitored in this age group in order to identify those with poor iron status and, thus, avoid the development of adverse health effects(39).
Zinc
Zinc is required for many diverse functions in the body including biochemical and immunological function(45). Zinc deficiency is common in older adults, particularly in those dependent on residential care(Reference Barnett, Dao and Hamer106). Factors such as low socioeconomic status, poor diet, inadequate chewing of food and impaired absorption in the gut all contribute to lower zinc intakes.
In Ireland, according to NANS average zinc intake in older adults is 10⋅4 mg/d from all sources and 8⋅7 mg/d from food alone. Thus, intakes are within the EFSA average requirement ranges (6⋅2–10⋅2 mg/d for women; 7⋅5–12⋅7 mg/d for men)(45) but below the EFSA population reference intake of 12⋅7 mg/d for women and 16⋅3 mg/d for men(42,45) .
High-protein foods, including ‘dark meats’, such as tuna, read meat and dark poultry meat (i.e. leg meat), cheese, eggs and nuts, also represent best sources of zinc and their consumption should be encouraged in this age group. Some older adults may also require zinc supplementation (15 mg/d) if high-protein foods are not regularly consumed(39).
Key conclusions and recommendations
This review identified many nutritional issues associated with ageing, such as increased vulnerability to chronic diseases (e.g. CVD, T2DM), polypharmacy, changes in body composition and loss of muscle mass. Many of these issues can be addressed through dietary interventions. The macro- and micronutrients of public health concern identified in this age group were protein, carbohydrate, fibre, fat, B vitamins (folate, vitamin B12, vitamin B6 and riboflavin), vitamin C, vitamin D, calcium, iron and zinc. While nutrient intake goals for the majority of these nutrients were the same as those for the general adult population, some important differences requiring specific dietary guidance were evident, e.g. protein quality and quantity and adequacy of B vitamin intake.
In conclusion, specific food-based dietary guidance is needed for this age group in order to address such issues. The key recommendations for this age group are outlined in Box 1.
Box 1 Older adults should be advised to:
Consume a more protein-dense diet than the general population to preserve muscle mass, and thus prevent, or delay, the onset of sarcopaenia and frailty. High-quality protein foods should be consumed in adequate quantities (0.4 g/kg bw) at two or more meals every day.
Avoid rapid weight-loss diets to safeguard muscle mass and prevent onset of frailty:
Limit weight reduction to older adults with health conditions that warrant weight loss and advise on how to ensure that this has minimal impact on lean body mass.
Ensure appropriate supervision of weight reduction with the objective of gradual weight loss accompanied by increased physical activity to whatever capacity possible.
Take physical activity daily, and resistance exercise in particular, to whatever capacity is possible to help ensure the maintenance of muscle mass and blood glucose control.
Consume high-fibre, low-free sugar carbohydrate foods as mixed meals (with protein and fat) to reduce the effect of carbohydrates on blood glucose levels.
Consume healthier fats that are protective against CVD, by using minimal amounts of oils and reduced-fat margarines rich in MUFA and PUFA; include foods, such as oily fish, nuts and seeds.
Consume fortified foods (e.g. high-fibre breakfast cereals, low fat milk) which will help to achieve many of the nutrient goals, especially as regards optimising B vitamin intakes and status.
Take a daily vitamin D supplement of 15 μg, all year round.
Consume adequate amounts of fluids; women need at least 1.6 l and men need at least 2 l of drinks per day. This can be provided from a number of sources – water, milk, tea, coffee and unsweetened fruit juice will all contribute to fluid intakes. Tea is best consumed between, rather than with, meals in order to avoid interference with iron absorption.
bw, body weight.
This work has formed the scientific basis to underpin the development of healthy eating guidelines for older adults living in Ireland. Next steps are to raise awareness among this population and their carers and provide accessible food-based advice that aligns with cultural habits.
Financial Support
This review paper received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
The authors had sole responsibility for all aspects of preparation of this paper.






