CVD is the leading cause of death worldwide, placing heavy economic and health burdens on society(Reference Ashtary-Larky, Rezaei Kelishadi and Bagheri1). Metabolic disorders such as obesity, hypertension, diabetes, metabolic syndrome, non-alcoholic fatty liver disease and dyslipidaemia lead to increased risk of CVD(Reference Ashtary-Larky, Bagheri and Ghanavati2,Reference Asbaghi, Choghakhori and Ashtary-Larky3) and are considered by many to be the most important component in cardiovascular pathologies. It has been shown that improvement in cardiovascular risk factors has significant effects on lowering CVD morbidity and mortality. Although it is well known that various pharmacotherapies can improve cardiovascular risk factors, they have been shown to have adverse side effects and complications in some individuals. Therefore, dietary supplement therapy can be considered an alternative or adjunctive treatment for CVD. One of these dietary supplements is CLA(Reference Asbaghi, Shimi and Shiraseb4,Reference Asbaghi, Ashtary-Larky and Naseri5) .
CLA is series of linoleic acid (18:2, n6; LA) isomers, with conjugated double bonds that unlike LA are not separated by a methylene group(Reference Lehnen, da Silva and Camacho6). Ruminant and dairy products are major dietary sources of CLA. Major isomers of CLA in food are Cis-9, trans-11-CLA (about 90 % of dietary CLA) and trans-10, cis-12-CLA (about 10 % of dietary CLA)(Reference Rubin, Herrmann and Much7). In ruminants, CLA is generated during ruminal biohydrogenation of LA via rumen bacteria, such as Butyrivibrio Fibrisolvens. It can also be synthesised in mammary tissues from vaccenic acid (11-trans octadecanoic acid; VA), another intermediate in the biohydrogenation of unsaturated fatty acids, by involving Δ9–desaturase(Reference Lehnen, da Silva and Camacho6,Reference Benjamin, Prakasan and Sreedharan8) . In humans, endogenous synthesis of CLA from VA is limited(Reference Wang, Li and Du9).
Nowadays, there is increasing demand for developing CLA-related products due to the spectrum of beneficial therapeutic properties attributed to this fatty acid. To produce CLA, food manufacturers have employed alkali isomerisation. In this technique, LA in LA-rich vegetable oils (such as corn, soybean and safflower oils) is isomerised to CLA, in alkaline situations(Reference Wang, Li and Du9). Supplementation of this compound, with different isomer ratios, has also attracted researchers. CLA, as a nutraceutical, has been shown to contribute to various biological processes, producing beneficial health effects. It can reduce cancer, boost immune function, prevent heart disease, modulate glucose and lipid metabolism and treat obesity by modifying body composition or increasing lean body mass(Reference Whigham, Cook and Atkinson10). Supplementation with CLA is also linked to CVD and associated risk factors(Reference Funck, Barrera-Arellano and Block11). Thus, investigating the effectiveness of CLA supplementation appears to be beneficial.
The efficacy of CLA on lipid profiles is still inconclusive. Based on the results of some meta-analyses, reduction of HDL(Reference Asbaghi, Ashtary-larky and Naseri12,Reference Moreno, Marquez and Oberg13) and an increase in TAG(Reference Moreno, Marquez and Oberg13) after CLA supplementation (particularly doses more than 4 g/day) may illustrate the negative effects, while decreased LDL levels with CLA supplement form (0·59–6·8 g/d) and foods enriched with CLA (1·17–73·7 g/d)(Reference Derakhshande-Rishehri, Mansourian and Kelishadi14) can demonstrate the beneficial effects of CLA on CVD risk factors. The health effect of CLA on blood pressure is also unclear. Animal studies have shown CLA to present positive effects in reducing blood pressure, but human clinical trials do not support any favourable effects on blood pressure regulation(Reference Yang, Wang and Zhou15,Reference Haghighat, Shimi and Shiraseb16) . Moreover, preclinical studies regarding the negative relationship between CLA and obesity is encouraging. However, clinical evidence in humans on CLA to reduce body weight and boost repartitioning of body fat and fat free mass (FFM) is insufficient(Reference Larsen, Toubro and Astrup17). The inconsistent findings observed across the studies can be related to the heterogeneity in the design, population and duration of the studies, variations in doses of CLA, dissimilarities in preparation of CLA isomer (or mixture) and differences in control groups.
Given the complexity of information about the efficacy of CLA on some parameters of CVD risk factors, we aimed to conduct a comprehensive systematic review and meta-analysis of published human randomised controlled trials (RCT) to investigate the effects of conjugated linoleic acid supplementation on cardiovascular risk factors in patients at risk of CVD.
Methods
This meta-analysis study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, as a practical method for reporting systematic reviews and meta-analyses(Reference Page, McKenzie and Bossuyt18). The PROSPERO registration code of this meta-analysis is: CRD42023426373.
Search strategy and study selection
An exhaustive search of the literature, in the various online databases, including ISI web of science, PubMed and Scopus was performed, up to January 2023, with no date and language limitation, to recognise associated articles. In order to prevent missing any publication, we did not limit our search strategy to CVD. In addition, for increasing the precision of finding eligible study, we checked the references of included studies and explored Google scholar manually. Therefore, these databases were searched using the following search items in titles and abstracts: (‘Conjugated linoleic acid’[Title/Abstract] OR ‘conjugated fatty acid’[Title/Abstract] OR ‘bovic acid’[Title/Abstract] OR ‘rumenic acid’[Title/Abstract] OR ‘CLA’[Title/Abstract]) AND (Intervention[Title/Abstract] OR ‘Intervention Study’[Title/Abstract] OR ‘Intervention Studies’[Title/Abstract] OR ‘controlled trial’[Title/Abstract] OR randomized[Title/Abstract] OR random[Title/Abstract] OR randomly[Title/Abstract] OR placebo[Title/Abstract] OR ‘clinical trial’[Title/Abstract] OR Trial[Title/Abstract] OR ‘randomized controlled trial’[Title/Abstract] OR ‘randomized clinical trial’[Title/Abstract] OR RCT[Title/Abstract] OR blinded[Title/Abstract] OR ‘double blind’[Title/Abstract] OR ‘double blinded’[Title/Abstract] OR trial[Title/Abstract] OR trials[Title/Abstract] OR ‘Pragmatic Clinical Trial’[Title/Abstract] OR ‘Cross-Over Studies’[Title/Abstract] OR ‘Cross-Over’[Title/Abstract] OR ‘Cross-Over Study’[Title/Abstract] OR parallel[Title/Abstract] OR ‘parallel study’[Title/Abstract] OR ‘parallel trial’[Title/Abstract] OR OR[Title/Abstract]). We applied Endnote software, for screening included studies.
Inclusion criteria
All studies that had these features were included in this meta-analysis: (1) RCTs evaluating the effects of CLA supplementation on CVD risk factors as an outcome (TAG, total cholesterol (TC), LDL, HDL, systolic blood pressure (SBP), diastolic blood pressure (DBP), body weight, BMI, waist circumference (WC), body fat percentage (BFP), FFM) with a control group, (2) studies carried out on adults (≥ 18 years) with risk of CVD including type 2 diabetes, obesity, metabolic syndrome, hypertension, hyperlipidaemia, atherosclerosis and fatty liver disease, (3) that received CLA supplementation as an intervention, (4) studies with at least 8 weeks of intervention duration, (5) parallel or crossover designs, (6) studies with outcome reporting at the start and the end of the intervention
Exclusion criteria
After the full text analysing of the studies, articles that possessed these criteria were excluded consequently: (1) review, animal, observational and ecological studies, (2) trials without placebo or control group or randomisation, (3) studies conducted on under 18 years individuals (4) Additionally, healthy participants or subjects with unrelated condition or disease were excluded
Data extraction
Two separate researchers extracted data from qualified articles. All extracted data possessed characteristics, including publication date and the main country of the execution, main designing structure, the name of the first author, the features of the subjects, such as mean age and BMI in both intervention and control groups, the sample size in both groups, gender of participants, the dosage and the duration of CLA supplementation from the beginning of the trial to the end, the mean changes and the sd of the markers throughout the study, for both the intervention and control groups. When a study provided multiple data at various time points, only the most recent one was considered.
Quality assessment
As a practical protocol for measuring the quality of included studies, we used Cochrane Collaboration modified risk of bias. In RCT, in seven fields, the risk of bias was assessed, including (1) random sequence generation, (2) allocation concealment, selective reporting bias, (3) selective reporting (4) blinding (participants and personnel) (5) blinding (outcome assessment) (6) incomplete outcome data and (7) other sources of bias(Reference Higgins, Altman and Gøtzsche19). Consequently, terms are defined and used as ‘Low’, ‘High’ or ‘Unclear’, for reporting the evaluation of each domain. If two of these seven fields had high risk of bias, the general risk of bias is defined ‘moderate’. Moreover, more than two fields with high risk of bias is defined as ‘High’ and less than two fields with high risk of bias is considered as ‘low’. Furthermore, after finding any probable dissemblance, it was resolved by the corresponding author.
Statistical analysis
To identify the overall effect size of CLA supplementation on TAG, TC, LDL, HDL, SBP, DBP, body weight, BMI, WC, BFP and FFM of each intervention and control group, which is reported as sd and mean difference, the random-effects model is used following the DerSimonian and Laird method(Reference DerSimonian and Laird20). Furthermore, when mean changes were not found, they were calculated by applying this formula:
Mean change = final values − baseline values, and also we computed sd changes by performing this formula(Reference Borenstein, Hedges and Higgins21):
 $$\eqalign{
& {\rm{SD}}\;{\rm{change}} = \cr
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\sqrt {\,[{{\left( {{\rm{SD}}\;{\rm{baseline}}} \right)}^2}\; + \;{{\left( {{\rm{SD}}\;{\rm{final}}} \right)}^2} - \left( {2{\rm{R}}\; \times \;{\rm{SD}}\;{\rm{baseline}}\; \times \;{\rm{SD}}\;{\rm{final}}} \right)} \cr} $$
$$\eqalign{
& {\rm{SD}}\;{\rm{change}} = \cr
& \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\sqrt {\,[{{\left( {{\rm{SD}}\;{\rm{baseline}}} \right)}^2}\; + \;{{\left( {{\rm{SD}}\;{\rm{final}}} \right)}^2} - \left( {2{\rm{R}}\; \times \;{\rm{SD}}\;{\rm{baseline}}\; \times \;{\rm{SD}}\;{\rm{final}}} \right)} \cr} $$
We considered correlation coefficient(R) = 0·8. The outcome variables of CLA supplementation on CVD risk factors (TAG, TC, LDL, HDL, SBP, DBP, body weight, BMI, WC, BFP and FFM) that were reported in mmol/l were converted to mg/dl by applying most common formulas. In addition, SEs, 95 % CIs and interquartile ranges were transformed to sd by carrying out the protocol of Hozo et al. (Reference Hozo, Djulbegovic and Hozo22). We performed random-effects model, which takes between-study variations into account to determine the overall effect size. Furthermore, between-studies heterogeneity was examined by Cochran’s Q test and was assessed by I-square (I2) statistic(Reference Higgins, Thompson and Deeks23). We considered I2 > 40 % or P-value < 0·05 as a high between-studies heterogeneity. To find potential sources of heterogeneity(Reference Higgins and Thompson24). Subgroup analyses were carried out following the pre-planned criteria, including duration of the investigation (< 12 weeks, ≥ 12 weeks), CLA dosage (≤ 6·4 g/d and ≥ 1·3 g/d), baseline levels of CVD risk factors (TAG, TC, LDL, HDL, SBP, DBP, body weight, BMI, WC, BFP, FFM), health status (metabolic syndrome, type 2 diabetes mellitus, hyperlipidaemia, hypertension, non-alcoholic fatty liver disease) and gender (male, female and both). The potential non-linear impacts of the CLA dosage (g/day) and the duration of the intervention (weeks) were assessed by using fractional polynomial modelling. Furthermore, the meta-regression examination was carried out for identifying the confounders and linear association between the effect and sample size, the duration and the dose of intervention(Reference Mitchell25). We performed a sensitivity analysis to assess the influence of each specific investigation on overall estimation(Reference Tobias26). The possibility of publication bias was checked by performing Egger’s regression examination. Additionally, the visually inspected test of the funnel plot was used(Reference Egger, Smith and Schneider27). Ultimately, by applying STATA, version 11.2 (Stata Corp), the statistical analyses were conducted. In all analyses, the P-values < 0·05 was considered statistically significant.
Certainty assessment
To assess the overall validity of evidence in studies, we used the Grading of Recommendations Assessment, Development and Evaluation guidelines Working Group. Additionally, following the corresponding assessment feature, we defined and categorised the quality of evidence as high, moderate, low and very low(Reference Gordon, Oxman and Vist28).
Results
Study selection
As illustrated in Fig. 1, at the first step of the search protocol, 8516 studies were found. As a result, 2185 studies were duplicates and were removed, subsequently. Afterwards, by evaluating the titles and abstracts, based on inclusion criteria, 6257 studies were deleted because of being irrelevant to the subject. After a comprehensive assessment of the full text of 74 studies, 60 studies were deleted due to the lack of necessary data reporting. Ultimately, 14 studies were qualified to conduct this meta-analysis.
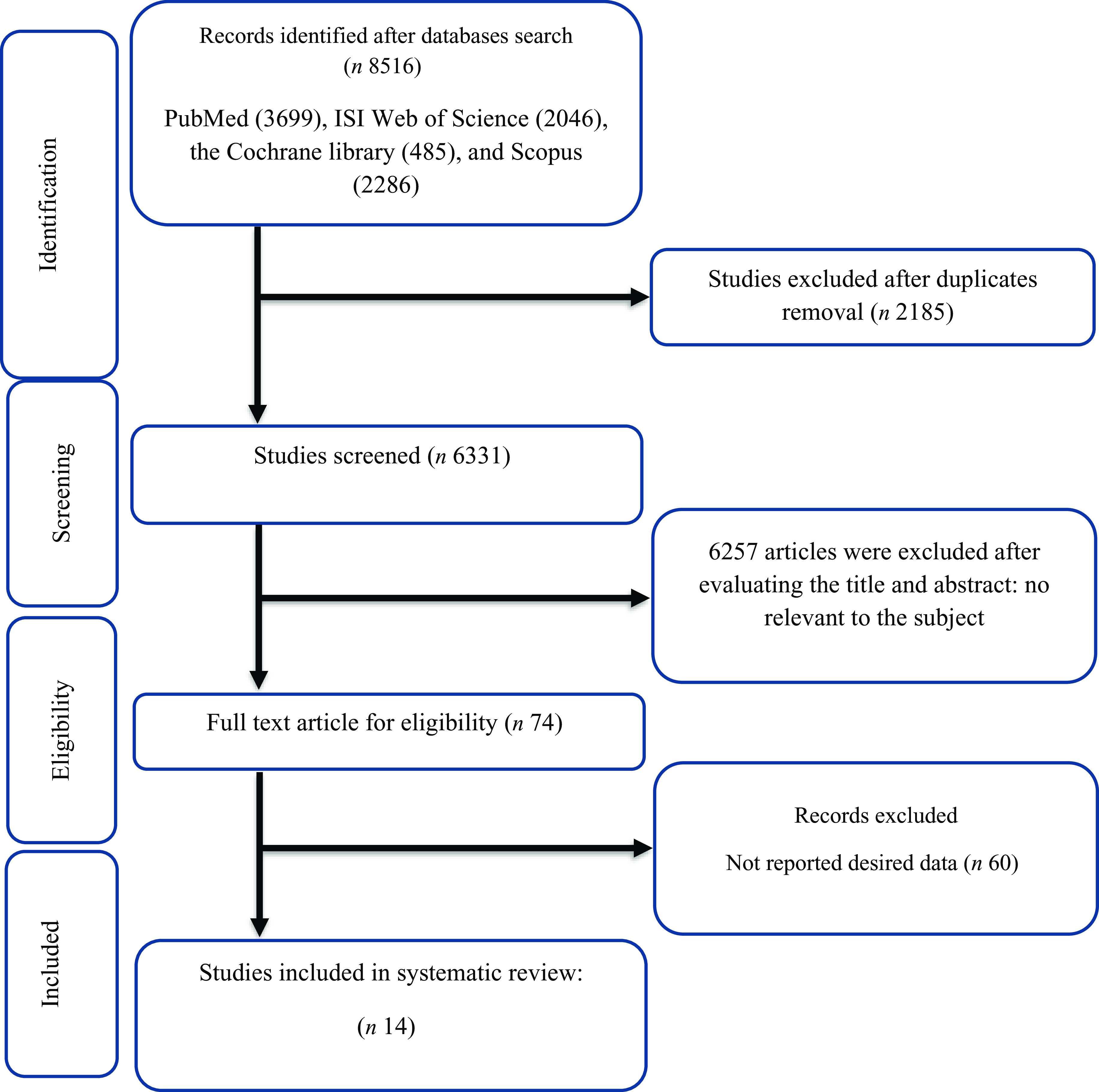
Fig. 1. Flow chart of study selection for inclusion trials in the systematic review.
Study characteristic
Fourteen RCT with 17 effect sizes including 772 overall individuals (373 cases and 399 controls) were included and qualified. All included studies were published between 1984 and 2022. In all qualified studies, the duration of intervention was from 8 to 16 weeks. In all qualified studies, the sample size differed from 14(Reference Carvalho, Uehara and Rosa29) to 80(Reference Zhao, Zhai and Wang30) participants. Moreover, all included studies were executed in parallel RCT or crossover designs. In this meta-analysis, various subjects were observed in qualified studies including, men with obesity and metabolic syndrome(Reference RISerus, Arner and Brismar31), patients with type 2 diabetes mellitus(Reference Schmitt, Ferry and Daniel32–Reference Shadman, Taleban and Saadat35), subjects being overweight and having LDL phenotype B(Reference Naumann, Carpentier and Saebo36), patients with obesity-related hypertension(Reference Zhao, Zhai and Wang30), postmenopausal women with type 2 diabetes mellitus(Reference Norris, Collene and Asp37), patients who were overweight and hyperlipidaemic(Reference Venkatramanan, Joseph and Chouinard38–Reference Joseph, Jacques and Plourde40), have atherosclerosis(Reference Eftekhari, Aliasghari and Babaei-Beigi41,Reference Eftekhari, Aliasghari and Beigi42) , individuals with metabolic syndrome(Reference Carvalho, Uehara and Rosa29) and patients with non-alcoholic fatty liver disease(Reference Ebrahimi-Mameghani, Jamali and Mahdavi43). The main countries that included studies were performed are the UK(Reference Moloney, Yeow and Mullen33), Iran(Reference Shadman, Rastmanesh and Taleban34,Reference Shadman, Taleban and Saadat35,Reference Eftekhari, Aliasghari and Babaei-Beigi41–Reference Ebrahimi-Mameghani, Jamali and Mahdavi43) , Netherlands(Reference Naumann, Carpentier and Saebo36), Canada(Reference Venkatramanan, Joseph and Chouinard38–Reference Joseph, Jacques and Plourde40), Sweden(Reference RISerus, Arner and Brismar31), Germany(Reference Norris, Collene and Asp37), Brazil(Reference Carvalho, Uehara and Rosa29), France(Reference Schmitt, Ferry and Daniel32) and China(Reference Zhao, Zhai and Wang30). Two studies were carried out on just females(Reference Carvalho, Uehara and Rosa29,Reference Norris, Collene and Asp37) , three studies on males(Reference RISerus, Arner and Brismar31,39,Reference Joseph, Jacques and Plourde40), and the others were executed on both(Reference Zhao, Zhai and Wang30,Reference Schmitt, Ferry and Daniel32–Reference Naumann, Carpentier and Saebo36,Reference Venkatramanan, Joseph and Chouinard38,Reference Eftekhari, Aliasghari and Babaei-Beigi41–Reference Ebrahimi-Mameghani, Jamali and Mahdavi43) . We mentioned the features of included studies in Table 1.
Table 1. Characteristic of included studies in meta-analysis (Mean values and sd)

Abbreviations: IG, intervention group; CG, control group; DB, double-blinded; SB, single-blinded; PC, placebo-controlled; CO, controlled; RA, randomised; NR, not reported; F, Female; M, Male; NR, not reported.
Quality assessment
Estimating the general risk of bias in qualified articles revealed that five studies acquired a moderate risk of bias(Reference Schmitt, Ferry and Daniel32,Reference Moloney, Yeow and Mullen33,Reference Shadman, Taleban and Saadat35,Reference Naumann, Carpentier and Saebo36,Reference Eftekhari, Aliasghari and Babaei-Beigi41) , two studies showed a low risk of bias(Reference Norris, Collene and Asp37,Reference Ebrahimi-Mameghani, Jamali and Mahdavi43) , and seven articles mentioned a high risk of bias(Reference Carvalho, Uehara and Rosa29–Reference RISerus, Arner and Brismar31,Reference Shadman, Rastmanesh and Taleban34,Reference Venkatramanan, Joseph and Chouinard38,Reference Joseph, Jacques and Plourde40,Reference Eftekhari, Aliasghari and Beigi42) (Table 2).
Table 2. Risk of bias assessment
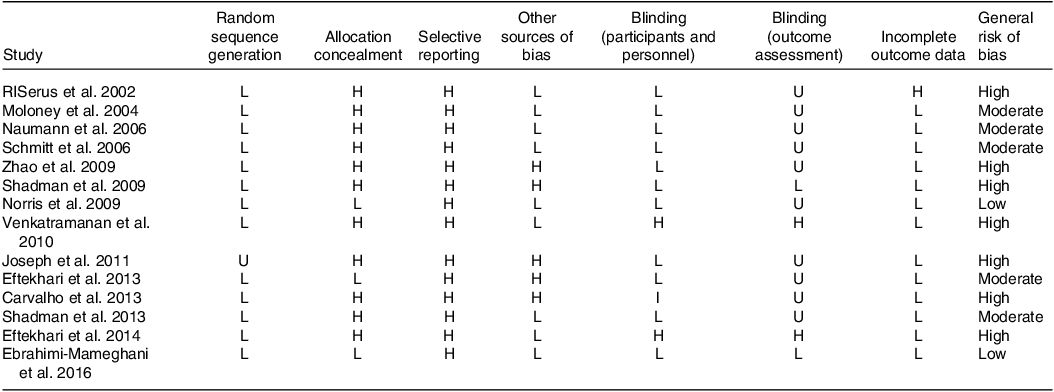
L; low risk of bias; H, high risk of bias; U, unclear risk of bias.
General Low risk < 2 high risk.
General moderate risk = high risk.
General high risk < 2 high risk.
Meta-analysis
Effect of conjugated linoleic acid supplementation on lipid profile
Assessing 15 overall effect sizes indicated that CLA supplementation had no significant effect on TAG levels (WMD: 1·57 mg/dl 95 % CI: −8·06, 11·21; P = 0·748). A significant degree of between-studies heterogeneity was also found (I2 = 99·7 %) (Table 3).
Table 3. Subgroup analyses of CLA supplementation on CVD risk factor in patients at risk of CVD (Weighted mean differences and 95 % CI)
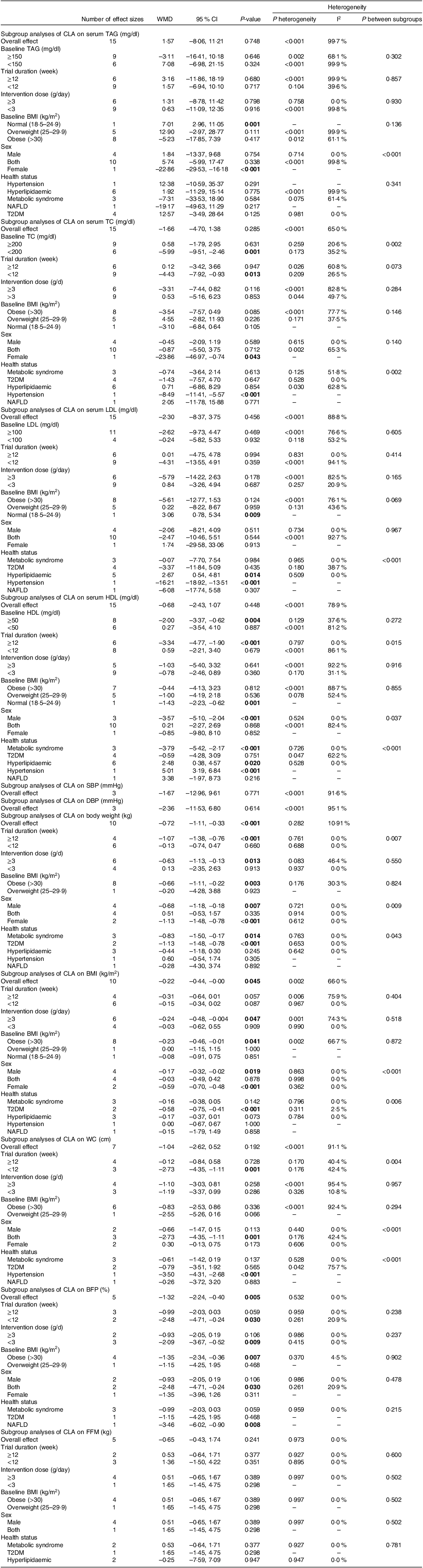
Abbreviations: WMD, weighted mean differences; TC, total cholesterol, ; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; FM, fat mass; BFP, body fat percentage; FFM, fat free mass; T2DM, type 2 diabetes.
Bold values denote statistical significance at the P < 0.05 level.
Pooled data from 14 overall effect sizes mentioned no significant impact of CLA supplementation on TC levels (WMD: −1·66 mg/dl; 95 % CI: −4·70, 1·38; P = 0·285). Moreover, we observed a moderate degree of heterogeneity among studies (I2 = 65 %). Subgroup analysis revealed that CLA supplementation in short-term intervention (< 12 weeks) or in participants with lower baseline levels of TC (< 200) diminished TC levels (Table 3).
The overall results from evaluating 15 overall effect sizes indicated no significant changes in LDL levels following the CLA supplementation (WMD: −2·30 mg/dl; 95 % CI: −8·37, 3·75 mg; P = 0·456). Moreover, a high degree of heterogeneity was observed among studies (I2 = 88·8 %). In the assessment of the outcomes of subgroup analysis, it was mentioned that CLA supplementation in patients with hyperlipidaemia, lowered LDL levels (Table 3).
After evaluating 14 overall effect sizes, it was found that CLA supplementation had no significant influence on HDL levels (WMD: −0·68 mg/dl; 95 % CI: −2·43, 1·07; P = 0·448). In addition, a significant between-studies heterogeneity was observed (I2 = 78·9 %). Moreover, the results of subgroup analysis demonstrated that in the long-term intervention (≥ 12 weeks), in individuals with higher baseline levels of HDL (≥ 50), or male participants, or among patients with hyperlipidaemia or metabolic syndrome, CLA supplementation altered HDL levels (Table 3).
Effect of conjugated linoleic acid supplementation on blood pressure
For estimating the effect of CLA supplementation on SBP and DBP, we evaluated three overall effect sizes for SBP and three for DBP, and then, it was revealed that CLA supplementation did not influence SBP (WMD: −1·67 mmHg 95 % CI: −12·96, 9·61; P = 0·771) or DBP, significantly (WMD: −2·36 mmHg 95 % CI: −11·53, 6·80; P = 0·614). We observed significant heterogeneity for both SBP (I2 = 91·6 %) and DBP (I2 = 95·1 %), among studies (Table 3).
Effect of conjugated linoleic acid supplementation on BMI and body mass
For reporting the impact of CLA supplementation on BMI and body mass, 10 overall effect sizes for body mass and 10 for BMI were assessed. The results mentioned that CLA supplementation had a significant lowering effect on body mass and BMI (for body weight WMD: −0·69 kg; 95 % CI: −1·10, −0·29; P = 0·001) (Fig. 2(a)), (for BMI WMD: −0·22 kg/m2; 95 % CI: −0·44, −0·01; P = 0·037) (Fig. 2(b)). Moreover, a high degree of heterogeneity for BMI (I2 = 60·5 %), but a low degree for body weight (I2 = 18·5 %) was observed among studies. According to the results of the subgroup analysis, CLA supplementation made significant reductions in body weight, long-term intervention (≥ 12 weeks), the higher dosage of CLA supplementation (≥ 3 g), patients with obesity (BMI > 30), type 2 diabetic, metabolic syndrome and females. Additionally, CLA supplementation in high dose (≥ 3 g), patients with obesity (BMI > 30) or type 2 diabetes, lowered BMI (Table 3).
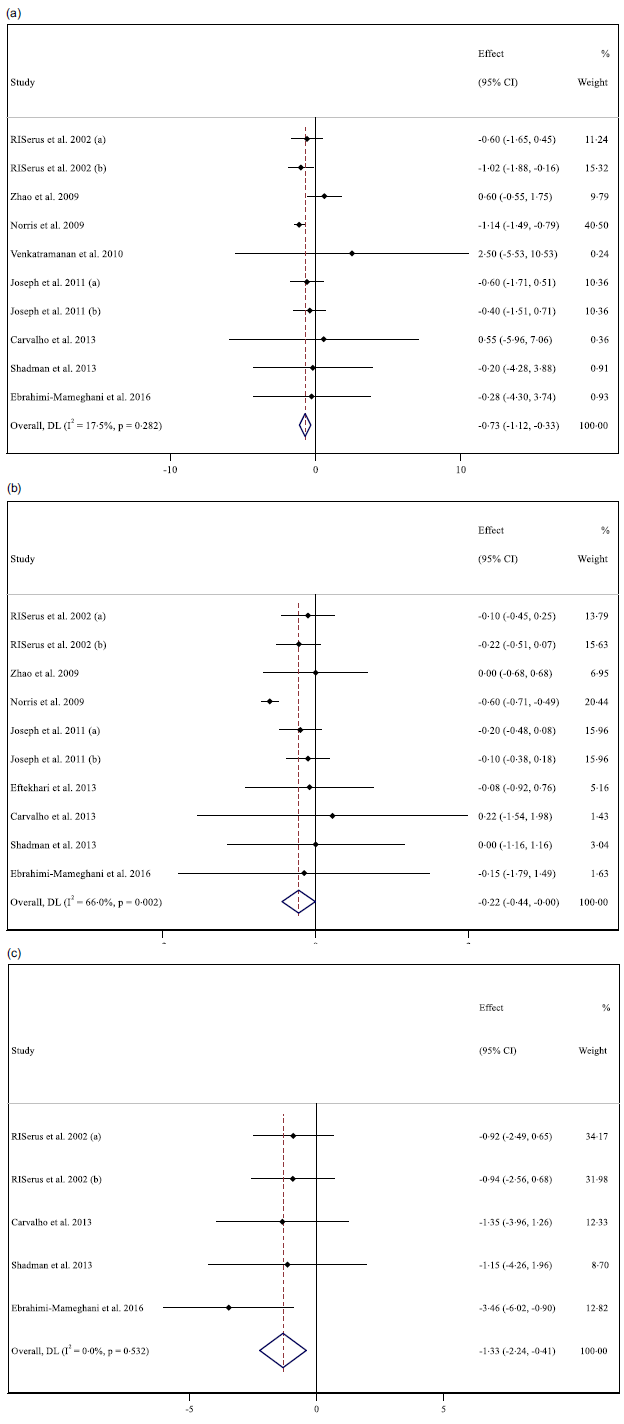
Fig. 2. Forest plot detailing weighted mean difference and 95 % CI for the effect of CLA supplementation on (a) body weight (kg); (b) BMI (kg/m2); and (c) BFP (%). *Effect in the figures is effect size that shows level of changes in variables after supplementation with CLA compared with control group.
Effect of conjugated linoleic acid supplementation on WC, body fat percentage and fat free mass
Analysing seven overall effect sizes for WC, five for BFP and five for FFM revealed that CLA supplementation had no significant impact on WC (WMD: −0·60 cm 95 % CI: −1·93, 0·72; P = 0·371) and FFM (WMD: −0·03 kg 95 % CI: −0·78, 0·71; P = 0·931), but reduced BFP, significantly (WMD: −1·32 kg 95 % CI: −2·24, −0·40; P = 0·005) (Fig. 2(c)). Furthermore, a high heterogeneity for WC (I2 = 88·9 %), a low for FFM (I2 = 18·7 %) and no heterogeneity for BFP (I2 = 00·0 %) were found among studies. Moreover, in short-term duration (< 12 weeks), lower dosage (< 3 g) of CLA supplementation and among patients with obesity (BMI > 30), BFP was diminished (Table 3).
Sensitivity analysis
To ascertain the impact of each study on the overall effect size, each included study was omitted from the analysis, respectively. By removing the studies, RISerus et al. 2002 (b)(Reference RISerus, Arner and Brismar31) (WMD: –0·21, 95 % CI: –0·46, 0·03, P = 0·087), Joseph et al. 2011 (a)(Reference Joseph, Jacques and Plourde40) (WMD: –0·22, 95 % CI: –0·46, 0·02, P = 0·087) and Ebrahimi-Mameghani et al. 2016(Reference Ebrahimi-Mameghani, Jamali and Mahdavi43) (WMD: –0·22, 95 % CI: –0·44, 0·00, P = 0·056), the overall results of BMI was altered significantly, following the CLA supplementation.
Publication bias
A significant publication bias was observed by inspecting the funnel plots and carrying out Egger’s test on studies assessing the impact of CLA supplementation on SBP (P = 0·004), BMI (P = 0·015) and body mass (P = 0·036) (Fig. 3(e), (g), (h)).
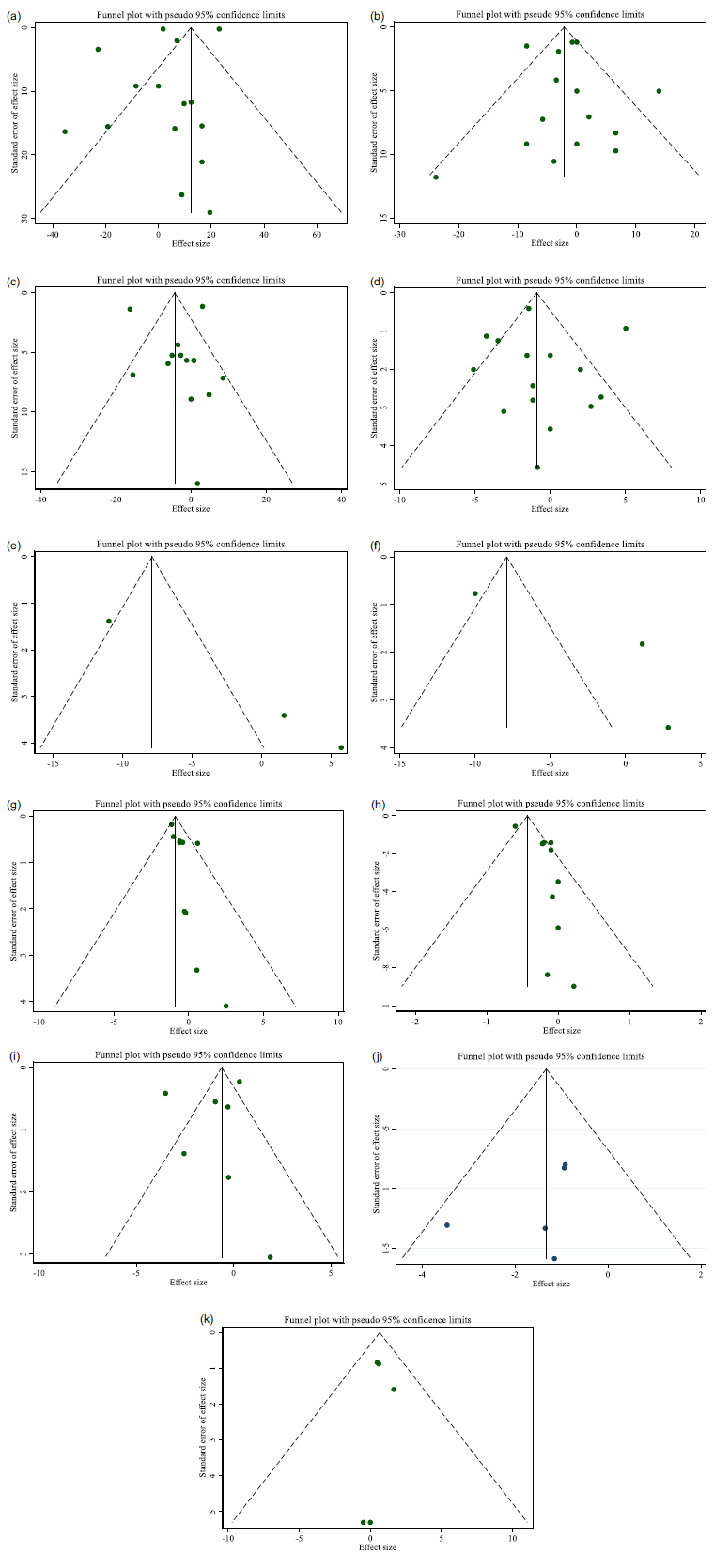
Fig. 3. Funnel plots for the effect of CLA supplementation on (a) TG (mg/dl); (b) TC (mg/dl); (c) LDL (mg/dl); (d) HDL (mg/dl); (e) SBP (mmHg); (f) DBP (mmHg); (g) body weight (kg); (h) BMI (kg/m2); (i) WC (cm); (j) BFP (%); and (k) FFM (kg).
Non-linear dose–response analysis
Non-linear dose-response analysis was conducted to find the relationship between changes in each variable, and dose (Supplementary file 2), as well as to investigate the association among all variables and the duration of the intervention (see Supplementary File 3). Assessing the outcomes of non-linear dose–response analysis demonstrated that alterations in TAG (coefficient = −79·61, P = 0·04) (coefficient = −0·39, P < 0·01) were associated significantly with the dosage of CLA supplementation (Table 4). Thus, by increasing the dose of CLA supplement from 1·3 grams per day, TAG and weight loss increased. Additionally, the intervention duration of the CLA supplementation was associated significantly with changes in BMI (coefficient = −938·08, P = 0·03). Supplementation for more than 12 weeks significantly reduced BMI.
Table 4. Linear and non-linear dose–response analysis
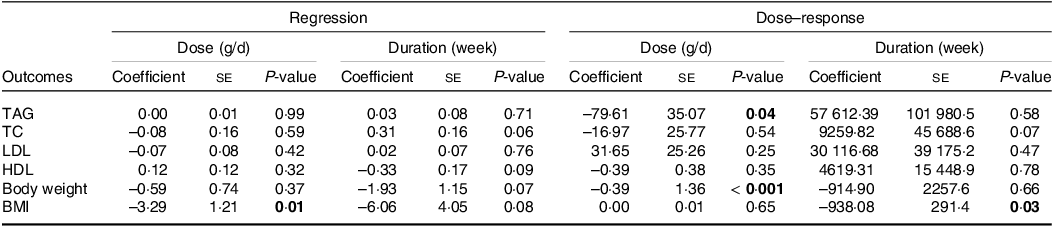
Bold values denote statistical significance at the P < 0.05 level.
Meta-regression analysis
To find the relationship between changes in variables, doses (online Supplementary file 4) and durations (online Supplementary file 5) of intervention, we perform linear meta-regression dose–response analysis. Evaluating the results of the meta-regression test indicated a significant association between the dosage of CLA supplementation and BMI changes (coefficient = −3·29, P = 0·010). We provided the results of meta-regression test in Table 4.
Grading of Recommendations Assessment, Development and Evaluation analysis
The Grading of Recommendations Assessment, Development and Evaluation protocol was executed in this meta-analysis to assess the quality of the evidence. The quality of the evidence in studies that aimed to reveal the effect of CLA supplementation on TAG, TC, LDL, HDL, SBP, DBP, BMI, WC, FM, BFP and FFM was low and very low. Moreover, the evidence quality in studies that had the objective to show the impact of CLA supplementation on body mass was upgraded to moderate (Table 5).
Table 5. Grading of Recommendations Assessment, Development and Evaluation profile of CLA for CVD risk factor in patients at risk of CVD
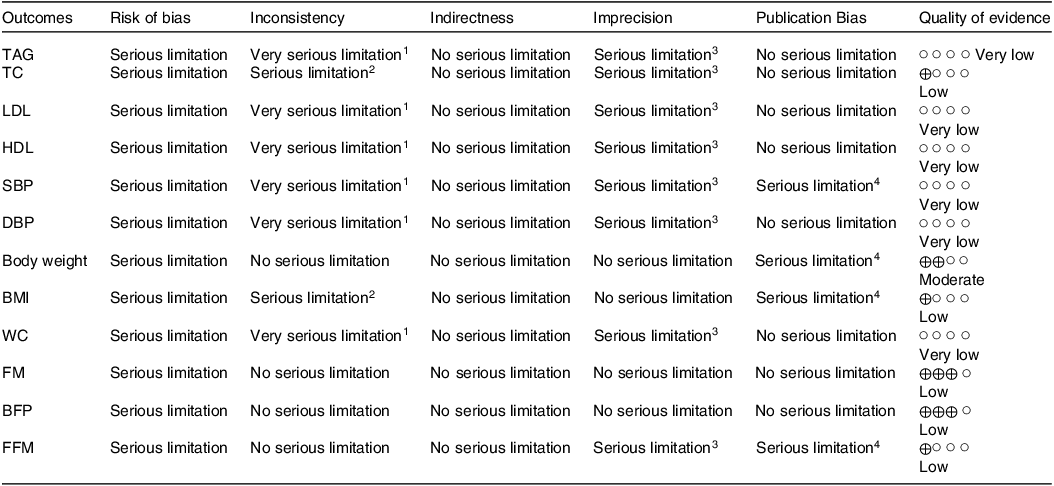
1There is high heterogeneity (I2 > 75 %) for TAG, LDL, HDL, SBP, DBP and WC.
2There is moderate heterogeneity (I2 > 40 %) for TC and BMI.
3There is no evidence of significant effects of CLA supplementation on TAG, TC, LDL, HDL, SBP, DBP, WC and FFM.
4There is a significant publication bias for SBP, body weight, BMI and FFM.
Discussion
To our knowledge, this is the first Grading of Recommendations Assessment, Development and Evaluation-assessed systematic review and dose–response meta-analysis to evaluate the effects of CLA supplementation on risk factors of CVDs, in adults ≥ 18 years at risk of CVDs. Our analysis suggested that CLA supplementation was associated with a small but significant decrease in body weight, BMI and BFP. No association was seen with lipid profiles, blood pressure, WC, FFM and CLA supplementation. According to subgroup analyses, CLA intake decreased TC levels in females with lower TC levels and a shorter intervention duration. Additionally, it was found that CLA supplementation significantly altered the level of HDL among male individuals, patients with metabolic syndrome, a more extended period of intervention and higher baseline levels of HDL.
Similar to our study, several interventional clinical studies have shown the anti-obesity and lowering abdominal adiposity effect of CLA supplementation in healthy individuals living with obesity and being overweight(Reference Rezvani, Montazeri and Baradaran44–Reference López-Plaza, Bermejo and Weber46). In addition, several human studies have indicated the role of CLA supplementation in increasing energy expenditure and lean body mass along with reducing the weight and/or fat gain(Reference Nazare, de la Perriere and Bonnet47,Reference Mądry, Malesza and Subramaniapillai48) . Moreover, similar to our findings, cellular and animal studies showed a reduction anthropometric indices, such as body weight and body fat mass following CLA supplementation. The favourable effects of CLA can be mediated by different mechanisms including decreasing the TAG uptake in adipocytes by reducing the stearoyl CoA desaturase and lipoprotein lipase activity(39), stopping the peroxisome-proliferator activated receptor activity and inducing the fat mass cell apoptosis(Reference Brown, Boysen and Jensen49), boosting the basal energy expenditure by increasing the uncoupling proteins(Reference West, Blohm and Truett50) and increasing the beta-oxidation rate of fatty acids by increasing activity of carnitine acetyltransferase(Reference Park, Albright and Liu51).
There are no consistent results on the favourable effects of CLA on blood pressure. Hypotensive effects of CLA have been reported in previous animal and human studies(Reference DeClercq, Taylor and Zahradka52,Reference DeClercq, Taylor and Wigle53) . For instance, Aryaeian et al. suggested that 2·5 g CLA equivalent to 2 g of cis 9-trans 11 and trans 10-cis12 CLAs could reduce SBP and mean arterial pressure, significantly(Reference Aryaeian, Shahram and Djalali54). However, several previous studies(Reference Shadman, Rastmanesh and Taleban34,Reference Asbaghi, Shimi and Naseri55,Reference Engberink, Geleijnse and Wanders56) , similar to this meta-analysis, did not support the overall favourable effect of CLA on blood pressure. Baseline blood pressure may be the reason that CLA failed to improve blood pressure. For example, the more studies included in our analysis were normotensive; therefore, further reducing SBP and/or DBP was unlikely. In two other human studies that CLA reduced blood pressure, it was taken together with a calcium supplement or ramipril tablet, which shows the importance of simultaneous use(Reference Zhao, Zhai and Wang30,Reference Herrera, Aréalo-Herrera and Shahabuddin57) .
In the current meta-analysis, we also did not find any significant decrease in TAG, TC, LDL and HDL concentration following CLA supplementation, overall and in more of their subgroups. Similar to this meta-analysis, several previous studies did not support the overall favourable effect of CLA on lipids profiles(Reference Shadman, Taleban and Saadat35,39,Reference Eftekhari, Aliasghari and Beigi42). However, other studies showed a significant effect of CLA supplementation on some of the components of lipid profile(Reference Moloney, Yeow and Mullen33,Reference Ebrahimi-Mameghani, Jamali and Mahdavi43) . An animal study showed that the pure cis-9, trans-11 isomer led to a decrease in free fatty acids and TAG, while the pure trans-10, cis-12 isomer(Reference Bhattacharya, Banu and Rahman58) decreased free fatty acids and LDL. Similar findings were found in humans(Reference RISerus, Arner and Brismar31). However, the results of other studies with equal ratios of CLA isomers are inconsistent(Reference Shadman, Taleban and Saadat35). This inconsistency can be due to the different doses of intervention and durations, various population and different proportions of the isomers of CLA. Overall, the impact of CLA supplementation on lipids metabolism is not well known, and more studies are needed to clarify the effect of CLA supplementation on lipid profile regulation and metabolism(Reference Shadman, Taleban and Saadat35).
This meta-analysis had several limitations including (1) only 14 randomised trials were included; thus, subgroup analyses were not performed in some of the CVDs risk factors, (2) more included studies were not primarily designed to investigate the CLA effect on CVDs and related risk factors. Therefore, the effects of other factors related to CVDs including inflammation, glycaemic profile and antioxidant-related markers of participants were unclear, (3) we failed to perform a subgroup analysis based on the type of CLA supplementation, (4) extra CLA intake from food was not controlled in more of the studies, due to a lack of controlling the diet of individuals, (5) the lack of variability in dose, small number of samples and heterogeneity in sample populations may affect the dose–response analysis. Our study had some strengths. We did not observe publication bias in this meta-analysis. All included studies were randomised and placebo-controlled trials, more of which were double-blind and this increased the internal validity and decreased the biases.
Conclusions
This systematic review and meta-analysis of 14 studies suggest that CLA supplementation exerts a beneficial effect on some of the anthropometric indices in patients at risk of CVDs. Moreover, CLA supplementation had no adverse effects on blood pressure or lipid profile in individuals with CVD. Additional long-term and well-designed RCT are necessary to further examine and confirm these findings.
Acknowledgements
Not applicable.
Funding not applicable.
O. A. and G. S. contributed in conception. M. E. and S. D. screening records, Z. B. D., H. S. O. and N. A. data extraction, D. A.-L., K. G. and N. R. contributed in manuscript drafting. All authors reviewed the manuscript.
All data generated or analysed during this study are included in this published article.
Ethical approval and consent to participate not applicable.
The authors declare that they have no competing interests.
Systematic review registration: https://www.crd.york.ac.UK/prospero/, identifier (CRD42023426373). PROSPERO registration code: CRD42023426373.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524001065











