No CrossRef data available.
Article contents
Impact of crown architecture on light availability, gas exchange, flowering and fruiting in jamun (Syzygium cumini [L.] Skeels)
Published online by Cambridge University Press: 27 November 2024
Abstract
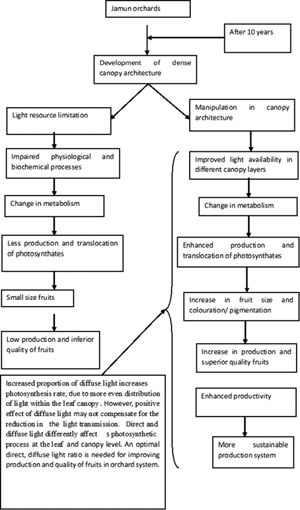
Experiments were conducted to assess the impact of crown architecture on light availability beneath the trees, flowering, fruiting, yield and quality of jamun (Syzygium cumini [L.] Skeels). Trees were maintained as control, palmette and open centre crown. Impact was evaluated for three consecutive years, i.e. 2017–2019. Diffuse light beneath the trees ranged from 69.7 ± 2.22 to 45.9 ± 1.45%, whereas direct light varied from 30.4 ± 0.97 to 54.1 ± 1.78%. At flowering and fruit development stage (June), photosynthesis rate (A) in control trees was 12.5 ± 0.43 μmol CO2/m2/s; however, at fruit maturity and dormancy (August), it was only 9.5 ± 0.35 μmol CO2/m2/s. Similarly, in palmette and open centre trees, photosynthesis rate at flowering and fruit development stage was 13.5 ± 0.46 and 15.7 ± 0.54 μmol CO2/m2/s, respectively; whereas at fruit maturity and dormancy, photosynthesis rate dropped to 10.5 ± 0.39 and 11.7 ± 0.43 μmol CO2/m2/s, respectively. Substantial variation in stomatal conductance (gs), vapour pressure deficit (VPD) and transpiration rate (E) was also found. Days to start flowering ranged from 92 ± 0.33 to 98 ± 0.33. Similarly, days to end flowering varied from 99 ± 0.07 to 107 ± 0.36, days to fruit set 132 ± 0.33 to 139 ± 0.33 and days to fruit maturity 176 ± 0.48 to 184 ± 0.63. Significant variation in fruit length, fruit width and fruit weight was also found. Total soluble solids in fruit pulp varied from 9.0 ± 0.15 to 12.2 ± 0.149°Brix and fruit yield 62.3 ± 1.5 to 86.7 ± 1.33 kg per tree. Noteworthy variation in fruit quality traits was also recorded. This study illustrates that crown architecture has considerable impact on gas exchange parameters, flowering, fruiting, yield and quality of jamun.
- Type
- Crops and Soils Research Paper
- Information
- Copyright
- Copyright © INDIAN COUNCIL OF AGRICULTURAL RESEARCH, 2024. Published by Cambridge University Press



