The longitudinal course of patients with late-onset schizophrenia remains uncertain, with studies reporting markedly increased (Reference HoldenHolden, 1987; Reference Craig and BregmanCraig & Bregman, 1988), intermediate (Reference Jorgensen and Munk-JorgensenJorgensen & Munk-Jorgensen, 1985) or normal rates of ‘organic deterioration’, cognitive decline or dementia (Reference Palmer, Bondi and TwamleyPalmer et al, 2003). Limitations of these studies of late-onset disease include the diversity in schizophrenia diagnoses, the lack of standard criteria for dementia diagnosis, and the short duration of follow-up. Whether people with late-onset schizophrenia, meeting strict criteria, have a high risk of developing dementia – and if so, which type – is of much clinical interest. We have previously reported cross-sectional data on a sample of 27 people with DSM–III–R-defined schizophrenia (American Psychiatric Association, 1987) with onset of illness at 50 years of age or later (Reference Brodaty, Sachdev and RoseBrodaty et al, 1999; Sachdev et al, Reference Sachdev, Brodaty and Rose1999, Reference Sachdev, Brodaty and Cheang2000). The present study reports the 5-year follow-up data on our original sample of late-onset schizophrenia patients and normal controls, including levels of dementia, current psychosis and functional ability.
METHOD
Sample
Patients with a known diagnosis of late-onset schizophrenia were recruited between 1992 and 1994 from local mental health services. The study was approved by several institutional research ethics committees. After complete description of the study to the participants, written informed consent was obtained. All late-onset schizophrenia patients met DSM–III–R criteria for schizophrenia, as determined independently by two psychiatrists using all available data. Late-onset schizophrenia was defined as schizophrenia with age of onset at or after the age of 50 years, including the prodromal phase, as confirmed by an informant. Normal controls were volunteers without a history of psychiatric illness recruited through advertisements in senior citizens’ clubs and older women's networks. All participants were White and were competent in the English language. The following led to exclusion:
-
(a) history of injected drug or alcohol misuse of 5 years or more, or of any duration within 5 years of the study;
-
(b) history of stroke, transient ischaemic attack, epilepsy, Parkinson's disease, other diagnosable brain disease or head injury with loss of consciousness for more than 30 min or with neurological sequelae;
-
(c) a score of less than 20 on the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975);
-
(d) learning difficulties;
-
(e) worse than mild tardive dyskinesia;
-
(f) current major depression or mania;
-
(g) lack of corroborative history.
A low MMSE threshold was set because of the effects of psychosis on test results. Results were reanalysed for subsamples of participants with higher baseline MMSE thresholds.
Of 112 participants assessed, 21 were excluded, leaving 27 late-onset schizophrenia patients (mean age of onset 66.4 years, range 50–87), 30 patients with early-onset disease (whose results are not included in this paper) and 34 normal controls (details described by Reference Brodaty, Sachdev and RoseBrodaty et al, 1999). One late-onset schizophrenia patient attained a score of 19 on the MMSE, but this was considered to be an underestimate. This patient was included in the study, and scored 23 at reassessment a year later, supporting the contention that the poor initial performance was due to psychosis rather than underlying cognitive disorder. The psychiatric, neurological, neuropsychological and magnetic resonance imaging (MRI) characteristics of the study sample have been reported by Sachdev et al (Reference Sachdev, Brodaty and Rose1999, Reference Sachdev, Brodaty and Cheang2000).
The late-onset schizophrenia and normal control groups were comparable on age, gender and socio-economic status at baseline. However, the patients had significantly fewer years of education (U z =-3.48, P=0.001) and were significantly more likely to have never been married (Yates's continuity correction CCχ2=6.53, d.f.=1, P=0.008) than the controls.
Assessments
The following standardised instruments were employed at 5-year follow-up: Global Assessment of Functioning (GAF; American Psychiatric Association, 1987), Instrumental Activities of Daily Living (IADL; Reference Lawton and BrodyLawton & Brody, 1969), Activities of Daily Living (ADL; Reference Katz and ApkonKatz & Apkon, 1976), Clinical Dementia Rating (CDR; Reference Hughes, Berg and DanzigerHughes et al, 1982), MMSE (Reference Folstein, Folstein and McHughFolstein et al, 1975), Cognitive Decline Scale (Reference Jorm, Mackinnon and HendersonJorm et al, 1995; a 10-item informant-rated scale, assessing aspects of cognitive decline), Cambridge Mental Disorders of the Elderly Examination (CAMDEX; Reference Roth, Tym and MountjoyRoth et al, 1986) and the Hachinski Ischaemia Scale (Reference Hachinski, Iliff and ZilkhaHachinski et al, 1975). A neurological examination and MRI scan were performed at baseline assessment (details described by Reference Sachdev, Brodaty and RoseSachdev et al, 1999).
Diagnosis of schizophrenia at 5-year follow-up was based on DSM–IV criteria (American Psychiatric Association, 1994). Current psychopathological symptoms were assessed using the Brief Psychiatric Rating Scale (Reference Overall and GorhamOverall & Gorham, 1962), an 18-item scale with each item rated from 0 (not present) to 6 (extremely severe). Diagnosis of dementia at 5-year follow-up was based on DSM–IV criteria, using information from the CDR, MMSE, Cognitive Decline Scale and items from the CAMDEX. This was reached by consensus in case conferences involving two psychiatrists experienced in diagnosing dementia (H.B. and P.S.) and a research psychologist. Participants’ identifying information was excluded from these conferences. Patients were considered to be diagnosable as having mild cognitive impairment at baseline if their score on the Logical Memory I or II subtest of the Wechsler Memory Scale (Reference WechslerWechsler, 1981) was 1.5 standard deviations below the Age Scale score.
Method of follow-up
Participants were assessed 1 year and 5 years after the baseline investigation. They were located by telephone and written contact, directly or through their next of kin, or were traced through the electoral roll (in Australia, registration on the electoral roll is compulsory). Death certificates and medical notes were obtained where possible for those who had died. Follow-up interviews with participants and/or their informants were conducted by a physician. The next of kin of those who had died were interviewed where possible.
Data analysis
The Statistical Package for the Social Sciences (SPSS) version 9 for Windows was used for all statistical analyses (SPSS, 1999). Two-sample t-tests were employed for between-group comparisons on continuous variables. In the case of IADL, ADL, Cognitive Decline Scale and CDR, age differences between the patient and control groups approached significance (P=0.059), and so age was used as a covariate in between-group analyses involving these variables. Mann–Whitney U-tests were used for skewed continuous data. Chi-squared analyses were used for between-group comparisons on categorical variables. For 2×2 tables, Yates's continuity correction (denoted by CCχ2) is reported. Fisher's exact test was used in the analysis of 2×2 tables with expected frequencies lower than 5 in two or more cells. Change within groups over time on outcome variables was analysed using repeated measures analysis of variance (ANOVA) or multivariate analysis of variance (MANOVA). Age at institutionalisation was compared between groups using Kaplan–Meier survival analysis. For all analyses, probability levels reported were two-tailed, and the level of significance was set at 0.05.
RESULTS
Sample characteristics
Of the baseline sample of 27 late-onset schizophrenia patients, 22 (82%) were followed up at 1 year, and 19 (70%) were also followed up at 5 years, including nine patients who had died and for whom information was provided by an informant. Causes of death were medical illness in eight patients, and an accidental fall in one patient. Post-mortem examinations were not carried out. Of the 34 original healthy controls, 31 (91%) were followed up at 1 year and 24 (71%) at 5 years. Of the eight late-onset schizophrenia patients not followed up at 5 years, six could not be located and two refused. Of the ten controls not followed up at 5 years, eight refused and two had died. Table 1 compares the baseline characteristics of participants followed up for 5 years with those not so followed. There was no significant difference between the two subgroups. Controls who were followed up had significantly more years of education and lower Hachinski Ischaemia Scale scores at baseline than those not followed up.
Table I Characteristics of study participants at baseline, comparing those followed up at 5 years with those not followed up
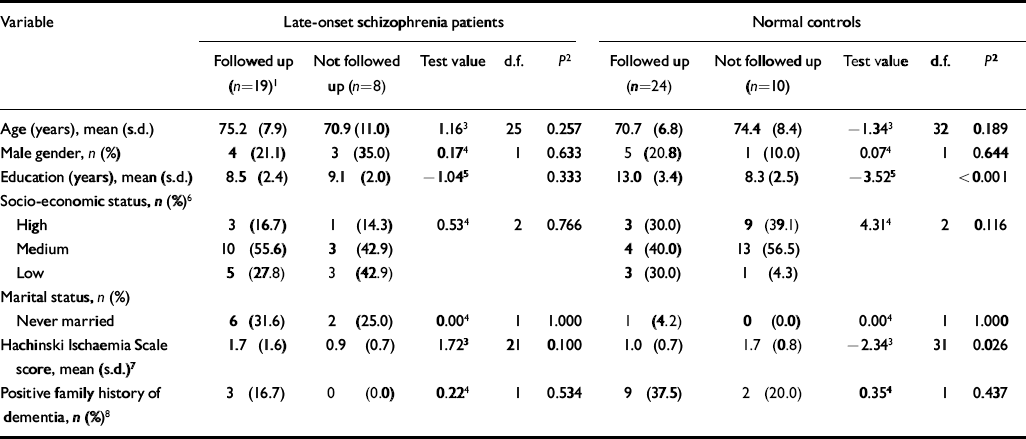
| Variable | Late-onset schizophrenia patients | Normal controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Followed up (n=19)1 | Not followed up (n=8) | Test value | d.f. | P 2 | Followed up (n=24) | Not followed up (n=10) | Test value | d.f. | P 2 | |
| Age (years), mean (s.d.) | 75.2 (7.9) | 70.9 (11.0) | 1.163 | 25 | 0.257 | 70.7 (6.8) | 74.4 (8.4) | -1.343 | 32 | 0.189 |
| Male gender, n (%) | 4 (21.1) | 3 (35.0) | 0.174 | 1 | 0.633 | 5 (20.8) | 1 (10.0) | 0.074 | 1 | 0.644 |
| Education (years), mean (s.d.) | 8.5 (2.4) | 9.1 (2.0) | -1.045 | 0.333 | 13.0 (3.4) | 8.3 (2.5) | -3.525 | <0.001 | ||
| Socio-economic status, n (%)6 | ||||||||||
| High | 3 (16.7) | 1 (14.3) | 0.534 | 2 | 0.766 | 3 (30.0) | 9 (39.1) | 4.314 | 2 | 0.116 |
| Medium | 10 (55.6) | 3 (42.9) | 4 (40.0) | 13 (56.5) | ||||||
| Low | 5 (27.8) | 3 (42.9) | 3 (30.0) | 1 (4.3) | ||||||
| Marital status, n (%) | ||||||||||
| Never married | 6 (31.6) | 2 (25.0) | 0.004 | 1 | 1.000 | 1 (4.2) | 0 (0.0) | 0.004 | 1 | 1.000 |
| Hachinski Ischaemia Scale score, mean (s.d.)7 | 1.7 (1.6) | 0.9 (0.7) | 1.723 | 21 | 0.100 | 1.0 (0.7) | 1.7 (0.8) | -2.343 | 31 | 0.026 |
| Positive family history of dementia, n (%)8 | 3 (16.7) | 0 (0.0) | 0.224 | 1 | 0.534 | 9 (37.5) | 2 (20.0) | 0.354 | 1 | 0.437 |
Change over 5 years
On most measures, the patients had a worse outcome after 5 years than did controls (Table 2). They were institutionalised at a younger age, their IADL and ADL scores declined more, and their level of cognitive decline, as measured by the CDR and Cognitive Decline Scale, was significantly worse than that of controls. Their mean MMSE score declined by 6.5 points over 5 years while that of controls remained stable. Of ten patients who were alive at 5-year follow-up, three patients were not interviewed. Of the seven who were interviewed, one met DSM–IV criterion A for schizophrenia at 5-year follow-up, and six out of seven patients assessed with the BPRS (including the patient meeting criterion A) had symptoms of psychosis: five with delusions or hallucinations, one with grandiose ideation and probable psychosis. Despite decline in other areas, global functioning (GAF score), while lower than that of controls, did not decline in the late-onset schizophrenia group as a whole over the 5-year period. However, patients with dementia at 5-year follow-up had a mean GAF score of 28.1 (s.d.=19.6, n=9), in contrast to a mean GAF of 61.6 (s.d.=18.9, n=10) in patients without dementia at 5 years. The late-onset schizophrenia patients and controls did not differ significantly in levels of neurological abnormality at 5-year follow-up.
Table 2 Change over 5 years in clinical and residential status of study participants
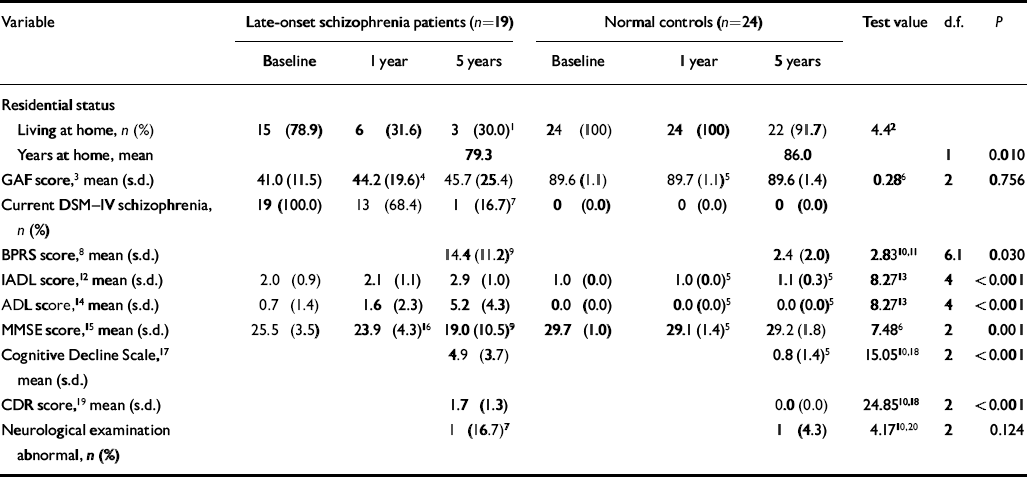
| Variable | Late-onset schizophrenia patients (n=19) | Normal controls (n=24) | Test value | d.f. | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 year | 5 years | Baseline | 1 year | 5 years | ||||
| Residential status | |||||||||
| Living at home, n (%) | 15 (78.9) | 6 (31.6) | 3 (30.0)1 | 24 (100) | 24 (100) | 22 (91.7) | 4.42 | ||
| Years at home, mean | 79.3 | 86.0 | 1 | 0.010 | |||||
| GAF score,3 mean (s.d.) | 41.0 (11.5) | 44.2 (19.6)4 | 45.7 (25.4) | 89.6 (1.1) | 89.7 (1.1)5 | 89.6 (1.4) | 0.286 | 2 | 0.756 |
| Current DSM-IV schizophrenia, n (%) | 19 (100.0) | 13 (68.4) | 1 (16.7)7 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| BPRS score,8 mean (s.d.) | 14.4 (11.2)9 | 2.4 (2.0) | 2.8310,11 | 6.1 | 0.030 | ||||
| IADL score,12 mean (s.d.) | 2.0 (0.9) | 2.1 (1.1) | 2.9 (1.0) | 1.0 (0.0) | 1.0 (0.0)5 | 1.1 (0.3)5 | 8.2713 | 4 | <0.001 |
| ADL score,14 mean (s.d.) | 0.7 (1.4) | 1.6 (2.3) | 5.2 (4.3) | 0.0 (0.0) | 0.0 (0.0)5 | 0.0 (0.0)5 | 8.2713 | 4 | <0.001 |
| MMSE score,15 mean (s.d.) | 25.5 (3.5) | 23.9 (4.3)16 | 19.0 (10.5)9 | 29.7 (1.0) | 29.1 (1.4)5 | 29.2 (1.8) | 7.486 | 2 | 0.001 |
| Cognitive Decline Scale,17 mean (s.d.) | 4.9 (3.7) | 0.8 (1.4)5 | 15.0510,18 | 2 | <0.001 | ||||
| CDR score,19 mean (s.d.) | 1.7 (1.3) | 0.0 (0.0) | 24.8510,18 | 2 | <0.001 | ||||
| Neurological examination abnormal, n (%) | 1 (16.7)7 | 1 (4.3) | 4.1710,20 | 2 | 0.124 | ||||
Incidence of dementia
At 5-year follow-up we found that nine of the 19 late-onset schizophrenia patients met DSM–IV criteria for dementia. Five of these met DSM–IV criteria for Alzheimer's disease and one met criteria for vascular dementia. The remaining three had dementia of unknown type. None of the controls was found to have dementia. A χ2 analysis comparing incidence of any dementia between the two groups was highly significant (CCχ2=11.7, d.f.=1, P < 0.001). Since odds ratios do not allow for zero cells, pseudo-Bayes estimates were calculated for this table (Reference Bishop, Fienberg and HollandBishop et al, 1975). The resulting odds ratio of 16.52 had a very wide 95% confidence interval, 1.85–147.8, which is not surprising considering the zero cell for the control group. If the ‘most extreme case scenario’ is considered and all patients (and controls) lost to follow-up did not have dementia, the analysis comparing the incidence of dementia between the two groups would remain highly significant (χ2=12.8, d.f.=1, Fisher's exact test P < 0.001). The pseudo-Bayes estimate for the odds ratio is 9.98 (95% CI 1.44–69.37).
Of the 12 patients who had a baseline MMSE score of 25 or over, five developed dementia, as did four of seven with initial MMSE scores below 25. If the rates of dementia are calculated only in participants whose MMSE scores were 25 or over at baseline, the rate of dementia at follow-up was still significantly higher in the patients than in the controls (5/12 v. 0/24; χ2=11.6, d.f.=1, Fisher's exact test P=0.002). Even restricting the comparison to those with MMSE scores of 28 or more, the rate of dementia was significantly higher among patients than controls (2/7 v. 0/23; χ2=7.0, d.f.=1, Fisher's exact test P=0.05).
We considered whether some of the patients might have been diagnosable as having mild cognitive impairment. All seven patients with baseline MMSE scores below 25 had scores on the Logical Memory I or II subtest of the Wechsler Memory Scale at least 1.5 s.d. below the Age Scale score, as did 7 of the 12 with MMSE scores or 25 or over. No control had diagnosable mild cognitive impairment using this criterion. Seven of the 14 late-onset schizophrenia patients with diagnosable mild cognitive impairment developed dementia, as did two of five patients without mild cognitive impairment.
We conducted post hoc comparisons of baseline characteristics between late-onset schizophrenia patients who went on to develop dementia (n=9) and those who did not (n=10). Patients who subsequently developed dementia were older at baseline, of lower socio-economic status, with longer duration of illness and with worse IADL, ADL and MMSE scores than those who did not develop dementia (Table 3), but none of these comparisons reached statistical significance. Re-analysis correcting for age differences did not change these findings. There were non-significant differences between the groups on baseline MRI variables, with the subsequently demented patients having had greater ventricle-to-brain ratio (see Reference Victoroff, Mack and GraftonVictoroff et al, 1994) and more periventricular and centrum semiovale hyperintensities (see Reference Fazekas, Chawluk and AlaviFazekas et al, 1987) on T 2-weighted imaging (Table 3).
Table 3 Characteristics at baseline of patients with late-onset schizophrenia with or without dementia at 5-year follow-up
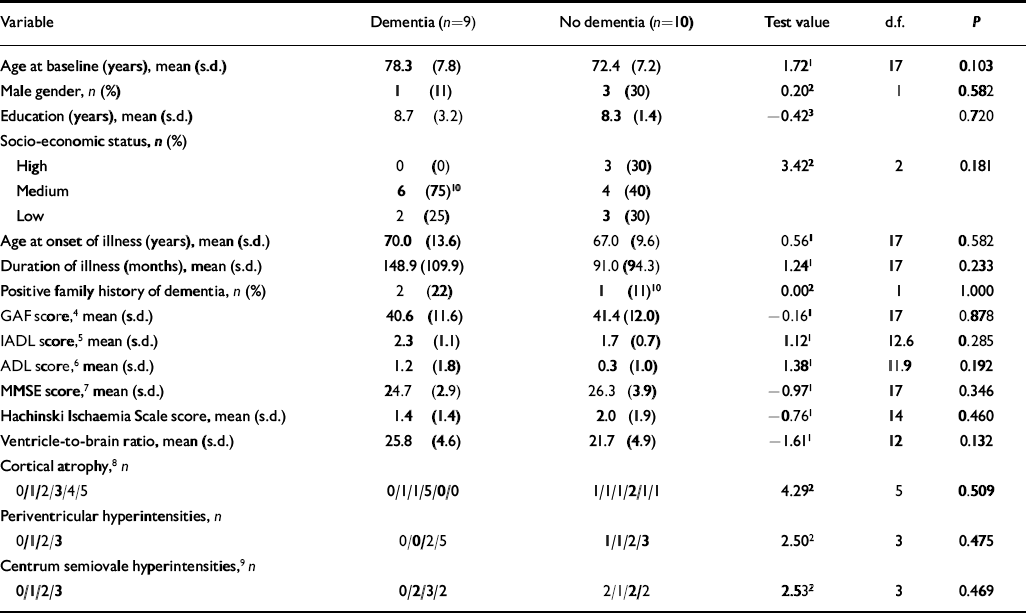
| Variable | Dementia (n=9) | No dementia (n=10) | Test value | d.f. | P |
|---|---|---|---|---|---|
| Age at baseline (years), mean (s.d.) | 78.3 (7.8) | 72.4 (7.2) | 1.721 | 17 | 0.103 |
| Male gender, n (%) | 1 (11) | 3 (30) | 0.202 | 1 | 0.582 |
| Education (years), mean (s.d.) | 8.7 (3.2) | 8.3 (1.4) | -0.423 | 0.720 | |
| Socio-economic status, n (%) | |||||
| High | 0 (0) | 3 (30) | 3.422 | 2 | 0.181 |
| Medium | 6 (75)10 | 4 (40) | |||
| Low | 2 (25) | 3 (30) | |||
| Age at onset of illness (years), mean (s.d.) | 70.0 (13.6) | 67.0 (9.6) | 0.561 | 17 | 0.582 |
| Duration of illness (months), mean (s.d.) | 148.9 (109.9) | 91.0 (94.3) | 1.241 | 17 | 0.233 |
| Positive family history of dementia, n (%) | 2 (22) | 1 (11)10 | 0.002 | 1 | 1.000 |
| GAF score,4 mean (s.d.) | 40.6 (11.6) | 41.4 (12.0) | -0.161 | 17 | 0.878 |
| IADL score,5 mean (s.d.) | 2.3 (1.1) | 1.7 (0.7) | 1.121 | 12.6 | 0.285 |
| ADL score,6 mean (s.d.) | 1.2 (1.8) | 0.3 (1.0) | 1.381 | 11.9 | 0.192 |
| MMSE score,7 mean (s.d.) | 24.7 (2.9) | 26.3 (3.9) | -0.971 | 17 | 0.346 |
| Hachinski Ischaemia Scale score, mean (s.d.) | 1.4 (1.4) | 2.0 (1.9) | -0.761 | 14 | 0.460 |
| Ventricle-to-brain ratio, mean (s.d.) | 25.8 (4.6) | 21.7 (4.9) | -1.611 | 12 | 0.132 |
| Cortical atrophy,8 n | |||||
| 0/1/2/3/4/5 | 0/1/1/5/0/0 | 1/1/1/2/1/1 | 4.292 | 5 | 0.509 |
| Periventricular hyperintensities, n | |||||
| 0/1/2/3 | 0/0/2/5 | 1/1/2/3 | 2.502 | 3 | 0.475 |
| Centrum semiovale hyperintensities,9 n | |||||
| 0/1/2/3 | 0/2/3/2 | 2/1/2/2 | 2.532 | 3 | 0.469 |
We examined the influence of possible confounding factors, namely intercurrent illness and medication usage, on dementia incidence. There was no significant difference between late-onset schizophrenia patients with and those without dementia as regards occurrence (between the 1-year and 5-year assessments) of any of the following: myocardial infarction, stroke, transient ischaemic attack, cerebrovascular disease, other neurological changes and surgery. There was no significant difference between the two groups in rates of current usage of psychoactive medications in general, or antipsychotics specifically. We did not have sufficient data on history of alcohol misuse to include it in our analyses, but no participant was alcohol-dependent.
DISCUSSION
Do patients with late-onset schizophrenia decline cognitively?
Although cognitive deficits are recognised as being integral to the syndrome of schizophrenia, they are generally regarded as being relatively stable, consistent with the notion of a static encephalopathy (Reference Goldberg, Hyde and KleinmanGoldberg et al, 1993). Whether the encephalopathy of late-onset schizophrenia is similarly static is controversial. Cross-sectional studies have generally reported that patients with late-onset schizophrenia have cognitive deficits that are similar to those seen in age-matched patients with early-onset schizophrenia (Reference Heaton, Paulsen and McAdamsHeaton et al, 1994; Reference Jeste, Harris and KrullJeste et al, 1995; Reference Sachdev, Brodaty and RoseSachdev et al, 1999). Comparisons of late-onset schizophrenia with Alzheimer's disease reported significant differences between the two patient groups (Reference Heaton, Paulsen and McAdamsHeaton et al, 1994). This has led to the conceptualisation of late-onset schizophrenia as a ‘non-dementia non-praecox dementia praecox’ (Reference Jeste, Harris and KrullJeste et al, 1995). Longitudinal studies of longer duration of late-onset schizophrenia (Reference Jorgensen and Munk-JorgensenJorgensen & Munk-Jorgensen, 1985; Reference HoldenHolden, 1987; Reference Craig and BregmanCraig & Bregman, 1988) have, however, not always been consistent with this.
Our study suggests that long-term follow-up of people with late-onset schizophrenia often yields a picture of progressive cognitive decline. Cross-sectional assessments in our patients at the time of entry into the study, as reported previously (Reference Sachdev, Brodaty and RoseSachdev et al, 1999), did not show significant differences in cognitive function between late- and early-onset schizophrenia, with both diagnostic groups performing worse than healthy individuals. Even at 1-year follow-up (Reference Brodaty, Sachdev and RoseBrodaty et al, 1999), we did not see evidence of decline in our late-onset schizophrenia sample. The picture was quite different at 5 years, with high rates of dementia and institutionalisation. Our study supports the findings of Craig & Bregman (Reference Craig and Bregman1988) and Holden (Reference Holden1987) that late-onset schizophrenia is a prelude to dementia in a high proportion of cases. Holden reported that 13 of 37 patients with a diagnosis of paranoid psychosis, and having a minimum score of 21 out of 43 on a scale of orientation, general knowledge and memory, progressed to dementia within 3 years (i.e. 35%, or 11.7% per annum compared with the present rate of 47.4% over 5 years or 9.5% per annum). Palmer et al (Reference Palmer, Bondi and Twamley2003) did not find evidence of decline in a group of patients with late-onset schizophrenia-spectrum disorders over 2 years of follow-up, but conceded that ‘longer follow-up periods could reveal that late onset schizophrenia disorder patients experience a very slow cognitive decline that is obscured by normal practice effects when observed over shorter periods’. Alternatively, sampling differences might explain the discrepancies in findings.
Possible explanations for cognitive decline in late-onset schizophrenia
Our findings do not appear to be a result of undiagnosed cases of dementia among participants with late-onset schizophrenia at baseline, as rates of dementia remained significantly higher when we compared late-onset schizophrenia subsamples with higher index MMSE scores or those without diagnosable mild cognitive impairment and controls. We were surprised that dementia cases were predominantly of Alzheimer type as evidenced by the patient's gradual decline in function, prominent disturbance of episodic memory and absence of clinical stigmata of cerebrovascular disease. Based on the finding of increased T 2-weighted hyperintensities in the white matter and subcortical nuclei in our patients at the start of the study, we had predicted an increased incidence of vascular dementia. This was not the case, as judged on a clinical basis. A limitation of our diagnostic process was the absence of neuroimaging at 5-year follow-up, which would have further increased confidence in the diagnosis. It is recognised that subcortical vascular dementia may present like Alzheimer's disease (Reference Jeste, Symonds and HarrisJeste et al, 1998) and we cannot rule out this possibility, except that hypertension was not in excess in the late-onset schizophrenia sample, and these patients’ clinical picture was not characterised by the ‘subcortical features’ of psychomotor slowing and frontal executive deficits. Our participants did not consent to post-mortem examination, which was not surprising since they needed much persuasion at every stage of the study.
Could psychosis have contributed to the development of Alzheimer's disease in our study group? Published research such as the EURODEM study (Reference Jorm, Mackinnon and HendersonJorm et al, 1995) on psychiatric risk factors for this disease did not find any such association. Nor was an excess of plaques and tangles found in a post-mortem study of patients with schizophrenia (Reference Purohit, Perl and HaroutunianPurohit et al, 1998). There is also no evidence, after more than 50 years of their usage, that antipsychotic agents contribute to the development of dementia (Reference Spohn and StraussSpohn & Strauss, 1989). One possible explanation for our finding is that we inadvertently included people with early dementia in our late-onset schizophrenia sample. Even on retrospective review of our early data, the diagnosis of dementia was not warranted in any case, at baseline or at 1-year follow-up. There was, however, a tendency for those who had dementia at 5-year assessment to have had slightly worse MMSE, ADL and IADL scores at baseline. It will be of great clinical interest to see whether the patients who were still cognitively intact at 5 years will also progress to dementia later in the course of their illness.
Pathogenesis of cognitive decline in late-onset schizophrenia
The occurrence of a schizophrenia-like psychosis for many years before dementia becomes manifest warrants speculation on its pathogenesis. It is parsimonious to argue that late-onset schizophrenia in these cases is a manifestation of changes associated with the dementing process. Delusions are common in Alzheimer's disease, but generally occur in the middle stages of the disease rather than being its presenting feature (Reference Jeste, Wragg and SalmonJeste et al, 1992). It is possible that in a few patients with Alzheimer's disease the brain regions affected in the early stages cause a propensity for the development of psychosis. In particular, lesions of the temporal lobes have been implicated (Reference LewisLewis, 1995). We must emphasise that dementia did not develop in all our patients, and about half of the sample were cognitively stable, suggesting that late-onset schizophrenia is a heterogeneous syndrome. This is further underscored by the clear gulf in global functioning scores between the demented and non-demented patients. While the mean GAF score of those with dementia declined, the mean score of those without dementia rose from 41.4 to 61.6 over the 5 years, which is likely to be due to the full or partial resolution of psychosis in many cases. The eventual fate of the non-demented group is, however, of future interest.
Implications of the study
Among the strengths of this study were the detailed nature of the follow-up assessment, which encompassed information from both participants and informants, and the strict consensus diagnoses of schizophrenia and dementia using DSM criteria. We were constrained somewhat by the availability of informant information only, in cases where the participant had died before the 5-year assessment. We were also limited by our small sample size and by the level of attrition. However, there was no difference at baseline between those followed up and those not. Although the small sample size limits the power of this study, it makes our finding of a high incidence of dementia all the more striking.
This study has implications for our understanding of the presentation and course of schizophrenia and dementia in the elderly. Further long-term follow-up of patients with late-onset schizophrenia, encompassing cognitive measures, functional neuroimaging and genotyping (e.g. for the apolipoprotein E ϵ4 isoform) would be of benefit in clarifying the possibility that late-onset schizophrenia is an early presentation of dementia. Why some patients present in this way should then become the focus of inquiry.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Almost half of people with a diagnosis of late-onset schizophrenia may go on to develop dementia, mainly of the Alzheimer type, within 5 years.
-
▪ Patients who are older, perform more poorly on cognitive performance or daily functioning, come from lower socio-economic classes, have a longer duration of illness or have more brain atrophy, appear to be more likely to develop dementia.
-
▪ Poor memory is common among people with late-onset schizophrenia, including those who do not go on to develop dementia.
LIMITATIONS
-
▪ The small sample size in this study was compounded by attrition through death or participants’ refusal of follow-up.
-
▪ Poor performance at baseline on the Mini-Mental State Examination in many patients may mean that the diagnosis of early dementia was missed.
-
▪ There was a lack of pathological diagnoses at post-mortem.
Acknowledgements
This research was supported by the National Health and Medical Research Council of Australia. We are grateful to Noelene Rose and Karen Berman for data collection.






eLetters
No eLetters have been published for this article.