Excoriation (skin-picking) disorder (SPD), also known as pathological skin-picking, neurotic/psychogenic excoriation and dermatillomania, is a body-focused repetitive behaviour characterised by compulsive picking of skin causing tissue damage. Reference Grant, Odlaug, Chamberlain, Keuthen, Lochner and Stein1 As a result of the growing body of research and recognition of SPD as a clinically significant condition over the past 10–15 years, SPD is now included in DSM-5 under the name ‘excoriation (skin-picking) disorder’ as a full disorder for the first time. 2 SPD is associated with a significant degree of psychosocial dysfunction, poor quality of life and medical complications Reference Odlaug and Grant3–Reference Tucker, Woods, Flessner, Franklin and Franklin4 and appears to be a fairly common disorder in the general population. In recent years, prevalence studies have indicated rates of SPD between 1.2 and 5.4% in population samples. Reference Hayes, Storch and Berlanga5–Reference Odlaug, Lust, Schreiber, Christenson, Derbyshire and Grant8 From a phenomenological perspective, SPD appears similar to trichotillomania and onychophagia (compulsive nail-biting), in that it is characterised by repetitive and excessive maladaptive grooming habits that are difficult for individuals to suppress. Reference Grant, Odlaug, Chamberlain, Keuthen, Lochner and Stein1 Indeed, these different types of symptoms commonly co-occur within individuals, leading to the notion that they be considered body-focused repetitive behaviours, which may share a common pathophysiological basis. Reference Stein, Chamberlain and Fineberg9 Another somewhat complementary perspective is that SPD be considered an obsessive–compulsive ‘spectrum’ disorder or disorder of the ‘impulsive–compulsive spectrum’ given the overlapping phenomenological and clinical characteristics of SPD and obsessive–compulsive disorder (OCD) and high rate of co-occurrence between the two disorders. In a study conducted in 901 patients with OCD, for example, 16.4% of the sample met criteria for concomitant SPD, while 4.9% met criteria for concomitant trichotillomania. Reference Lovato, Ferrão, Stein, Shavitt, Fontenelle and Vivan10 Recent aetiological research examining proposed genetic and environmental risk factors for the development of the obsessive–compulsive spectrum disorders found a high rate of overlap between these disorders, especially SPD and trichotillomania. Reference Monzani, Rijsdijk, Harris and Mataix-Cols11 Therefore, the relationship between SPD and OCD may be particularly pertinent in terms of such a conceptualisation.
Little is known regarding the neurobiological mechanisms or constructs involved in the aetiology of SPD. Recent structural imaging of SPD indicated reduced fractal anisotropy in tracts distributed bilaterally, which included white matter close to the anterior cingulate cortices, compared with controls. Reference Grant, Odlaug, Hampshire, Schreiber and Chamberlain12 These regions were remarkably similar to those separately found to be abnormal in trichotillomania. Reference Chamberlain, Hampshire, Menzies, Garyfallidis, Grant and Odlaug13,Reference Chamberlain, Menzies, Fineberg, Del Campo, Suckling and Craig14 For OCD, there exists a large body of literature, implicating dysregulation of the striatum (involved in habit generation) coupled with a lack of top–down input from cortical regions (including both medial and lateral prefrontal regions) responsible for various cognitive processes. Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore15 Patients with OCD often show behavioural impairments in executive planning (see for example Chamberlain et al, Tükel et al and Kashyap et al Reference Chamberlain, Fineberg, Blackwell, Clark, Robbins and Sahakian16–Reference Kashyap, Kumar, Kandavel and Reddy18 ). Such deficits extend to relatives of patients with OCD who are clinically asymptomatic, highlighting the centrality of this cognitive function and its implicated neural substrates in the pathophysiology – it may well represent an intermediate biological ‘vulnerability marker’ for OCD. Reference Delorme, Goussé, Roy, Trandafir, Mathieu and Mouren-Siméoni19,Reference Cavedini, Zorzi, Piccinni, Cavallini and Bellodi20 As such, planning-related functional magnetic resonance imaging (fMRI) tasks represent a useful means of probing frontostriatal integrity in OCD and related conditions, since they challenge salient distributed neural circuitry. Decreased responsiveness in the (mainly dorsolateral) prefrontal cortex and caudate nucleus was found during fMRI executive planning tasks in people with OCD compared with controls. Reference van den Heuvel, Veltman, Groenewegen, Cath, van Balkom and van Hartskamp21
The objective of this study was to probe the integrity of fronto-striatal circuitry in people with SPD compared with matched healthy controls using an fMRI executive planning paradigm. We hypothesised that SPD would be associated with underactivation during planning in the striatum and (dorsolateral) prefrontal cortex v. controls, supporting a neurobiological relationship between SPD and OCD.
Method
Study participants
Men and women aged 18–54 with a primary diagnosis of SPD based on DSM-5 criteria and a structured clinical interview with a board-certified psychiatrist with expertise in SPD and body-focused repetitive behaviours were recruited by newspaper and poster advertisements. All participants were recruited and underwent neuroimaging procedures at the University of Minnesota Medical Center.
Inclusion criteria included: (a) participants met DSM-5 criteria for SPD for at least the past 12 consecutive months: (i) recurrent skin-picking resulting in skin lesions; (ii) repeated attempts to decrease or stop skin-picking; (iii) picking causes clinically significant distress or impairment in social, occupational or other important areas of functioning; (iv) picking is not because of the direct physiological effects of a substance (for example (meth)amphetamine, cocaine) or a general medical condition (such as scabies); and (v) skin-picking is not restricted to the symptoms of another mental disorder (such as skin-picking because of fixed beliefs about skin infestation in delusional disorder or parasitosis, preoccupation with appearance in body dysmorphic disorder); (b) a minimum score of >16 on the Yale Brown Obsessive Compulsive Scale modified for Neurotic Excoriation (NE-YBOCS); Reference Arnold, Mutasim, Dwight, Lamerson, Morris and McElroy22,Reference Grant, Odlaug and Kim23 and (c) picking behaviour occurred daily for at least 30 min consistently over the past 12 months.
Exclusion criteria in those with SPD comprised: (a) unstable medical illness or clinically significant abnormalities on physical examination; (b) current pregnancy or lactation; (c) lifetime history of bipolar disorder type I or II, dementia or any psychotic disorder; (d) any current (past 12 months) DSM-5 psychiatric disorder including OCD, body dysmorphic disorder, trichotillomania, gambling disorder, nicotine dependence and disruptive, impulse control and conduct disorders; (e) initiation of psychotherapy or pharmacotherapy within 3 months prior to study entry and, if taking medication, no dose changes for the preceding 3 months; (f) history of head injury or neurologic disorders; and (g) any contraindications to MRI based on safety screening and clinical history. In addition, no participants had a history of hypertension or diabetes, conditions which may interfere with brain imaging.
After a complete description of the study to the participants, written informed consent was obtained. No secondary consents were allowed for the study (i.e. parent/guardian consent was not allowed). A full institutional review board approved the consent procedures and all study procedures were carried out in accordance with the ethical principles for medical research involving human participants established in the 2008 Declaration of Helsinki. Age- and gender-matched healthy controls were recruited via word of mouth, poster and newspaper advertisements. All control group participants were free of any lifetime psychiatric disorder according to the Structured Clinical Interview for DSM-IV (SCID-I) Reference First, Spitzer and Gibbon24 and DSM-5 criteria for SPD.
Assessments
Participant interviews and scale administration were conducted by a board-certified psychiatrist with expertise in the assessment and treatment of body-focused repetitive behaviours, OCD and disorders of impulse control. Psychiatric comorbidity was assessed using the SCID-I. The severity of SPD was assessed using the NE-YBOCS. All participants underwent a safety screening for contraindication for MRI at study entry and at the scanning facility to ensure safety.
Functional imaging was completed within 7 days of the intake assessment. We conducted one fMRI task based on our desire to probe the integrity of striatal and frontal lobe function in SPD. As such, the Tower of London task of frontal lobe integrity was chosen given that this paradigm had previously been found to be sensitive to brain dysfunction in those with gambling disorder, Reference de Ruiter, Veltman, Goudriaan, Oosterlaan, Sjoerds and van den Brink25,Reference Kräplin, Bühringer, Oosterlaan, van den Brink, Goschke and Goudriaan26 obsessive–compulsive disorder, Reference Cavedini, Zorzi, Piccinni, Cavallini and Bellodi20 Parkinson's disease, Reference Nombela, Rowe, Winder-Rhodes, Hampshire, Owen and Breen27,Reference Williams-Gray, Hampshire, Robbins, Owen and Barker28 and National Football League players with executive deficits. Reference Hampshire, MacDonald and Owen29 Given significant time and funding constraints, this task was chosen based on our hypotheses of executive function deficits in the SPD group and this previous research. The Tower of London task has been validated in other clinical contexts but never before applied to SPD. Reference Williams-Gray, Hampshire, Robbins, Owen and Barker28,Reference Kempton, Ettinger, Foster, Williams, Calvert and Hampshire30 The task lasted for approximately 10 min, during which planning and subtracting problems were shown on a computer screen (inter-trial interval jittered 5–15 s). For each trial, participants were presented with two sets of tubes, with a random assortment of three coloured balls inserted in the tubes. Participants were prompted with a cue screen displaying the word ‘plan’ or ‘subtract’ before each new problem was presented. For ‘plan’ trials, participants were instructed to mentally determine the minimal number of ball moves required to make the top set of tubes match the bottom set of tubes. Participants indicated their response using a button-box placed in their right hand by pressing one of four buttons numbered 1–4; as such, trial duration was response driven (for further details see Williams-Gray et al Reference Williams-Gray, Hampshire, Robbins, Owen and Barker28 ). For ‘subtract’ trials, participants were instructed to count the number of balls in the top row and subtract them for the number of balls in the lower row. Again, responses were indicated using a button-box. Both tasks used only problems with correct responses of 2, 3 or 4. Participants were not provided with feedback regarding the correctness of their responses in order to minimise any possible influence of error-related feedback on brain activation. Task difficulty varied via a predefined pseudo-randomised sequence. All participants underwent a training session before scanning to ensure that they understood the rules of the task and were able to perform it adequately, and to minimise the risk of between-group behavioural differences in the scanner, which can represent major confounds in interpreting fMRI group differences.
All participants were scanned at the University of Minnesota using a 3-Tesla Siemens (Munich, Germany) TIM Trio MRI scanner. In total 330 T 2-weighted echo-planar images depicting blood oxygen level-dependent (BOLD) signal were acquired, with the first 10 being discarded to avoid T 1-equilibrium effects. The fMRI sequence data were as follows: acquisition time 2 s (per volume); type: 2D; slices: 32; slice gap: 25%; slice thickness: 3 mm; slice order: descending (32,31,…,1); field of view: 192×192 mm; matrix: 64×64; resolution: 3×3 mm; repetition time: 2000 ms; echo time: 30 ms; flip angle: 78°; bandwidth: 2232 Hz/Px; echo spacing: 0.51 ms.
Data analysis
Potential differences between the study groups on demographic and clinical measures of interest were explored using independent sample t-tests or alternative non-parametric tests as indicated, with significance defined as P<0.05 uncorrected for these purposes. Imaging data were pre-processed using the methods as previously reported. Reference Williams-Gray, Hampshire, Robbins, Owen and Barker28 In brief, the scan data were motion-corrected, slice-time acquisition corrected, co-registered to structural scans (standard MPRAGE), and normalised to the standard Montreal Neurological Institute echo-planar imaging template. The resulting images were smoothed with a 8 mm full-width at half-maximum Gaussian kernel. We first examined whether the task activated the expected frontostriatal planning network by conducting a whole brain analysis using SPM (version 5) for the plan minus subtract contrast, collapsing across all study participants (P<0.05 false discovery rate-corrected). The general linear model included the onsets and durations of the task events convolved with the canonical haemodynamic response function. It also included movement parameters and a constant term. There has been some debate in the neuroimaging field regarding the choice of FDR v. the alternative family-wise error-correction method for multiple comparisons (for discussion see for example Bennett et al Reference Bennett, Wolford and Miller31 ). Our choice of FDR correction was in order to avoid the elevated risk of false positives if using family-wise error, and in order to maintain consistency of approach with prior work (for example Williams-Gray et al and Hampshire et al Reference Williams-Gray, Hampshire, Robbins, Owen and Barker28,Reference Hampshire, MacDonald and Owen29 ).
In order to explore potential differences between the groups in terms of fMRI activation, we used the contrast of task minus rest (since this typically yields greater variance for detection of cross-group effects and is more easily interpretable for such analyses, as compared with the count minus subtract contrast). We used two complementary analysis approaches.
Select region of interest (ROI) analysis
Mean activation data were extracted from the activation clusters and compared cross-group using the MarsBaR Regions of Interest toolbox (random effects). Reference Brett, Anton, Valabregue and Poline32 Regions of interest were defined as 10 mm spheres based on peak activation coordinates derived from an independent healthy volunteer data-set (the first 50 healthy controls participating in Hampshire et al Reference Hampshire, MacDonald and Owen29 ); note that this dataset was entirely independent with no participants taking part in both studies. The region of interest (ROI) coordinates were: dorsolateral prefrontal cortex, left −22, 14, 56; dorsolateral prefrontal cortex, right 26, 26, 56; posterior parietal cortex, left −36, −76, 38; posterior parietal cortex, right 44, −70, 34; frontoparietal cortex, right 28, 50, 2. MarsBaR is a particularly sensitive approach when there is a strong prior hypothesis, because it averages values from all voxels within the ROI. This eschews the need to correct for many voxel-wise comparisons and thereby increases the sensitivity for detecting positive effects, mitigating the risk of false negatives.
Whole-brain unconstrained analysis
Cross-group differences were examined using robust permutation modelling (Cambridge Brain Analysis Software (CamBA), random effects). To provide stringent control for multiple comparisons, cluster correction was applied such that the expected number of false-positive clusters for the contrast of interest was less than one. Unlike parametric methods (for example those used in SPM), permutation modelling using CamBA is robust against outliers and non-normally distributed data. Moreover, CamBA offers a more advanced correction approach than SPM. More specifically, it uses cluster weights as opposed to extents and controls for multiple comparisons at the cluster level with permutation modelling.
We also examined correlations between clinical severity using the NE-YBOCS and (a) behavioural task measures; and (b) extracted mean ROI and cluster values from the imaging analyses (Pearson's r, uncorrected P<0.05). Correlations were also conducted between age at symptom onset and these measures. Exploratory t-tests (uncorrected P<0.05) were used to explore the possible moderating influence of medication status and lifetime history of other psychiatric disorders in the SPD individuals, with respect to disease severity and any behavioural or brain activation measures that differed between groups.
Results
Participant characteristics
In total, 18 participants (mean age 29.9 years, s.d. = 9.7, 100% women) with SPD and 15 age-matched controls (mean age 32.9 years, s.d. = 14.7, 86.7% women) met inclusion criteria and underwent the clinical assessment and functional neuroimaging (Table 1). The SPD group had a mean age at onset of 11.3 years (s.d. = 4.1) and a mean duration of illness of 18.6 years (s.d. = 9.1) years. A total of nine (50%) participants in the SPD group met DSM-5 criteria for another lifetime psychiatric condition: n = 6 major depressive disorder; n = 2 major depressive disorder and other specified anxiety disorder; n = 1 generalised anxiety disorder. Per our exclusion criterion, however, none of the participants had a current psychiatric disorder, other than SPD. Five participants in this group were taking stable doses of psychotropic medications at the time of imaging: three were taking a selective serotonin reuptake inhibitor (SSRI) and two were taking a selective noradrenergic reuptake inhibitor (SNRI). Of the three participants on an SSRI, one was taking citalopram augmented with aripiprazole and another was taking citalopram augmented with buspirone. Of the two participants on an SNRI, this was augmented with bupropion for one participant and with lamotrigine for the other.
Table 1 Characteristics of skin-picking disorder (SPD) and control group

| Baseline | ||||
| Variable | SPD group (n =18) | Control group (n = 15) | t-test | P |
| Age, years: mean (s.d.) | 29.9 (9.7) | 32.9 (14.7) | 0.703 | 0.488 |
| Gender, female, n (%) | 18 (100) | 13 (86.7) | 0.199 a | |
| White, n (%) | 17 (94.4) | 12 (80) | 0.308 a | |
| Marital status, single, n (%) | 13 (72.2) | 10 (66.7) | 1.000 a | |
| Education, n (%) | 0.283 a | |||
| Less than a college degree | 7 (38.9) | 3 (20) | ||
| College degree or higher | 11 (61.1) | 12 (80) | ||
| Right handedness, n (%) | 15 (83.3) | 13 (86.7) | 1.000a | |
| Age at onset, years: mean (s.d.) | 11.3 (4.1) | – | ||
| Any lifetime psychiatric comorbidity, n (%) | 9 (50) | – | ||
| Yale Brown Obsessive Compulsive
Scale Modified for Neurotic Excoriation, total score: mean (s.d.) |
21.9 (4.6) | – | ||
a. Fisher's exact test.
No significant demographic or clinical severity differences were found between those in the SPD group with a history of a lifetime psychiatric disorder and those without such a history (all P>0.010) (age: P = 0.641, age at onset: P = 0.507, NE-YBOCS: P = 0.243) or between those taking a psychotropic medication v. those not taking medication (age: P = 0.108, age at onset: P = 0.510, NE-YBOCS: P = 0.569).
Imaging results
No individually clinically significant MRI structural abnormalities were identified. On the Tower of London task, the SPD group did not differ significantly from the control group in terms of response times (subtract trials: SPD group mean 3.6 s (s.d. = 0.8), control group mean 4.9 s (s.d. = 2.8), P = 0.10; plan trials: SPD group mean 8.4 s (s.d. = 3.0), control group 8.7 s (s.d. = 2.5), P = 0.73). Similarly, the SPD and control groups did not differ significantly in terms of percentage correct on the task (subtract trials: SPD group mean 82.5% (s.d. = 11.0%), control group mean 71.7% (s.d. = 17.7%), P = 0.05; plan trials: SPD group mean 74.9% (s.d. = 19.5%), control group mean 70.2% (s.d. = 20.4%), P = 0.51).
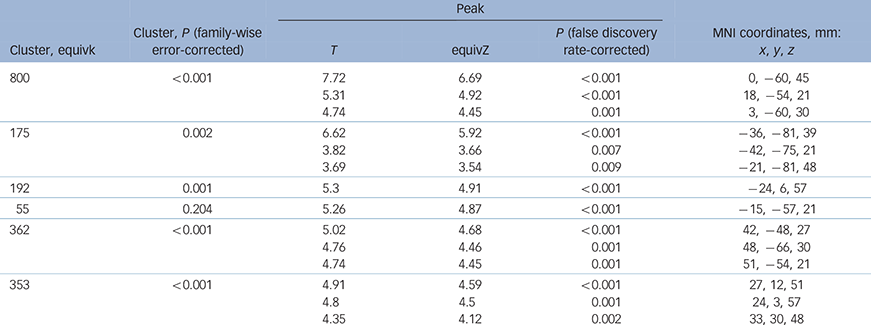
As expected, the Tower of London task activated a network of brain regions during executive planning, including frontal, parietal and striatal brain regions based on the plan minus subtract contrast. In close concordance with previous studies that have used this paradigm, subtracting trials from planning trials rendered a dorsal subset of these regions including dorsolateral prefrontal cortex and posterior parietal cortex (Table 2 and online Fig. DS1). In the ROI (MarsBaR) analysis, no significant activation differences were evident between the two groups within the dorsal planning network (Table 3, P>0.05 uncorrected; Fig. 1).
Table 2 Significant activation across all participants for plan minus subtract contrast, in dorsolateral prefrontal cortex and posterior parietal cortex
| Peak | |||||
|---|---|---|---|---|---|
| Cluster, equivk | Cluster, P
(family-wise error-corrected) |
T | equivZ |
P (false
discovery rate-corrected) |
MNI coordinates, mm: x, y, z |
| 800 | <0.001 | 7.72 | 6.69 | <0.001 | 0, −60, 45 |
| 5.31 | 4.92 | <0.001 | 18, −54, 21 | ||
| 4.74 | 4.45 | 0.001 | 3, −60, 30 | ||
| 175 | 0.002 | 6.62 | 5.92 | <0.001 | −36, −81, 39 |
| 3.82 | 3.66 | 0.007 | −42, −75, 21 | ||
| 3.69 | 3.54 | 0.009 | −21, −81, 48 | ||
| 192 | 0.001 | 5.3 | 4.91 | <0.001 | −24, 6, 57 |
| 55 | 0.204 | 5.26 | 4.87 | <0.001 | −15, −57, 21 |
| 362 | <0.001 | 5.02 | 4.68 | <0.001 | 42, −48, 27 |
| 4.76 | 4.46 | 0.001 | 48, −66, 30 | ||
| 4.74 | 4.45 | 0.001 | 51, −54, 21 | ||
| 353 | <0.001 | 4.91 | 4.59 | <0.001 | 27, 12, 51 |
| 4.8 | 4.5 | 0.001 | 24, 3, 57 | ||
| 4.35 | 4.12 | 0.002 | 33, 30, 48 | ||
Equivk, cluster size of voxels; equivZ, Z-score equivalent of the statistic; MNI, Montreal Neurological Institute.
Table 3 Region of interest (ROI) approach (Marsbar): comparison of activation between the skin-picking disorder group and control group within the frontoparietal network, task minus rest contrast

| ROI (MNI coordinates) | Contrast value | t statistic | Uncorrected P |
|---|---|---|---|
| Left dorsolateral prefrontal cortex (−22,14, 56) | 0.04 | 0.32 | 0.375 |
| Right dorsolateral prefrontal cortex (26, 26, 56) | −0.009 | −0.67 | 0.746 |
| Left posterior parietal cortex (−36, −76, 38) | 0.04 | 0.23 | 0.409 |
| Right posterior parietal cortex (44, −70, 34) | −0.03 | −0.16 | 0.563 |
| Right frontoparietal cortex (28, 50, 2) | 0.05 | 0.36 | 0.359 |
MNI, Montreal Neurological Institute.
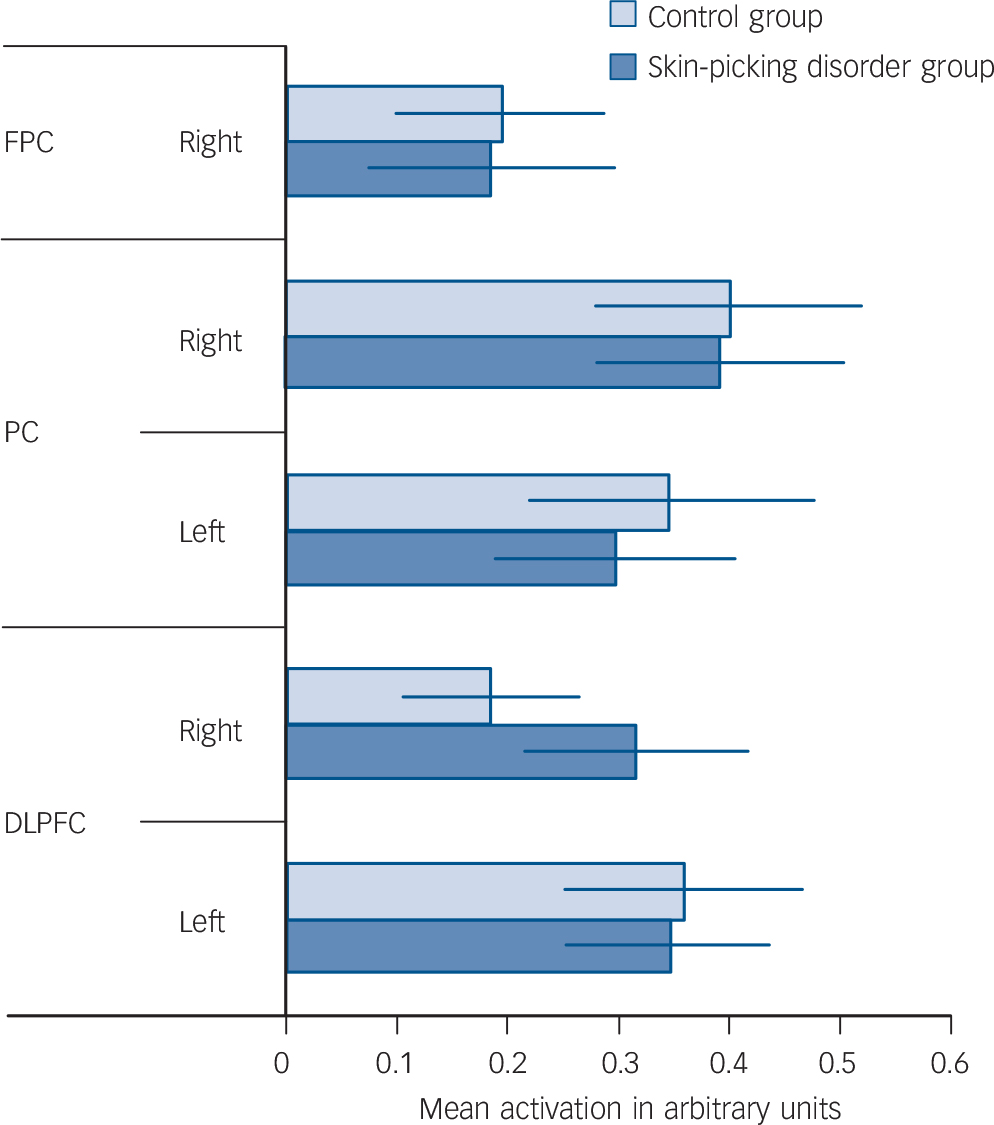
Fig. 1 Tower of London task: task minus rest collapsed across difficulty level.
FPC, frontoparietal cortex right; PC, parietal cortex; DLPFC, dorsolateral prefrontal cortex.
Whole brain analysis indicated that SPD was associated with significant underactivation during the task, compared with the control group, in a single cluster (Table 4 and online Fig. DS2). The cluster was maximal in the right caudate nucleus (14, 24, −8), and included the bilateral caudate, right putamen, bilateral anterior cingulate cortices, bilateral olfactory lobes, and right frontal (superior medial and middle orbital) regions. In exploratory analyses, mean activation in the identified brain cluster did not differ significantly as a function of medication status (P = 0.676) nor as a function of past history of psychiatric comorbidities in the SPD group (P = 0.702).
Table 4 Cambridge Brain Analysis (CamBA) software approach, comparison of activation between the skin-picking disorder and control group, task minus rest contrast

| MNI coordinates | Voxels | |
| Cluster 1 | 1607 | |
| Caudate_R, max −3.937232 | 14, 24, −8 | 72 |
| BA6: Frontal_Sup_Orb_R | 17 | |
| BA21: Olfactory_L | 18 | |
| BA22: Olfactory_R | 69 | |
| BA24: Frontal_Sup_Medial_R | 6 | |
| BA26: Frontal_Mid_Orb_R | 39 | |
| BA28: Rectus_R | 83 | |
| BA31: Cingulum_Ant_L | 9 | |
| BA32: Cingulum_Ant_R | 38 | |
| BA71: Caudate_L | 123 | |
| BA72: Caudate_R | 256 | |
| BA74: Putamen_R | 32 | |
| Space outside regions | 917 | |
MNI, Montreal Neurological Institute; R, right; BA, Brodmann area; Sup, superior; Orb, orbital; L, left; Mid, middle; Ant, anterior.
No significant correlation was found between mean brain activation in the identified cluster and task behavioural measures (P = 0.603); nor between NE-YBOCS total scores and task behavioural measures (both P>0.10). Furthermore, no significant correlation was identified between mean brain activation in the identified cluster and NE-YBOCS total scores (P = 0.211) nor age at symptom onset (P = 0.542).
Since the control group contained two men whereas the SPD group had none, supplementary analyses investigated whether gender had any influence on activation in the identified cluster. Activation in the identified cluster was remarkably similar as a function of gender in the control group and the SPD–control difference in activation remained highly significant even when the two male controls were excluded (online Fig. DS3).
Discussion
Main findings
This study is the first functional imaging study to be conducted in SPD, a fairly common disorder newly formalised in the DSM-5. Using a task of executive planning, no significant differences were found between the SPD and control groups in terms of task performance or brain activation within the neural network typically activated by this cognitive process, using a ROI approach (task minus rest contrast). Using unconstrained analysis across the whole brain (permutation cluster analysis with stringent correction for multiple comparisons, for the contrast of task minus rest), SPD was associated with significant underactivation in distributed neural circuitry including the bilateral dorsal striatum, bilateral anterior cingulate and right frontal regions. These neural abnormalities appeared to be unrelated to symptom severity or age at symptom onset.
Comparison with findings from other studies
Brain imaging has been helpful in furthering our understanding of the potential neurobiological mechanisms involved in other, similar compulsive behaviours such as OCD (see for example Brennan et al Reference Brennan, Rauch, Jensen and Pope33 ). There is an ongoing search in psychiatry for models of the neurobiological circuitry implicated in given disorders. Greater understanding of such circuitry is likely to have ramifications for novel treatments and more appropriate diagnostic classification systems. Reference Borairi and Dougherty34,Reference Tolin, Stevens, Villavicencio, Norberg, Calhoun and Frost35 Although caution is warranted when comparing findings across studies that have used somewhat different methodologies, it is interesting to contrast the intact planning performance we observed in SPD coupled with normal activation in the dorsal planning network according to the ROI analysis, to previous findings of deficient executive planning and hypoactivation in this network in patients with OCD v. controls. Reference van den Heuvel, Veltman, Groenewegen, Cath, van Balkom and van Hartskamp21 Viewed together, these results militate against a primary planning deficit in SPD. Rather, the data suggest that SPD is associated with abnormal function of neural regions outside the usual ‘planning’ network: specifically, as indicated by the permutation cluster analysis, hypoactivation of the dorsal striatum, anterior cingulate cortices and right frontal regions. These regions are anatomically close to those found to be structurally abnormal in previous SPD and also trichotillomania research. Reference Grant, Odlaug, Hampshire, Schreiber and Chamberlain12–Reference Chamberlain, Menzies, Fineberg, Del Campo, Suckling and Craig14 Thus, it appears that SPD is associated not only with structural but also functional brain abnormalities in regions involved in habit generation, action monitoring and top–down inhibitory control processes. Reference Grant, Odlaug, Hampshire, Schreiber and Chamberlain12 In OCD, dysfunction extends from medial to lateral prefrontal networks whereas in SPD, dysfunction may be more restricted to medial sectors of this circuitry.
These results suggest that cognitive fMRI tasks germane to other cognitive processes, such as motor inhibition and habit generation, may shed more light on the pathophysiology of SPD than executive planning tasks. In terms of the state v. trait nature of the currently identified neural abnormalities, we could not detect a significant correlation between activation and disease severity. The study may have been too small to detect such a relationship; alternatively, the hypoactivation could be ‘trait’ in nature: i.e. it may exist in people at risk of SPD even before symptoms develop. This lack of relationship between fMRI measures and disease severity could also theoretically be a consequence of the severity scale measure used. Since the NE-YBOCS (although a standard tool for measuring symptom severity for this disorder) only assesses severity over the past 7 days, it may not be a good reflection of more of a ‘trait’ cognitive dysfunction that arguably underlies SPD. Future work could address this issue by utilising an ‘endophenotyping’ design in which patients are recruited along with clinically unaffected first-degree relatives.
Limitations
The demographics, clinical characteristics and severity of our SPD sample accord well with the characteristics of previously reported samples of patients with SPD, Reference Odlaug and Grant3 suggesting that our sample and findings may be representative of the female SPD population at large. However, several limitations to this current study should be considered. First, the relatively small sample size of both groups limits the power to make any definitive statements about neural processing in SPD. As a result of the limited sample size, we opted for the dual complementary approaches of utilising both an ROI analysis in conjunction with FDR correction (to maximise power and minimise type II error) and a separate whole brain permutation cluster analysis (to evaluate potential abnormalities in an unconstrained fashion, but at potential risk of diminished power). The cluster identified in the permutation analysis appeared to contain some white matter, a finding not uncommon in fMRI studies more broadly (see Gawryluk et al Reference Gawryluk, Mazerolle, Brewer, Beyea and D'Arcy36 for discussion). This could be because of imprecision in the localisation of fMRI signal (particularly with gap slices) in that the regions could nonetheless represent grey matter activation differences; alternatively they may truly reflect abnormal activation associated with white matter, there being evidence elsewhere of structural white matter abnormalities in SPD. Reference Grant, Odlaug, Hampshire, Schreiber and Chamberlain12 White matter findings using fMRI are, however, controversial and potentially problematic to interpret. Second, half of our sample had a lifetime psychiatric history and nearly one-third was taking a stable dose of a psychotropic medication at the time of imaging. This study was neither designed nor powered to address possible influences of medications and comorbidities on brain activation. Nonetheless, exploratory analyses showed that these variables did not significantly have an impact on brain activation in the identified cluster in the SPD group. Future studies will need to formally address this issue with larger sample sizes before firm conclusions are drawn about the observed abnormal activation patterns being entirely attributable to disease rather than potential confounds. Given that people with SPD report high rates of psychiatric comorbidity, Reference Grant, Odlaug, Chamberlain, Keuthen, Lochner and Stein1,Reference Tucker, Woods, Flessner, Franklin and Franklin4 however, we felt that excluding these participants would result in a less-generalisable sample than the SPD population at-large, as well as diminishing power for an already relatively small study for which funding is scarce. Finally, as the control group were, for the most part, recruited in advance of the SPD group, by chance two control participants were male, whereas none of the SPD group were male (and we had anticipated some males in the SPD group). Analysis confirmed, however, that inclusion of these two male controls did not affect the imaging results, in that the SPD–control group difference in activation in the identified cluster remained highly significant even when both male controls were excluded.








eLetters
No eLetters have been published for this article.