Conjugated polymers combine flexibility and ease of processing with good optoelectronic properties which make them ideal for organic devices such as solar cells. Introducing quantum dots as electron acceptors in such devices can improve electron mobility, but blends with polymers are often hampered by phase separation and slow charge transfer at the inorganic–organic interface. In the April edition of Angewandte Chemie International Edition (DOI: 10.1002/anie.201100200; p. 3818), Z. Lin and co-workers at Iowa State University and Georgia Institute of Technology describe a simple “click” reaction for chemically bonding these two materials and facilitating the electronic interaction between them.
Their research employs the widely studied conjugated polymer poly(3-hexylthiophene) (P3HT), and semiconductor nanorods of cadmium selenide. Elongating one dimension of quantum dots to form nanorods has proved to be beneficial in many device applications because of reduced electron conduction pathways and an extended range of absorbed wavelengths. The researchers promote the growth of their CdSe particles into 40-nm long rods by using bromobenzylphosphonic acid as a surface passivating ligand. The acid group binds to the crystal surface while the terminating bromines are left available for further reaction with NaN3 to convert them to azides. These groups can then be coupled to ethynyl-terminated P3HT through a Huisgen cycloaddition reaction which belongs to the direct, catalyst-free, bond-forming reactions of click chemistry. The success of this step was confirmed by proton nuclear magnetic resonance of the coupled ligand and polymer after detaching them from the crystal surface.
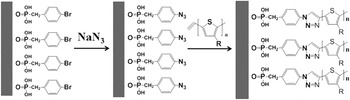
Grafting P3HT onto bromobenzylphosphonic acid functionalized CdSe nanorods by a catalyst-free click reaction. Reproduced with permission from Angewandte Chemie International Edition (DOI: 10.1002/anie.201100200). © 2011 Wiley-VCH Verlag GmbH & Co. KGaA
Electron microscopy of the resulting composite reveals that there is no phase separation, and therefore a much larger interface than for an equivalent physically blended material. Efficient charge transfer between the two materials is evident from almost complete quenching of the polymer fluorescence and much faster fluorescence lifetime for the chemically bound composite.
The use of a cycloaddition to couple conjugated polymers to the surface of a nanocrystal provides an effective alternative to more established ligand-exchange methods, and results in over twice the density of attached polymers. Chemical bonding appears to considerably enhance charge separation at the material interface, and is therefore promising for hybrid solar cells. This research demonstrates the potential of click chemistry when applied to materials, allowing even further scope for chemical tailoring to device applications.


