Despite the high water content of the human body, it is necessary to maintain water levels within a relatively narrow range in order to avoid the adverse health consequences that are associated with both hyperhydration and hypohydration(Reference Greenleaf1). Significant reductions in body water content occurring as a result of disease or from excessive sweating during exercise should be replaced promptly and effectively. While it is accepted that full replacement of water losses requires ingestion of a volume greater than that lost along with replacement of any Na losses(Reference Shirreffs, Taylor and Leiper2), the maintenance of fluid balance is affected by the composition of an ingested solution. Current advice is similar for rehydration following water losses that result from disease(Reference Farthing3, Reference Leiper4) and exercise(Reference Sawka, Burke and Eichner5). This includes ingestion of hypotonic glucose–Na solutions, as the addition of small quantities of carbohydrate and Na assists in the rapid assimilation of ingested water into the extracellular fluid.
Shafiee et al. (Reference Shafiee, Charest and Cheema-Dhadli6) have reported that reducing the overall rate of fluid absorption by reducing the rate at which water was ingested was beneficial in terms of maintaining fluid balance due to the avoidance of a large reduction in the plasma Na concentration, which would lead to a diuresis. In addition, a recent study has shown that restoration of water balance following moderate levels of dehydration induced by exercise in the heat was achieved more effectively by the ingestion of a hypertonic 10 % glucose–electrolyte solution than by the ingestion of the same volume of a hypotonic 2 % glucose–electrolyte solution and an electrolyte-only solution(Reference Evans, Shirreffs and Maughan7). The greater efficacy of the hypertonic 10 % glucose–electrolyte solution was attributed to the avoidance of large reductions in serum osmolality and increases in plasma volume that resulted from the rapid absorption of the other drinks: these changes would cause suppression of circulating vasopressin concentrations and lead to diuresis. Another recent study by Osterberg et al. (Reference Osterberg, Pallardy and Johnson8) has demonstrated that the addition of carbohydrate to a rehydration solution resulted in an enhanced retention of fluid over a 4 h recovery period when compared with a placebo. Although fluid retention was not different between 3, 6 or 12 % carbohydrate solutions, ingestion of the 12 % carbohydrate solution resulted in greater fluid retention than a placebo–electrolyte solution. It should be noted that the participants in this study ingested a volume of fluid equivalent to 100 % body mass lost during exercise over a 60 min period.
Fluid uptake is dependent on the combined rates of gastric emptying and intestinal absorption. Reducing the rate at which fluid is emptied from the stomach and/or reducing the rate at which water is absorbed in the intestine will therefore reduce the overall rate of fluid uptake.
The rate of gastric emptying of liquids is affected by a number of factors. Of these, the volume of fluid in the stomach seems to be of most importance(Reference Noakes, Rehrer and Maughan9). The composition, and more specifically, the energy content of an ingested solution also seems to be of importance. Vist & Maughan(Reference Vist and Maughan10) observed that increasing the energy density of a solution by the addition of varying amounts of carbohydrate resulted in a reduction in the rate of gastric emptying. A subsequent study by the same authors(Reference Vist and Maughan11) showed that the gastric emptying rate was reduced at high solution osmolalities when energy density remained constant.
Intestinal absorption of water is a passive process governed by osmotic gradients. Consequently, the osmolality of an ingested solution is an important factor in determining the rate and direction of water movement within the intestine. Shi et al. (Reference Shi, Summers and Schedl12) demonstrated that perfusion of a hypotonic carbohydrate solution resulted in greater net water absorption than perfusion of a hypertonic carbohydrate solution. Leiper & Maughan(Reference Leiper and Maughan13) reported that perfusion of a hypertonic solution resulted in net secretion of water and electrolytes into the lumen, whereas perfusion of an isotonic glucose–electrolyte solution promoted water and electrolyte uptake. A recent study(Reference Evans, Shirreffs and Maughan14) has shown that ingestion of a 10 % glucose solution with an osmolality of 565 (sd 5) mOsm/kg resulted in an acute reduction in plasma volume, most likely due to the net secretion of water from the extracellular fluid into the intestinal lumen.
The current advice on ingesting hypotonic carbohydrate–electrolyte solutions during rehydration is based on observations that such solutions are emptied rapidly from the stomach into the intestine(Reference Vist and Maughan10, Reference Vist and Maughan11) and are rapidly absorbed in the intestine due to their low osmolality(Reference Shi, Summers and Schedl12, Reference Leiper and Maughan13). In addition, the rate of water uptake in the intestine is enhanced in the presence of carbohydrate and Na due to the active co-transport from the intestinal lumen(Reference Schedl, Maughan and Gisolfi15). These factors lead to a rapid increase in plasma volume(Reference Shi, Summers and Schedl12, Reference Evans, Shirreffs and Maughan14). However, the rapid appearance of ingested water in the circulation can lead to an increased urine output due to acute reductions in circulating fluid retention hormone concentration(Reference Nose, Mack and Shi16, Reference Nose, Mack and Shi17), which would be considered undesirable for the maintenance of fluid balance. It seems reasonable to suggest that reducing the overall rate of fluid uptake by reducing the rate of gastric emptying and/or reducing the rate of intestinal absorption may be beneficial in preventing a prompt diuresis and thus maintaining a fluid balance for a prolonged period of time. However, it is currently unclear whether reducing the rate of gastric emptying or reducing the rate of intestinal absorption is the more important factor in this process.
The aim of the present study was to evaluate fluid absorption characteristics of a hypotonic 2 % glucose–electrolyte and hypertonic 10 % glucose–electrolyte solution following repeated fluid ingestion.
Methods
A total of three male and five female subjects (mean age 26 (sd 6) years, height 170 (sd 10) cm, body mass 66 (sd 9) kg) volunteered to take part in the present investigation. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Loughborough University Ethics Advisory Committee (reference no. R06/P96). Written informed consent was obtained from all subjects.
The subjects were familiarised with the study protocol before beginning the experimental trials. The subjects orally inserted a 14 g gastric tube before the stomach was emptied and washed with distilled water. A recovery test was performed, which involved the instillation of 100 ml of distilled water before the stomach contents were mixed by removing and immediately aspirating between 30 and 50 ml of the stomach contents at least ten times. The stomach contents were subsequently removed. The gastric tube was considered to be appropriately positioned at the base of the stomach, if 80–110 ml were removed. Finally, the subjects drank 500 ml of water to ensure that they were able to drink with the gastric tube in position. Since one subject was unable to drink with the tube in position, the test solutions were instilled via the gastric tube for this subject during the experimental trials.
Each subject participated in two experimental trials that involved ingestion of a solution with a different carbohydrate content and osmolality. Solutions contained glucose, at final concentrations of 2 and 10 %, Na and flavouring; the measured osmolalities were 189 (sd 3) and 654 (sd 3) mOsm/kg, respectively. The composition of each solution is outlined in Table 1. The trials were separated by a period of at least 7 d and began at the same time of day. The subjects followed similar physical activity and nutritional intake patterns in the 24 h before the start of each trial. They were instructed to ingest 500 ml of water approximately 1 h before the start of the fluid restriction period during each trial in an effort to maintain an adequate and consistent state of euhydration.
Table 1 Composition of test drinks used*
(Mean values and standard deviations)
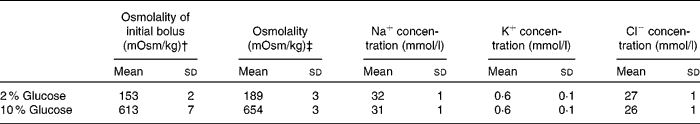
* Drinks contained distilled water, glucose, lemon flavouring and 25 mm NaCl.
† ‘Osmolality of the initial bolus’ refers to the osmolality of the first bolus’ of the test solution containing 10 g 2H2O.
‡ ‘Osmolality refers to the osmolality of the subsequent aliquots of test solution that contained no 2H2O.
The subjects arrived at the laboratory in the evening and provided a urine sample before nude body mass was measured to the nearest 10 g (Adam Equipment, Milton Keynes, UK). A cheese and tomato pizza was provided and the subjects ate ad libitum during the first experimental trial. The same amount of pizza (430 (sd 178) g) was consumed during the second experimental trial. During each trial, 201 (sd 2) ml of water was provided. The subjects left the laboratory after being instructed to refrain from eating or drinking anything further until arrival at the laboratory the following morning.
The subjects returned to the laboratory 13 h after the initial measurement of body mass. A urine sample was obtained before the measurement of nude body mass. The subjects inserted a gastric tube before the stomach was emptied, washed and the recovery test was performed. The subjects remained seated for a period of 15 min during which time a 21 g cannula was inserted into a vein in the back of the hand. The cannula remained in position for the duration of the trial and was kept patent between sample collections by flushing with heparinised isotonic saline. We collected two 7 ml blood samples over a 5 min period. The stomach was emptied once again during this time. Following the collection of the second resting blood sample, the subject was given 1 minute to ingest 495 ml of the test solution. This initial bolus of the test solution contained 10 g of 2H2O and 50 mg/l phenol red. Further boli of the test solution were provided 15, 30 and 45 min after the initial bolus and were ingested over periods of 1, 5 and 5 min, respectively. These boli contained no 2H2O or phenol red. Total drink volume amounted to 3 % of initial body mass during the first experimental trial. The subjects ingested 494 (sd 90), 496 (sd 94) and 496 (sd 90) ml during the 2nd, 3rd and 4th boli, respectively. Blood samples (7 ml) were obtained 2, 5, 10, 15, 20, 30, 45, 60, 75, 90, 105 and 120 min after the ingestion of the first bolus of test solution. A urine sample was obtained at the end of the trial after the stomach had been emptied. The subjects remained seated in a comfortable environment (24·5 ± 1·4°C) for the duration of the trial. Subjects were seated in an upright position in order to avoid the previously reported postural differences in blood and plasma volume(Reference Hagan, Diaz and Horvath18, Reference Shirreffs and Maughan19), and the hand was immersed in hot water (>42°C) in an attempt to ensure arterialisation of the collected blood samples.
Gastric volumes were measured at 15 min intervals throughout the experimental protocol using a modified double sampling technique of George(Reference George20) by Beckers et al. (Reference Beckers, Rehrer and Brouns21). A sample of the initial bolus of the test solution was retained for analysis. Following the ingestion of the initial bolus, the stomach contents were mixed by removing and immediately reinjecting between 30 and 50 ml of fluid ten times. A sample of the stomach contents was retained. The residual volume present in the stomach before ingestion of the initial bolus of test solution was calculated from the change in dye concentration between the phenol red concentration of the test drink and the phenol red concentration of the stomach contents immediately after drinking. At each sampling point, the stomach contents were mixed as described previously, and a sample retained before a small volume of a phenol red solution was added to the stomach contents. The stomach contents were then mixed again and a further sample was retained. From the changes in phenol red concentrations, the total volume of fluid and the volume of the test drink remaining in the stomach can be calculated (Beckers et al. (Reference Beckers, Rehrer and Brouns21)). The difference between these two values corresponds to the volume of gastric secretions produced in the test period. The concentration of phenol red solution added to the stomach was increased throughout the trial: 5 ml of 0·5 g/l phenol red were added at time points 15, 30 and 45; 2·5 ml of 2·0 g/l phenol red were added at time points 60 and 75; 5 ml of 2·0 g/l phenol red were added at time points 90, 105 and 120.
Sample analysis
Blood samples were analysed for Hb concentration by the cyanmethaemoglobin method, packed cell volume by microcentrifugation and glucose concentration by the glucose oxidase peroxidase amino-antipyrine phenol (GOD-PAP) method (Randox, Crumlin, UK). Hb and packed cell volume values were used to estimate the percentage of changes in blood, erythrocytes and plasma volumes as described by Dill & Costill(Reference Dill and Costill22).
Blood, gastric contents and drink 2H concentration were analysed by separating water from samples by vacuum distillation before 2H was measured using infrared spectrophotometry as described previously by Lukaski & Johnson(Reference Lukaski and Johnson23).
An aliquot of whole blood was centrifuged at 1500 g for 15 min at 4°C before the serum was removed and kept for analysis of osmolality by freezing point depression (Gonotec Osmomat 030 Cryoscopic Osmometer; Gonotec, Berlin, Germany) and Na concentration by flame photometer (Corning Clinical Flame Photometry 410C; Corning Limited, Halstead, Essex, UK).
Gastric aspirate and drink samples were analysed for phenol red concentration by spectrophotometry after dilution (1:20) with NaOH–NaHCO3 (200:500 mmol/l) buffer.
Urine and gastric samples were analysed for osmolality using the method described earlier. Drink samples were analysed for potassium concentration by flame photometry, and chloride concentration was analysed by coulometric titration (J Chloride Meter, Jenway Limited, Dunmow, Essex, UK).
All analyses were performed in duplicate, with the exception of the packed cell volume measurements, which were made in triplicate.
Statistical analysis
All datasets were found to be normally distributed using the Kolmogorov–Smirnov test and are, therefore, presented as means and standard deviations.
To evaluate the differences between trials, two-factor repeated-measures ANOVA was used. To analyse changes in one variable over time, one-way ANOVA followed by Dunnett's pair-wise comparisons was used for parametric post hoc tests. t-Tests were used to locate differences between trials. Level of significance was taken as P < 0·05, and this level of significance applies unless otherwise stated.
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) for Windows.
Results
The subjects were adequately hydrated before the 13 h fluid restriction period as urine osmolality was 241 (sd 193) and 350 (sd 150) mOsm/kg on the 2 and 10 % glucose trials, respectively. This was not different between trials (P = 0·206). The 13 h overnight fluid restriction period induced a similar (P = 0·244) reduction in body mass on both trials amounting to 0·6 (sd 0·3) kg.
No difference between trials in Hb concentration, packed cell volume, blood glucose concentration, serum Na concentration or serum osmolality was observed for either of the pre-ingestion samples. No difference in Hb concentration, packed cell volume, serum Na concentration or serum osmolality was observed between the two pre-ingestion blood samples on either trial. Blood glucose concentration of the two pre-ingestion samples during the 2 % glucose trial was similar, but the glucose concentration of the second pre-ingestion blood sample (6·0 (sd 0·1) mmol/l) on the 10 % glucose trial was slightly greater (P = 0·018) than the first pre-ingestion blood sample (5·8 (sd 0·2) mmol/l). The second pre-ingestion blood sample was used as a baseline value as this was collected immediately before drinking.
Gastric volumes
Residual gastric volume amounted to 28 (sd 21) ml and was not different between trials (P = 0·723). The calculated total volume of fluid in the stomach at the end of the trial was the same as the volume of fluid manually emptied from the stomach at the end of the trial (2 %: P = 0·098; 10 %: P = 0·390). The total volume of fluid in the stomach was greater during the 10 % glucose trial than on the 2 % glucose trial from 30 min after ingestion of the initial bolus of the test solution until the end of the experimental protocol (Fig. 1). This pattern was the same for the volume of test meal present in the stomach (Fig. 2) and for the 2H concentration of the gastric aspirate. The total volume of gastric secretions varied greatly between individuals but tended to be greater on the 10 % glucose trial (1395 (sd 685) ml) than on the 2 % glucose trial (744 (sd 350) ml; P = 0·084).

Fig. 1 Total volume of fluid in stomach (ml) during the 2 % (–□–) and 10 % (–▲–) glucose trials. Values are means, with standard deviations represented by vertical bars. *Mean values were significantly different on the 10 % glucose trial than on the 2 % glucose trial (P < 0·05). Drinking periods started at 0, 15, 30 and 45 min.

Fig. 2 Volume of test drink in stomach (ml) during the 2 % (–□–) and 10 % (–▲–) glucose trials. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different on the 10 % glucose trial than on the 2 % glucose trial (P < 0·05). Drinking periods started at 0, 15, 30 and 45 min.
The total amount of glucose delivered to the intestine was greater during the 10 % glucose trial than on the 2 % glucose trial (P < 0·001) and amounted to 105 (sd 24) and 38 (sd 6) g, respectively.
Blood 2H concentration
Blood 2H concentration increased from baseline levels 10 min after ingestion of the first bolus of test solution on both trials and remained elevated for the duration of the protocol. Blood 2H concentration was significantly greater on the 2 % glucose trial than on the 10 % glucose trial from 10 min after ingestion of the initial bolus of test solution until the end of the experimental protocol with the exception of 105 min, when a tendency (P = 0·057) for blood 2H concentration to be greater on the 2 % glucose trial was observed (Fig. 3).

Fig. 3 Blood 2H concentration (parts-per-million, ppm) during the 2 % (–□–) and 10 % (–▲–) glucose trials. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different on the 2 % glucose trial than on the 10 % glucose trial (P < 0·05). Drinking periods started at 0, 15, 30 and 45 min.
The maximum blood 2H concentration (C max) was greater (P = 0·005) and the time until C max was achieved (T max) was faster (P = 0·011) during the 2 % glucose trial than on the 10 % glucose trial. Consequently, the rate of blood 2H accumulation (slope) was greater (P = 0·005) during the 2 % glucose trial than on the 10 % glucose trial (Table 2).
Table 2 Maximum blood 2H concentration (C max), time to C max (T max) and rate of blood 2H accumulation (slope) during 2 and 10 % glucose trials
(Mean values and standard deviations)

ppm, parts-per-million.
Changes in blood, erythrocytes and plasma volume
Blood volume was greater during the 2 % glucose trial than on the 10 % glucose trial from 30 to 105 min after ingestion of the initial bolus of test solution. Plasma volume was greater during the 2 % glucose trial than on the 10 % glucose trial from 20 min after ingestion of the initial bolus of test solution until the end of the experimental protocol. No differences in blood volume from baseline levels were observed on either trial, but plasma volume was elevated from baseline levels 75 min after ingestion of the initial bolus of the test solution on the 2 % glucose trial and decreased from baseline levels 60 and 75 min after ingestion of the initial bolus of the test solution on the 10 % glucose trial (Fig. 4). No differences between trials or over time were observed in erythrocytes volume on either trial.

Fig. 4 Percentage of change in plasma volume during 2 % (–□–) and 10 % (–▲–) glucose trials. Values are means, with standard deviations represented by vertical bars. * Mean values were significantly different on the 2 % glucose trial than on the 10 % glucose trial (P < 0·05). Mean values were significantly different from baseline levels 60 min after ingestion of initial bolus on the 10 % glucose trial (†) and at 75 min on both trials (‡; P < 0·05). Drinking periods started at 0, 15, 30 and 45 min.
Serum measurements
Pre-ingestion serum osmolality was not different between trials and amounted to 281 (sd 8) and 282 (sd 7) mOsm/kg on the 2 and 10 % glucose trials, respectively. Serum osmolality was greater during the 10 % glucose trial than on the 2 % glucose trial from 15 min after ingestion of the initial bolus of the test solution until the end of the experimental protocol. The average serum osmolality from 15 min after ingestion until the end of the trial was 274 (sd 7) and 283 (sd 6) mOsm/kg during the 2 and 10 % glucose trials, respectively. No differences from baseline levels were observed on either trial. Pre-ingestion serum Na concentration was not different between trials and amounted to 142 (sd 3) and 141 (sd 1) mmol/l on the 2 and 10 % glucose trials, respectively. Serum Na concentration was greater during the 10 % glucose trial than on the 2 % glucose trial 75 and 90 min after ingestion of the initial bolus of the test solution. Serum Na concentration was reduced from baseline levels 90 min after ingestion of the initial bolus of the test solution during the 2 % glucose trial.
Gastric aspirate osmolality
Gastric aspirate osmolality was greater during the 10 % glucose trial than on the 2 % glucose trial for the whole experimental protocol. During the 2 % glucose trial, gastric aspirate osmolality increased relative to the osmolality of the initial bolus from 30 min after ingestion until the end of the trial. During the 10 % glucose trial, gastric aspirate osmolality decreased relative to the osmolality of the initial bolus immediately after drinking until the end of the trial.
Blood glucose
Pre-ingestion blood glucose concentration was not different between trials and amounted to 5·9 (sd 0·5) and 6·0 (sd 0·1) mmol/l on the 2 and 10 % glucose trials, respectively. Blood glucose concentration was elevated from baseline levels 20, 30, 45, 60 and 75 min after ingesting the first bolus during the 2 % glucose trial and elevated from 15 min after ingesting the first bolus during the 10 % glucose trial until the end of the trial. Blood glucose concentration was greater during the 10 % glucose trial than on the 2 % glucose trial 15, 20, 30, 75, 90, 105 and 120 min after ingestion of the initial bolus of the test solution.
Urine measurements and free water clearance
Urine volume and osmolality were similar between trials before and after the 13 h fluid restriction period. Following the trials, urine volume was greater (2 %, 741 (sd 221) ml; 10 %, 413 (sd 293) ml; P = 0·006) and urine osmolality was lower (P = 0·024) during the 2 % glucose trial than on the 10 % glucose trial.
Free water clearance at the end of the trials was calculated using the equation
where V is the urinary flow rate, U osm is the urine osmolality and S osm is serum osmolality. Free water clearance was greater on the 2 % glucose trial than on the 10 % glucose trial (P = 0·011).
Discussion
The present results suggest that the reduced rate of fluid uptake observed following repeated ingestion of a 10 % glucose–electrolyte solution when compared with a 2 % glucose–electrolyte solution is due primarily to a reduction in the gastric emptying rate rather than a reduction in the rate of intestinal absorption. However, during the first 15 min after ingestion, it would seem that the rate of intestinal absorption was a limiting factor in fluid uptake following ingestion of the 10 % glucose–electrolyte solution.
The present study included a period of 13 h of fluid restriction before fluid ingestion in order to induce a moderate level of hypohydration. This 13 h period of fluid restriction resulted in a reduction in body mass of 0·60 (sd 0·30) kg, which is equivalent to a reduction of 0·89 (sd 0·38) % of the initial body mass. Urine osmolality following the 13 h fluid restriction period was 887 (sd 98) mOsm/kg. These changes are consistent with those reported by Shirreffs et al. (Reference Shirreffs, Merson and Fraser24) who observed similar reductions in body mass and increases in urine osmolality following a 13 h period of fluid restriction. The magnitude of change in body mass and measured urine osmolality would suggest that the participants were moderately dehydrated before drinking, and there is a stronger rationale for investigating fluid replacement from a situation of hypohydration rather than euhydration.
Vist & Maughan(Reference Vist and Maughan11) reported that a single 600 ml bolus of a dilute (40 g/l) glucose solution emptied from the stomach in an exponential manner, while the same volume of a concentrated (188 g/l) glucose solution emptied from the stomach in a linear manner. In the present study, the gastric emptying characteristics of the 10 % glucose–electrolyte solution during the second hour followed a linear pattern, whereas the 2 % glucose–electrolyte solution followed an exponential trend as expected. During the final 60 min of the present study, 45 (sd 10) % of the test drink was emptied from the stomach every 15 min on the 2 % glucose trial, whereas 11 (sd 6) % was emptied from the stomach on the 10 % glucose trial.
During the first 60 min, the subjects ingested a total of 1968 (sd 274) ml. At the end of the second 60 min, the calculated volume of test meal remaining in the stomach was 98 (sd 79) ml during the 2 % glucose trial and 928 (sd 264) ml during the 10 % glucose trial. This equates to 4 (sd 4) and 46 (sd 10) % of the total volume of the test drinks during the 2 and 10 % glucose trials remaining in the stomach at the end of the experimental protocol with the rest having emptied from the stomach into the intestine for absorption. As has been observed by earlier authors(Reference Vist and Maughan11, Reference Costill and Saltin25), gastric secretions tend to be greater following ingestion of hypertonic solutions. In the present study, gastric secretions were greater during the 10 % glucose trial than during the 2 % glucose trial, although this was not deemed to be significant (P = 0·084). An important consideration is that, as the rate of gastric emptying was slower during the 10 % glucose trial, these secretions will mix with the stomach contents, whereas during the 2 % glucose trial gastric secretions will be emptied into the intestine due to the relatively fast rate of gastric emptying.
The concentration of 2H in the blood following ingestion of the test solutions containing the label showed an initial phase of rapid accumulation followed by a plateau(Reference Davis, Burgess and Slentz26, Reference Maughan, Leiper and Vist27). This pattern was observed in the present study during both trials. Blood 2H concentration increased from baseline levels 10 min after ingestion of the first bolus of the test solution, indicating a significant rate of fluid absorption during both trials. The rate of fluid absorption, as determined by the time taken to reach maximum blood 2H concentration, was greater during the 2 % glucose trial than on the 10 % glucose trial. The reduced rate of blood 2H accumulation during the 10 % glucose trial is likely to be due to the reduced rate of gastric emptying on this trial. However, it is interesting to observe that blood 2H concentration was greater during the 2 % glucose trial than on the 10 % glucose trial 10 min after ingestion of the initial bolus of the test solution, but the volume of fluid and test drink in the stomach at 15 min was not different. This would suggest that in the early stages of the present study, fluid absorption was limited by the rate of intestinal absorption rather than the rate of gastric emptying. The present results are in agreement with those of Murray et al. (Reference Murray, Bartoli and Eddy28) who observed that, following ingestion of a single bolus of the solution during exercise, plasma accumulation of 2H2O was significantly slower when a solution containing 20 % glucose was ingested than when a 6 % glucose solution or water was ingested. This difference was attributed, in part, to a reduced rate of gastric emptying.
Previous investigations(Reference Leiper and Maughan13) have shown that perfusion of the intestine with a hypertonic solution results in a net secretion of water and electrolytes into the intestinal lumen. Subsequent investigations(Reference Evans, Shirreffs and Maughan14) have suggested that ingestion of hypertonic solutions results in a transient reduction in plasma volume, which is likely due to the net secretion of water into the intestine. In the present study, as the rate of gastric emptying during the initial 15 min of the protocol was similar during both trials, the lower blood 2H concentration during the 10 % glucose trial than during the 2 % glucose trial may be ascribed to the low rate of intestinal absorption due to the movement of water from the extracellular fluid into the intestinal lumen.
The relatively slow rate of fluid uptake during the 10 % glucose trial compared with the 2 % glucose trial resulted in a lower volume of urine being excreted at the end of the trial. Free water clearance provides an indication of circulating concentrations of arginine vasopressin. Calculation of free water clearance in the present study suggests that the lower urine output observed during the 10 % glucose trial is likely due to greater circulating concentrations of arginine vasopressin. Further evidence for this can be found when looking at the serum osmolality data presented. Plasma osmolality has a profound influence on circulating arginine vasopressin concentrations(Reference Baylis29) and, therefore, the reabsorption of water in the nephron. As serum osmolality was significantly lower on the 2 % glucose trial than on the 10 % glucose trial throughout the experimental protocol, the lower urine output at the end of the 10 % glucose trial is likely due to higher circulating concentrations of arginine vasopressin.
Previous investigations have shown that hypertonic glucose–electrolyte solutions are effective in maintaining fluid balance when a fixed volume of fluid is ingested following exercise-induced dehydration(Reference Evans, Shirreffs and Maughan7, Reference Osterberg, Pallardy and Johnson8) and when ad libitum fluid is consumed(Reference Evans, Shirreffs and Maughan30). The present results suggest that the success of these solutions in maintaining fluid balance is due to a reduced overall rate of fluid uptake and, in particular, due to a relatively slow rate of gastric emptying. However, it should be noted that ingestion of hypertonic solutions does lead to secretion of water into the intestinal lumen, which may not be considered ideal in situations where rapid restoration of plasma volume is the main objective. This has potential implications for the addition of other substrates to the rehydration solutions.
In conclusion, the present results suggest that repeated ingestion of a 10 % glucose–electrolyte solution with an osmolality of 654 (sd 3) mOsm/kg resulted in a slower rate of fluid uptake than ingestion of a 2 % glucose–electrolyte solution with an osmolality of 189 (sd 3) mOsm/kg. During the early stages following drinking, this was largely due to a slower rate of intestinal absorption, but the relatively slow rate of gastric emptying following ingestion of the 10 % glucose solution is likely to be the main reason for the reduced rate of fluid uptake over the duration of the experimental period.
Acknowledgements
None of the authors has any conflict of interest to report. G. H. E. was supported by a Loughborough University PhD studentship. There was no other funding for the present study. G. H. E. and S. M. S. contributed to data collection. All authors contributed to the study design, data interpretation and manuscript writing.








