One-carbon metabolism is a complex metabolic network. Dietary factors involved in this pathway include folate, methionine, vitamin B6 and vitamin B12. Folate is thought to play an important role in cancer prevention(Reference Ulrich1). As a carbon donor in one-carbon metabolism, folate is essential for normal DNA synthesis and repair. Adequate folate status is also important for the production of S-adenosylmethionine for DNA methylation. Moreover, methionine is the precursor for the endogenous production of S-adenosylmethionine. Additionally, one-carbon metabolism may involve as many as twenty-five enzymes, some of which require the presence of vitamin B6 and vitamin B12 as co-enzymes(Reference Ziegler and Lim2). For example, vitamin B12 acts as a cofactor for the enzyme methionine synthase, and vitamin B6 serves as a cofactor for folate-dependent enzymes in the one-carbon metabolism pathway.
Folate digestion and absorption are affected by many factors such as diseases or medications. Alcohol is a known folate antagonist. It can impair folate absorption and metabolism(Reference Halsted, Villanueva and Devlin3), and plausibly increase an individual's requirement for folate intake. Some studies, but not all, have found that alcohol consumption modifies the effect of folate intake on breast cancer risk(Reference Larsson, Giovannucci and Wolk4).
Several epidemiological studies have examined the association of dietary folate with breast cancer, but the results are inconsistent. While some studies have reported a negative association between dietary folate intake and breast cancer risk(Reference Adzersen, Jess and Freivogel5–Reference Freudenheim, Marshall and Vena13), other studies have not observed a significant relationship(Reference Baglietto, English and Gertig14–Reference Stevens, McCullough and Sun24). In addition to folate, a few studies have investigated the relationships between other one-carbon metabolism-related nutrients (such as vitamin B6, vitamin B12 and methionine) and breast cancer risk(Reference Lajous, Lazcano-Ponce and Hernandez-Avila8, Reference Levi, Pasche and Lucchini9, Reference Shrubsole, Jin and Dai11, Reference Feigelson, Jonas and Robertson16, Reference Maruti, Ulrich and White23, Reference Kabat, Miller and Jain25–Reference Lin, Lee and Cook31). But the results remain inconclusive. Therefore, further investigation of these issues is warranted.
Oestrogen receptor (ER) and progesterone receptor (PR) are the most widely studied markers in breast tissue. Recent studies have raised the hypothesis that the association of dietary factors with breast cancer may differ by ER or PR status(Reference Colditz, Rosner and Chen32–Reference Zhang, Hankinson and Hunter34). Folate may influence the methylation of ER or PR genes and thereby affecting the silencing of these genes(Reference Ericson, Borgquist and Ivarsson35, Reference Zhu, Davidson and Hunter36). The associations between folate intake and breast cancer risk may therefore differ according to ER or PR status of tumours. So far, only a few studies have examined folate intake in relation to breast cancer risk according to ER and/or PR status(Reference Larsson, Bergkvist and Wolk22, Reference Maruti, Ulrich and White23, Reference Cho, Holmes and Hankinson28, Reference Zhang, Hankinson and Hunter34, Reference Sellers, Vierkant and Cerhan37, Reference Ma, Iwasaki and Kobayashi38), and the results are inconsistent.
Moreover, the majority of these studies have been performed in Western populations. Unlike their Western counterparts, most Chinese women consume natural (unfortified and unprocessed) foods, and they seldom take vitamin supplements or drink regularly. Thus, Chinese dietary habits may allow a better assessment of nutrient intake and minimise potential misclassifications in epidemiological studies. The present study aimed to evaluate the association between dietary folate and other one-carbon metabolism-related nutrient intake and the risk of breast cancer characterised by ER and PR status among Chinese women in Guangdong Province.
Materials and methods
Study subjects
A hospital-based case–control study was conducted in Guangdong Province, China, from June 2007 to August 2008. The selection of cases and controls has been described in detail elsewhere(Reference Zhang, Ho and Chen39, Reference Zhang, Ho and Chen40). In brief, potential case subjects were recruited from patients admitted to the surgical units of two affiliated hospitals of Sun Yat-sen University, Guangzhou, China. Inclusion criteria were female subjects aged 25–70 years and natives of Guangdong Province or having lived in Guangdong for at least 5 years, with incident, primary, histologically confirmed breast cancer diagnosed no more than 3 months before the interview. Women were excluded if they could not understand or speak Mandarin/Cantonese or with a prior history of breast cancer or other cancers. In total, 438 (96 %) cases out of 455 eligible cases were successfully interviewed.
As well as the 438 eligible patients, 438 control subjects with no history of cancer and admitted to the same hospitals during the same time period as the case subjects were interviewed. They were frequency-matched by age (5-year interval) and residence (rural/urban) to the case patients. These patients presented with a wide spectrum of non-neoplastic conditions including eye disorders (glaucoma, uveitis, keratitis, pterygium, dacryocystitis and optic neuritis), ear, nose and throat diseases (sudden deafness, acute bacterial/viral otitis media, sinusitis, deviation of nasal septum, tonsillitis), trifacial neuralgia, varicose veins, traumatic skeletal disorders, osteoarthritis, degenerate joint disease, orthopaedics and acute appendicitis. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethical Committee of the Chinese University of Hong Kong. Written informed consent was obtained from all participants.
Data collection
Data on sociodemographic characteristics, anthropometrics, menstrual and reproductive history, family history of breast cancer, physical activity, smoking habits, alcohol use and prior disease history were collected from each subject by in-person interviews using a structured questionnaire. Regular drinking was defined as alcohol drinking at least once per week over the past year. Relevant medical information, medical diagnosis, histological findings and ER or PR status were abstracted from the hospital medical records. Information on the ER and PR status of the tumour was available for 399 (91·1 %) cases.
Dietary assessment
Dietary information was obtained from an eighty-one-item interviewer-administered FFQ covering the habitual diet of participants during the previous year. Food photographs were used to help participants quantify the portions consumed. Daily dietary nutrient intakes were estimated using the China Food Composition Table(Reference Yang, He and Pan41, Reference Yang, Wang and Pan42). Information on frequency of intake and portion size was used to calculate the amount of each food item consumed on average (g/d). Total dietary intakes of folate, vitamin B6, vitamin B12 and methionine were calculated by summing the product of the frequency of consumption, usual portion consumed and micronutrient content of each food item. Information on cooking method was not collected in the present study, as stir-frying and boiling are the two most common methods of cooking green leafy vegetables and animal foods in China.
The validity and reproducibility of the FFQ has been described in detail elsewhere(Reference Zhang and Ho43). A total of sixty-one female subjects completed 3 d dietary records at intervals of 2 months during a 12-month period and two FFQ administered 1 year apart. The correlation coefficients comparing the second FFQ and 18 d dietary records were 0·35 for folate, 0·26 for vitamin B6, 0·50 for vitamin B12 and 0·36 for methionine. The correlation coefficients between the two FFQ were 0·60 for folate, 0·57 for vitamin B6, 0·60 for vitamin B12 and 0·49 for methionine. These results showed that the reproducibility and validity of our FFQ seem to be comparable with the values reported by others(Reference Shu, Yang and Jin44, Reference Willett, Sampson and Stampfer45).
Statistical analysis
All statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Dietary folate, vitamin B6, vitamin B12 and methionine intakes were adjusted for total energy intake using the residual method(Reference Willett, Howe and Kushi46) and then categorised into quartiles based on the distribution among the control subjects. Unconditional logistic regression models were used to estimate the OR and 95 % CI of each quartile, using the lowest quartile group as the reference after adjusting for the various potential confounding factors. Risk factors identified by comparison of baseline characteristics between cases and controls as independently associated with breast cancer risk were adjusted in the multivariate models. These included age at menarche (continuous), live births and age at first live birth ( ≤ 19, 20–24, 25–29, ≥ 30 years and nulliparous), months of breast-feeding (nulliparous and never breast-feeding, 1–3, 4–11, 12–23 and ≥ 24 months), BMI (continuous), family history of breast cancer in a first-degree relative (yes/no), history of benign breast disease (yes/no), passive smoking from a husband (yes/no) and physical activity (categorical, never, occasional and ≥ 1 time per week). Tests for trend were performed by entering the categorical variables as continuous variables in the models. Analyses stratified by ER/PR status and menopausal status were conducted to evaluate whether these factors modified the associations of dietary folate intake with breast cancer risk. The potential interactions (each quartile of folate × menopausal status) between folate intake and breast cancer risk by menopausal status were also examined. Our sample of 230 cases and 218 controls in two quartiles (Q1 and Q4) gave us 99 % power to detect the OR of 0·32 for the association between folate intake and breast cancer risk at P < 0·05 (two-tailed). We had greater than 98 % power to detect OR of 0·31 and 0·29 for the association between folate intake and ER+ and PR+ breast cancer. However, the power was lower than 65 % among ER − and PR − tumours.
Results
Table 1 presents the sociodemographic and established breast cancer risk factors of the study populations. Compared with controls, cases had an earlier age at menarche, older age at first live birth, fewer months of breast-feeding and higher BMI. Cases were more likely to have a history of breast cancer in a first-degree relative, history of benign breast disease and history of passive smoking from a husband, and were less likely to be physically active than controls. All of the aforementioned variables were considered to be potential confounding factors and controlled for in subsequent analyses. No significant differences were found between the case and control subjects in sociodemographic factors, including educational level, occupational status, marital status and household income, or in reproductive factors, including nulliparity, number of live births, age at menopause and use of an oral contraceptive.
Table 1 Comparison of breast cancer cases and controls on sociodemographic and selected characteristics among Chinese women
(Mean values, standard deviations, number of patients and percentages)

* Among parous women.
† Among menopausal women.
The median intake of folate was 228·3 μg/d, vitamin B6 was 0·86 mg/d, vitamin B12 was 1·54 μg/d and methionine was 1·14 μg/d in the control group (Table 2). Compared with controls, the consumption of dietary folate and vitamin B6 was significantly lower in the case subjects. No significant differences between cases and controls were observed for vitamin B12 and methionine intake.
Table 2 Comparison of dietary folate, vitamin B6, vitamin B12 and methionine intake between breast cancer cases and controls
(Mean values, standard deviations, 25th (P25), 50th (P50) and 75th (P75) percentiles)

* Wilcoxon's rank-sum test comparing the median consumption levels between cases and controls.
The associations between the intake of dietary folate, vitamin B6, vitamin B12 and methionine and the risk of breast cancer are shown in Table 3. After adjustment for the various potential confounders, a significant inverse association was observed between the intake of dietary folate and vitamin B6 and the risk of breast cancer. The OR for the highest quartile of intake compared with the lowest were 0·32 (95 % CI 0·21, 0·49; P trend < 0·001) for folate intake and 0·46 (95 % CI 0·30, 0·69; P trend < 0·001) for vitamin B6 intake. Associations for vitamin B12 and methionine were not statistically significant.
Table 3 Folate, vitamin B6, vitamin B12 and methionine intake and breast cancer risk among Chinese women
(Odds ratios and 95 % confidence intervals)
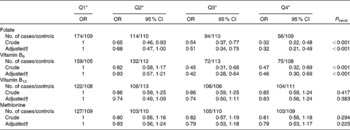
Q, quartile.
* Quartile cut-off points were based on residual energy-adjusted intake among the control subjects.
† OR were adjusted for age at menarche, live births and age at first live birth, months of breast-feeding, BMI, history of benign breast disease, mother/sister/daughter with breast cancer, physical activity, passive smoking and total energy intake.
The associations between folate intake and breast cancer risk according to sources of folate are shown in Table 4. There was a consistent inverse association between folate intake from different food sources (e.g. vegetables, fruits, soya, grains and animal foods) and breast cancer risk.
Table 4 Folate intake from different food sources and breast cancer risk among Chinese women
(Odds ratios and 95 % confidence intervals)
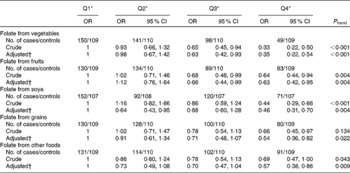
Q, quartile.
* Quartile cut-off points were based on residual energy-adjusted intake among the control subjects.
† OR were adjusted for age at menarche, live births and age at first live birth, months of breast-feeding, BMI, history of benign breast disease, mother/sister/daughter with breast cancer, physical activity, passive smoking and total energy intake.
Among the case subjects with information on hormone receptor status, 292 (73·2 %) and 348 (87·2 %) were for ER+ and PR+, respectively; 275 (68·9 %) were ER+/PR+; seventeen (4·3 %) were ER+/PR − ; seventy-three (18·3 %) were ER − /PR+; and thirty-four (8·5 %) were ER − /PR − . Table 5 shows the impact of dietary folate consumption on the risk of breast cancer characterised by ER and PR status. The inverse association between folate intake and breast cancer risk was observed in all subtypes of ER and/or PR status, although the association was statistically non-significant among women with PR − , ER+PR − and ER − PR − breast cancer tumours due to the relatively small numbers.
Table 5 Dietary folate intake and breast cancer risk stratified by oestrogen receptor (ER)/progesterone receptor (PR) status
(Odds ratios and 95 % confidence intervals)
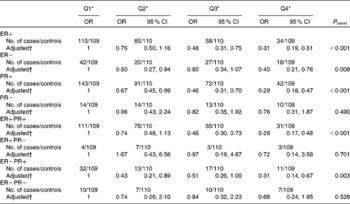
Q, quartile.
* Quartile cut-off points were based on residual energy-adjusted intake among the control subjects.
† OR were adjusted for age at menarche, live birth and age at first live birth, months of breast-feeding, BMI, history of benign breast disease, mother/sister/daughter with breast cancer, physical activity, passive smoking and total energy intake.
Because pre- and postmenopausal breast cancers probably have a separate disease aetiology, a stratified analysis by menopausal status was conducted. The inverse association of folate intake with breast cancer risk did not vary by menopausal status. The multivariate-adjusted OR were 0·34 (95 % CI 0·21, 0·58) among premenopausal women and 0·28 (95 % CI 0·12, 0·65) among postmenopausal women, comparing the fourth quartile with the first quartile. No interaction was observed between menopausal status and folate intake (P interaction = 0·570, data not shown).
A number of sensitivity analyses were performed to examine the association between dietary folate intake and breast cancer risk. Ductal carcinoma was the most frequent histological type (415, 94·7 %), and restricting the analysis to these cases yielded very similar results. It was observed that seventy cases (16·1 %) and eighty controls (18·3 %) reported ever taking nutritional supplements. Sensitivity analysis that excluded women with nutritional supplement use revealed similar results as compared with the analyses that included nutritional supplement users. In the present study, only twelve (2·7 %) cases and ten (2·3 %) controls were regular drinkers. The results of sensitivity analyses excluding women with alcohol intake were essentially the same. Further analyses restricted to subjects reporting no dietary change showed no substantial change in the observed folate intake and breast cancer association.
Discussion
In the present hospital-based case–control study, we observed a statistically significant inverse association between dietary folate, vitamin B6 intake and the risk of breast cancer after adjusting for various confounding factors. The inverse association of folate intake with breast cancer risk was observed in all subtypes of ER and/or PR status. No association was found with vitamin B12 and methionine intake.
The association of dietary folate intake with breast cancer risk has been examined in many epidemiological studies. Of the fifteen case–control studies(Reference Adzersen, Jess and Freivogel5–Reference Freudenheim, Marshall and Vena13, Reference Thorand, Kohlmeier and Simonsen27, Reference Chen, Gammon and Chan29, Reference Zhu, Davidson and Hunter36, Reference Ma, Iwasaki and Kobayashi38, Reference Potischman, Swanson and Coates47, Reference Sharp, Little and Schofield48), nine have(Reference Adzersen, Jess and Freivogel5–Reference Freudenheim, Marshall and Vena13) reported the protective effect of dietary folate intake on breast cancer risk. A case–control study conducted in Shanghai, China, showed that participants in the highest quintile of dietary folate had a 38 % decrease in breast cancer risk compared with those in the lowest quintile(Reference Shrubsole, Jin and Dai11). The findings of the present study are consistent with these studies. No differences were observed in the inverse association between folate intake and breast cancer risk according to folate intake from different food sources. However, some prospective cohort studies(Reference Baglietto, English and Gertig14–Reference Stevens, McCullough and Sun24) did not find a significant inverse association of dietary folate intake with breast cancer risk. A meta-analysis including nine prospective studies and fourteen case–control studies has shown a negative association between dietary folate intake and breast cancer risk in case–control studies but not in prospective studies(Reference Larsson, Giovannucci and Wolk4). Methodological differences may partially explain the inconsistent findings.
Relatively few epidemiological studies have evaluated the association of vitamin B6, vitamin B12 and methionine intake with breast cancer. Most of the studies have not found a significant association of methionine intake(Reference Shrubsole, Jin and Dai11, Reference Feigelson, Jonas and Robertson16, Reference Maruti, Ulrich and White23, Reference Kabat, Miller and Jain25–Reference Cho, Holmes and Hankinson28), vitamin B6 or vitamin B12 intake(Reference Lajous, Lazcano-Ponce and Hernandez-Avila8, Reference Levi, Pasche and Lucchini9, Reference Shrubsole, Jin and Dai11, Reference Maruti, Ulrich and White23, Reference Ma, Iwasaki and Junko26, Reference Chen, Gammon and Chan29–Reference Lin, Lee and Cook31) with breast cancer risk. In agreement with these results, neither vitamin B12 nor methionine was associated with breast cancer in the present study. However, higher vitamin B6 intake was found to be inversely associated with breast cancer risk. Since plant foods are the major sources of vitamin B6 and folate and their intakes are highly correlated, the effect of confounding may exist. Thus, further investigation into the effect of vitamin B6 on breast cancer risk is warranted.
In one case–control study(Reference Ma, Iwasaki and Kobayashi38) and five prospective cohort studies(Reference Larsson, Bergkvist and Wolk22, Reference Maruti, Ulrich and White23, Reference Cho, Holmes and Hankinson28, Reference Zhang, Hankinson and Hunter34, Reference Sellers, Vierkant and Cerhan37) that have investigated the association of folate with breast cancer according to the ER and/or PR status of breast tumour, the results remained inconclusive. In the Nurses' Health Study and the Vitamins and Lifestyle Cohort study(Reference Maruti, Ulrich and White23, Reference Zhang, Hankinson and Hunter34), folate intake was inversely associated with the risk of developing ER − but not ER+ tumours. In the Swedish Mammography Cohort Study(Reference Larsson, Bergkvist and Wolk22), high folate intake was associated with the decreased risk of developing ER+/PR − breast cancer but not ER+/PR+ and ER − /PR − tumours. However, no overall association was found between folate intake and ER − or ER+ breast cancer tumours in the Nurses' Health Study(Reference Cho, Holmes and Hankinson28), the Iowa Women's Health Study(Reference Sellers, Vierkant and Cerhan37) and one case–control study conducted in Japan(Reference Ma, Iwasaki and Kobayashi38). In the present study, the protective effect of folate intake on breast cancer was observed on all subtypes of the ER/PR status of breast cancer. However, in the present study, the power to detect the interactions between dietary folate intake and ER − and PR − status was low, as the number of ER − and PR − tumour subtypes was small. Further studies with larger sample sizes are thus needed to confirm this result.
Some(Reference Levi, Pasche and Lucchini9, Reference Negri, La Vecchia and Franceschi10, Reference Rohan, Jain and Howe17, Reference Zhang, Hunter and Hankinson21), but not all(Reference Cho, Spiegelman and Hunter15, Reference Stevens, McCullough and Sun24, Reference Lajous, Romieu and Sabia30), epidemiological studies have found a statistically significant reduction in breast cancer risk for high v. low folate intake among women with high alcohol intake. In the present study, however, the inverse association of dietary folate intake with breast cancer risk remained significant after excluding women with alcohol intake. Therefore, the present study did not support the evidence of the moderating effect of alcohol on folate intake. The reasons for the differences between the present study and others are unclear. The prevalence of alcohol intake is low in Chinese women, and only twelve (2·7 %) cases and ten (2·3 %) controls were regular drinkers in the present study. Further investigation in populations with a low prevalence of alcohol intake is warranted to help clarify this issue.
Folate intake is strongly influenced by various cooking methods. For example, the method of cooking of green vegetables has been found to have marked effects on folate retention(Reference McKillop, Pentieva and Daly49), and green vegetables are the major food sources for folate in our population. Therefore, the 68 % reduction in risk associated with a high intake of folate that we observed in the present study may be a conservative estimate. It has been suggested that genetic polymorphisms in folate metabolic enzyme genes could influence breast cancer risk(Reference Ma, Iwasaki and Junko26). It is biologically plausible that folate-related gene–nutrient interactions might play a role in breast cancer risk. Therefore, further studies with polymorphisms relevant to folate metabolism will help clarify the mechanism of breast caner risk. In China, the recommended nutrient intakes for folate, vitamin B6 and vitamin B12 are 400 μg/d, 1·2 mg/d and 2·4 μg/d, respectively(Reference Yang, Wang and Pan42). The median intake of dietary folate, vitamin B6 and vitamin B12 in the control group of the present study was 228·3 μg/d, 0·86 mg/d and 1·54 μg/d, respectively. These results showed the potential deficiency of one-carbon metabolism nutrients in the study population.
The present study has some limitations. Selection bias is a potential limitation in hospital-based case–control studies. We recruited controls from several conditions with no apparent association with a dietary cause to reduce this bias. Moreover, the high participation rate (96 and 98 % for cases and controls, respectively) and high comparability in sociodemographic factors between the case and control subjects also decreased the potential influence of selection bias on the present results. Recall bias is also of concern in case–control studies. To minimise this bias, we tried to interview the patients as soon as diagnosis was made. We also provided photographs with usual intake portions of foods to help participants quantify the amount of food consumed. The possible non-differential misclassification bias due to dietary folate intake may attenuate the estimated association between dietary intake and breast cancer risk. Therefore, some of the null associations observed in the present study may be due to random measurement error in dietary assessment.
In summary, the present study found that intakes of dietary folate and vitamin B6 were inversely associated with breast cancer risk. The inverse association was similar by ER and/or PR status. No associations were observed for vitamin B12 and methionine intake and breast cancer risk.
Acknowledgements
The present study was supported by the Centre of Research and Promotion of Women's Health of the School of Public Health and Primary Care of the Chinese University of Hong Kong. We gratefully acknowledge the assistance of our student helpers and the participation of the study subjects, without them the study would not have been possible. The authors would also like to thank the following doctors for their kind permission to interview patients in their hospitals: Kong-jia Luo and Hong Yang in Sun Yat-sen University Cancer Centre; Shu-wen Wu, Rui-yu Zheng, Feng-jiao Yan and Li-jing Hu in the First Affiliated Hospital, Sun Yat-sen University. C.- X. Z. constructed the project design, and was involved in the data collection and writing of the manuscript. S. C. H. supervised and contributed to the writing of the manuscript. Y.-M. C. provided significant advice regarding the analyses and interpretation of the data. S.-Z. C., J.-H. F. and F.-Y. L. were responsible for connecting and coordinating the fieldwork. The authors have no conflicts of interest.







