Research on autism spectrum disorder (ASD) has been dominated by a focus on risks and deficits, despite evidence of striking heterogeneity in patterns of onset, course, symptoms, adaptive functioning, and signatures of risk (Elsabbagh & Johnson, Reference Elsabbagh and Johnson2010; Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014; Rogers, Reference Rogers2009; Szatmari et al., Reference Szatmari, Chawarska, Dawson, Georgiades, Landa, Lord and Halladay2016). This is understandable given the significant challenges to social development and adaptive function faced by individuals on this spectrum, coupled with evidence on its early emergence, associated lifelong difficulties, and familial heritability. These features have generated urgency in the search for knowledge that informs preemptive interventions to avert problems early in development. Much of this work has focused on identifying signatures of early risk for ASD and its prodromal developmental course. This approach has yielded knowledge vital to early identification and support services. Nonetheless, widespread phenotypic heterogeneity observed in ASD early in life and growing interest in resilience perspectives are beginning to shift the focus of ASD research, particularly for studies of infants at high risk (HR) for the disorder. The purpose of this paper is to examine the potential benefits of integrating a developmental systems resilience framework into the study of ASD, illustrating the possibilities for research on infants at HR for the disorder. We discuss ways that a resilience framework could open up critical new avenues for investigating risk and adaptive processes associated with ASD over time, expanding earlier research to improve knowledge and outcomes for this disorder, while also enriching theory and knowledge in the broader domains of developmental psychopathology and resilience science.
First, we briefly review deficit models in ASD research and introduce the HR infant siblings research design. Then we discuss evidence of heterogeneity associated with ASD that motivates a shift away from exclusively deficit-focused risk models toward more integrated models of ASD. Such models would include positive pathways and cascades, as well as attention to promotive and protective influences on development. Drawing on examples from early ASD research, we describe key components of a developmental systems resilience framework. Subsequently, to illustrate the potential of this approach, we describe how a resilience perspective could expand the search for processes that may account for the observed female protective effect (FPE) in ASD, to include general and ASD-specific female advantages or protective influences as well as processes of male vulnerabilities. Finally, we advocate for future research elucidating markers of positive functioning and adaptation, developmental pathways and cascades during sensitive periods, reframing of existing findings, and considering diverse contexts of risk. We argue that integrating resilience perspectives into existing programs of HR infant siblings ASD research could transform our understanding of developmental mechanisms and inform clinical interventions, much as it has done in the past for other realms of developmental psychopathology.
ASD models focused on risk and deficit
ASD, currently estimated to affect 1 in 54 individuals (Maenner, Reference Maenner2020), is characterized by social communication and interaction difficulties as well as restricted and repetitive behaviors that can result in significant functional impairments in everyday life (American Psychiatric Association, 2013). Individuals with ASD have difficulty with reciprocal social exchange, performing daily tasks, adjusting to unexpected changes, and forming relationships–difficulties that compound over development to influence a host of life course outcomes. Those with ASD often struggle in academic settings (Migliore et al., Reference Migliore, Timmons, Butterworth and Lugas2012), maintaining a job (Hendricks, Reference Hendricks2010), and living independently (Anderson et al., Reference Anderson, Shattuck, Cooper, Roux and Wagner2014; Barnard et al., Reference Barnard, Harvey, Potter and Prior2001). They also report poorer wellbeing (Deserno et al., Reference Deserno, Borsboom, Begeer and Geurts2018; Hedley & Uljarević, Reference Hedley and Uljarević2018; Schmidt et al., Reference Schmidt, Kirchner, Strunz, Broźus, Ritter, Roepke and Dziobek2015) and an increased likelihood for a host of severe health problems (Croen et al., Reference Croen, Zerbo, Qian, Massolo, Rich, Sidney and Kripke2015) and premature death (Hirvikoski et al., Reference Hirvikoski, Mittendorfer-Rutz, Boman, Larsson, Lichtenstein and Bölte2016). Annual direct and indirect costs associated with carrying an ASD diagnosis are projected to reach $461 billion for 2025 in the US (Leigh & Du, Reference Leigh and Du2015).
Deficit model
Given the functional impairment associated with receiving an ASD diagnosis and the profound difficulties that infiltrate many domains of daily functioning over the ASD life course, it is understandable and not surprising that a deficit model has dominated ASD research. The majority of research studying the emergence of early ASD has focused on risk, or the elevated probability of a collection of negative outcomes associated with the diagnosis of ASD. We have gained important scientific insight into the defining elements of the diagnosis (persistent deficit in social communication and interactions, restricted/repetitive patterns of behavior, and impairments in key domains of function, including social relationships, school, or work) and associated problems (e.g., increased likelihood for various forms of psychopathology; Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016; Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014; Johnson et al., Reference Johnson, Gliga, Jones and Charman2015a; Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Varcin & Jeste, Reference Varcin and Jeste2017).
Clinicians, neuroscientists, geneticists, and developmental psychologists alike were motivated to investigate risk factors and processes in order to mitigate or prevent serious consequences of the disorder over time. Past work suggests that core ASD traits may lie on a continuum within the general population, with clinical levels of ASD disability representing the severe end of such a distribution, rather than a categorical trait (Constantino, Reference Constantino2011). To understand the complex and varied ways in which ASD manifests over the life course, research efforts focused on characterizing the core deficits across multiple domains exhibited by those with ASD compared to those with other disorders and their typically developing counterparts.
Familial ASD risk and the HR infant sibling studies
ASD is one of the most genetically associated of all neurodevelopmental disorders, yet efforts to explicate the architecture of genetic risk for ASD are ongoing (e.g., Satterstrom et al., Reference Satterstrom, Kosmicki, Wang, Breen, De Rubeis and An2020). To date, one of the best-established predictors for developing ASD is familial risk, or the degree of one’s genetic relation to someone with a diagnosis (Sandin et al., Reference Sandin, Lichtenstein, Kuja-Halkola, Larsson, Hultman and Reichenberg2014). Genetically informed designs point to the heritability of both ASD and the population-wide distributions of ASD-related traits (Ritvo et al., Reference Ritvo, Jorde, Mason-Brothers, Freeman, Pingree, Jones and Mo1989; Ronald & Hoekstra, Reference Ronald and Hoekstra2011; Rosenberg et al., Reference Rosenberg, Law, Yenokyan, McGready, Kaufmann and Law2009). Parents who have one child with ASD have about an 18% likelihood of having another child with the disorder (Ozonoff et al., Reference Ozonoff, Young, Carter, Messinger, Yirmiya, Zwaigenbaum and Stone2011). As such, the younger siblings of children with ASD are termed HR because of their increased likelihood of developing the disorder and related difficulties.
Additionally, at the present time, ASD cannot be reliably diagnosed until the ages of 18 (Ozonoff et al., Reference Ozonoff, Young, Landa, Brian, Bryson, Charman and Iosif2015) to 24 months (although see also Ozonoff et al., Reference Ozonoff, Young, Brian, Charman, Shephard, Solish and Zwaigenbaum2018) and in practice it is often not diagnosed until much later (Mandell et al., Reference Mandell, Novak and Zubritsky2005). Yet multiple lines of evidence, from parent reports to home video analysis, pointed to the emergence of ASD-related atypicalities earlier in life (Adrien et al., Reference Adrien, Faure, Perrot, Hameury, Garreau, Barthelemy and Sauvage1991; Chawarska et al., Reference Chawarska, Paul, Klin, Hannigen, Dichtel and Volkmar2007; Kozlowski et al., Reference Kozlowski, Matson, Horovitz, Worley and Neal2011; Maestro et al., Reference Maestro, Casella, Milone, Muratori and Palacio-Espasa1999; Rogers & DiLalla, Reference Rogers and Dilalla1990; Rosenthal et al., Reference Rosenthal, Massie and Wulff1980; Volkmar et al., Reference Volkmar, Stier and Cohen1985; Werner et al., Reference Werner, Dawson, Osterling and Dinno2000).
Evidence for the familial clustering of ASD and the possibility of early signs of the disorder catalyzed a widespread program of prospective research studying the younger infant siblings of children with ASD, known as HR infant sibling studies. Within this framework, a key line of research emerged with the goal of improving the identification of risk markers for ASD early in life. Investigators aimed to inform basic science and also to provide families with resources and access to early interventions, in order to ameliorate challenges of the disorder (Barbaro & Dissanayake, Reference Barbaro and Dissanayake2009; Boyd et al., Reference Boyd, Odom, Humphreys and Sam2010; Koegel et al., Reference Koegel, Koegel, Ashbaugh and Bradshaw2014; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson and Garon2013, Reference Zwaigenbaum, Bauman, Stone, Yirmiya, Estes and Hansen2015). Implicit (and sometimes explicit) in these studies is the idea that better function or outcomes will be facilitated by earlier identification of more effective risk markers. A review of research on HR infant siblings is beyond the scope of this discussion but can be found elsewhere (e.g., Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016; Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014; Johnson et al., Reference Johnson, Gliga, Jones and Charman2015a; Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Varcin & Jeste, Reference Varcin and Jeste2017). Theoretical models (e.g., Dawson, Reference Dawson2008) and empirical evidence point to the importance of capitalizing on early brain plasticity by detecting prodromal ASD risk factors and intervening early to enhance adaptive and cognitive functioning (Dawson et al., Reference Dawson, Rogers, Munson, Smith, Winter, Greenson and Varley2010), reduce long-term costs and impairment associated with the disorder (Jacobson & Mulick, Reference Jacobson and Mulick2000; Järbrink & Knapp, Reference Järbrink and Knapp2001), and better equip parents (Georgiades et al., Reference Georgiades, Szatmari, Zwaigenbaum, Bryson, Brian, Roberts and Garon2013; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Lord, Rogers, Carter, Carver and Yirmiya2009). In addition, it is thought that early intervention has the potential to disrupt or prevent cascading consequences of early difficulties in one or more domains of foundational competence for later development (Zwaigenbaum et al., Reference Zwaigenbaum, Bauman, Stone, Yirmiya, Estes and Hansen2015). Although our discussion is situated primarily in this well-studied landscape of HR infant siblings research, these integrated risk and resilience concepts and future directions also apply to other ASD research designs and risk contexts.
Phenotypic heterogeneity
Striking phenotypic heterogeneity is a common and puzzling observation noted from the earliest days of clinical descriptions of ASD (Kanner, Reference Kanner1943), despite the identification of shared core diagnostic features. Marked individual differences permeate ASD and complicate efforts to delineate etiology and the mechanisms by which the disorder emerges early in life. For instance, between 30% and 50% of individuals with the diagnosis are functionally nonverbal (Maenner, Reference Maenner2020), a group partially overlapping with as many as 50% of those diagnosed with an intellectual disability (Charman et al., Reference Charman, Pickles, Simonoff, Chandler, Loucas and Baird2011; CDC, 2016; Maenner, Reference Maenner2020), while 3% exhibit above average intelligence (Charman et al., Reference Charman, Pickles, Simonoff, Chandler, Loucas and Baird2011). Further, individuals across the ASD spectrum often have uneven profiles of functioning with relative strengths and weaknesses that vary by domain (Akshoomoff, Reference Akshoomoff2006; Charman et al., Reference Charman, Drew, Baird and Baird2003; Chawarska et al., Reference Chawarska, Klin, Paul, Macari and Volkmar2009; Joseph et al., Reference Joseph, Tager-Flusberg and Lord2002; Landa & Garrett-Mayer, Reference Landa and Garrett-Mayer2006). This well-documented phenotypic heterogeneity contributed to, and also stems from, the widening of the ASD diagnostic criteria in recent history and the quest to understand different possible “autisms” (Happé & Frith, Reference Happé and Frith2020).
Studies of HR infant siblings provide additional evidence of heterogeneity in prodromal ASD phenotypes and diagnostic outcomes early in life. Even in infancy, there are remarkable individual differences in the type and timing of ASD risk markers, rates of early development, and manifestation of core ASD symptoms (Elsabbagh & Johnson, Reference Elsabbagh and Johnson2010; Rogers, Reference Rogers2009; Szatmari et al., Reference Szatmari, Chawarska, Dawson, Georgiades, Landa, Lord and Halladay2016). Reviews have noted the substantial variation in rates of change over time such as delays and atypicalities that may only begin during the first year of life and appear more gradually (Rogers, Reference Rogers2009), resulting in many possible routes to an ASD diagnosis and associated problems (Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014) in addition to the marked heterogeneity of symptoms across individuals. Additionally, studies document that 70%–90% of ASD diagnoses are accompanied by various comorbidities, such as anxiety and attention-deficit hyperactivity disorder (Lundström et al., Reference Lundström, Reichenberg, Melke, Råstam, Kerekes, Lichtenstein, Gillberg and Anckarsäter2015; Simonoff et al., Reference Simonoff, Pickles, Charman, Chandler, Loucas and Baird2008).
Findings also have revealed notable phenotypic heterogeneity in HR siblings of children with ASD who do not go on to receive a diagnosis. For example, of those HR siblings who do not develop ASD, about 11% exhibit mild-to-moderate developmental delay, 30% exhibit elevated ASD-related subthreshold symptoms, and 7% will exhibit some form language delay when assessed at 3 years of age, collectively suggestive of what is known as the “broader autism phenotype” (BAP; Charman et al., Reference Charman, Young, Brian, Carter, Carver and Chawarska2017; Rubenstein & Chawla, Reference Rubenstein and Chawla2018). Some report that as many as 20% of HR siblings who do not go on to meet criteria for ASD will exhibit BAP features (Georgiades et al., Reference Georgiades, Szatmari, Zwaigenbaum, Bryson, Brian, Roberts and Garon2013; Messinger et al., Reference Messinger, Young, Ozonoff, Dobkins, Carter, Zwaigenbaum and Sigman2013). Researchers studying ASD in the HR infant sibling context have suggested the need to focus on identifying not only the markers for ASD, broadly defined, but also the indicators of processes that may account for the significant heterogeneity observed early in life and over the long term (Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014).
The study of heterogeneity has become a focus of ASD research (Geschwind, Reference Geschwind2009) with findings of variability in prodromal profiles, symptom presentation at all stages of development (Masi et al., Reference Masi, DeMayo, Glozier and Guastella2017), strengths and difficulties associated with the disorder, and long-term outcomes (Eaves & Ho, Reference Eaves and Ho2008; Helt et al., Reference Helt, Kelley, Kinsbourne, Pandey, Boorstein, Herbert and Fein2008; Howlin et al., Reference Howlin, Goode, Hutton and Rutter2004; Rutter, Reference Rutter1970; Seltzer et al., Reference Seltzer, Shattuck, Abbeduto and Greenberg2004). Importantly, heterogeneity implies that in addition to group or profile averages, there is considerable variation in both negative (maladaptive) and positive (adaptive) directions. As noted by Happé and Frith (Reference Happé and Frith2020) in a recent review of the field, efforts to parse heterogeneity have thus far been relatively unsuccessful. Along with others, we suggest that this may be in part because existing research parsing heterogeneity primarily focuses on maladaptation (e.g., variation in who develops an ASD diagnosis). In other words, research on heterogeneity in the early phenotypic patterning of ASD within and across time has been dominated by a focus on risks, symptoms, and categorical diagnoses, with little attention to the significance of positive aspects of variation and adaptation despite the presence of risks.
Shifting focus to integrate resilience processes
Decades of resilience-focused research have transformed theory and practice in multiple domains of basic and applied research concerned with children’s mental health and wellbeing. Deficit-oriented approaches focused on pathways to disorder gave way to more complex models that included adaptive processes and pathways, in keeping with the emergence of developmental psychopathology (Masten & Cicchetti, Reference Masten and Cicchetti2016). A similar process appears to be underway in the study of ASD, where solely deficit-oriented perspectives are shifting as researchers consider adaptive developmental pathways and heterogeneity (Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016; Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Johnson et al., Reference Johnson, Jones and Gliga2015b; Livingston & Happé, Reference Livingston and Happé2017), as well as individuals who eventually lose their diagnosis (Fein et al., Reference Fein, Barton, Eigsti, Kelley, Naigles, Schultz and Tyson2013).
In research on HR infant siblings, investigators emphasize the clinical importance of leveraging scientific knowledge to promote adaptive development of those with ASD and at risk for the disorder as well as provide support for their families (Constantino & Charman, Reference Constantino and Charman2016). Interest in resilience processes has grown with efforts to account for the variability in prodromal phenotypes, timing of onset of atypical features, and ASD symptom presentation in the first few years of life. Evidence suggested not only that there were multiple pathways to an ASD diagnosis, but also potentially unmapped pathways of adaptation (e.g., Elsabbagh, Reference Elsabbagh2020; Lai & Szatmari, Reference Lai and Szatmari2019). As we discuss in greater detail in later sections, these findings raise the possibility that positive, adaptive development could emerge in multiple ways: from fewer or decreasing risk factors, and/or the presence of factors that might mitigate effects of manifested risk on ASD outcomes. Although resilience perspectives remain limited in research on ASD, there is growing recognition that this framework could illuminate adaptive processes among children and adolescents with ASD and may be particularly relevant to the goals of early ASD research, offering novel translational opportunities (Elsabbagh, Reference Elsabbagh2020; Lai & Szatmari, Reference Lai and Szatmari2019; McCrimmon & Montgomery, Reference McCrimmon, Montgomery, Prince-Embury and Saklofske2014; Molnar-Szakacs et al., Reference Molnar-Szakacs, Kupis and Uddin2021; Szatmari, Reference Szatmari2018). In the following section, we describe a developmental systems resilience framework, drawing on themes and questions central to resilience science, illustrated by examples drawn from research on ASD in early childhood.
A developmental systems resilience framework
Resilience science and developmental psychopathology more broadly are centrally concerned with understanding variation in how individuals adapt and manifest different outcomes (Cicchetti, Reference Cicchetti, Cicchetti and Cohen2006; Masten, Reference Masten2007; Rutter, Reference Rutter2006). Historically, resilience science emerged from observations of marked variation in the development of children from “high-risk” groups of individuals (Masten & Cicchetti, Reference Masten and Cicchetti2016; Rutter, Reference Rutter1987). It was soon clear in longitudinal studies that some individuals with elevated risk for psychopathology manifested adaptive development or recovery in spite of their exposure to a variety of risk factors and adversities, such as maltreatment, premature birth, poverty, marginalization, harsh parenting, or mental illness in the family (Luthar, Reference Luthar2006; Marks et al., Reference Marks, Woolverton and García Coll2020; Masten, Reference Masten2015; Ungar & Theron, Reference Ungar and Theron2020). Resilience frameworks for research and practice expanded models of adaptation and intervention to include promotive and protective processes as well as risk and vulnerability, and motivated greater attention to explaining pathways toward adaptive adjustment and successful development as well as psychopathology (Luthar & Eisenberg, Reference Luthar and Eisenberg2017; Masten, Reference Masten2011; Sandler et al., Reference Sandler, Ingram, Wolchik, Tein and Winslow2015).
Defining resilience for developmental science
Over the decades of research focused on understanding positive adaptation in the context of risks for psychopathology, definitions of resilience varied and changed, reflecting the increasing influence of developmental systems theory and multisystem approaches to investigating the origins, outcomes, prevention and treatment of mental health problems in human development (Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021; Ungar & Theron, Reference Ungar and Theron2020). For the purposes of this paper, we adopt a contemporary systems definition of resilience, broadly referring to the capacity of a dynamic system to adapt successfully through multisystem processes to challenges that threaten the function, survival, or development of the system, whether the system is an individual, family, community or other complex adaptive system (Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021). Resilience of individuals continually changes due to ongoing interactions that shape human interactions and development, from cellular to social levels. The study of resilience in developmental psychopathology focuses on elucidating processes underlying positive variation in adaptive functioning and the course of development among individuals in groups with well-established risk factors for disorders, psychological problems, and other difficulties (Kaboski et al., Reference Kaboski, McDonnell and Valentino2017; Masten & Cicchetti, Reference Masten and Cicchetti2016).
This approach emphasizes that the current function of a system and the pathways of system function over time are shaped by reciprocal interactions and “co-actions” across multiple system levels, famously depicted in Gottlieb’s (Reference Gottlieb1992) illustration of bidirectional influences on development across genetic, neural, behavioral, and environmental levels. Thus, development emerges from the interplay of multiple systems, and because the influences on development arise from interactions of other systems that are continually changing, the course of development is probabilistic rather than deterministic (Gottlieb, Reference Gottlieb2007; Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021). Resilience from a developmental systems perspective is dynamic and distributed across multiple system levels, both internal and external to the person, manifesting through multiple possible pathways over time (Masten & Cicchetti, Reference Masten and Cicchetti2016).
The dynamic nature of resilience in development is especially important in the context of understanding emerging ASD during a period of rapid early development. Resilience changes as the organism develops and as the interactions with its context are changing, for many different reasons. Adaptive capacity changes with development and also with context. For instance, resilience processes during the infancy years emerge and function quite differently than adaptive processes during the school-age years when the organism, context, and demands are different. It is possible that manifestations of risk and resilience change or augment as children age and engage with increasingly complex environments.
Considering resilience processes at multiple levels of analysis also is vital for studying ASD as a highly heritable neurodevelopmental disorder with roots and distinct features spanning levels from the cellular, to the neural structural and functional, to the behavioral. It is likely that these processes interact in potentially non-linear ways across systems and at varying timescales to influence developmental outcomes and, as a result, resilience also could manifest at multiple levels. The interactive nature of systems also suggests that there may be multiple avenues to adaptation and many ways through which the life course could be influenced during salient developmental windows.
In resilience theory, multisystem interactions across levels and time also produce developmental cascades whereby changes in one level or system or domain of functioning spread to affect other systems (Masten & Cicchetti, Reference Masten and Cicchetti2010). Cascading changes can have cumulative effects on the course of a system’s development–particularly at sensitive periods in the course of development, at the junctions that Waddington (Reference Waddington1957) might describe as branching canals or forks in the road of an epigenetic landscape. Negative cascades in the development of psychopathology have been noted for a number of problems, such as the pathway from noncompliance or aggression in childhood to school failure, antisocial behavior or violence later in development (Dodge et al., Reference Dodge, Greenberg and Malone2008; Patterson et al., Reference Patterson, Reid and Dishion1992). However, developmental cascades also can lead to adaptive pathways of development. Positive cascade effects arising from interacting systems have been invoked to explain why “competence begets competence” in human development (Heckman & Mosso, Reference Heckman and Mosso2014; Huebner et al., Reference Huebner, Boothby, Aber, Darmstadt, Diaz, Masten, Yoshikawa, Redlener, Emmel, Pitt, Arnold, Barber, Berman, Blum, Canavera, Eckerle, Fox, Gibbons, Hargarten, Landers and Zeanah2016; Masten, Reference Masten2011). It is likely that the core processes, as well as the timing, of positive and negative cascades in development differ. Adaptive processes or interventions in development can instigate positive cascades or interrupt negative cascades (Masten & Cicchetti, Reference Masten and Cicchetti2010; Rutter, Reference Rutter1987). It is notable that interventions initially designed to interrupt negative cascades also provided evidence of positive developmental cascades. For example, efforts to improve parenting as a strategy for addressing child compliance resulted in spreading adaptive consequences in the family, some expected and others unexpected (Patterson et al., Reference Patterson, Forgatch and DeGarmo2010).
In the context of ASD, studies suggest that, in most cases, a small set of risk markers present early in life may interact to either amplify or decrease each other’s effects early in development (Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014). Illustrating a negative cascade, Elsabbagh and Johnson (Reference Elsabbagh and Johnson2010) and Dawson (Reference Dawson2008) discuss how a few initial and oftentimes subtle deviations in brain and behavior become compounded, giving rise to risk processes which lead development down an increasingly atypical and less social path during a sensitive period in development, resetting developmental trajectories on a course toward an ASD outcome. In contrast, a Cochrane Review of parent-mediated interventions for young children with ASD by Oono et al. (Reference Oono, Honey and McConachie2013) noted the effectiveness of parent-mediated early interventions reducing target symptoms and producing language gains. These findings could result from disruption of a negative cascade from early ASD-related social disability as well as instigation of a positive cascade for improved social interactions with cascading effects on language. In sum, positive and negative developmental cascades at multiple levels might help to explain how a small set of risk or adaptive processes that occur early in life with particular developmental timing could exert widespread and lasting effects on the life course in the context of ASD risk.
Dual threat in ASD
In resilience theory, the early years of human development are critically important for nurturing and optimizing fundamental adaptive systems and the capacity they represent for resilience over the life course (Masten, Reference Masten2018). Severe compromise to neural, cognitive, and/or social systems in early childhood threatens not only current functioning of a child in the present but also potentially jeopardizes future resilience by disrupting the developmental processes that build resilience capacity. Threats can be internal to the child, such as in the case of emerging neurodevelopmental disorder, that likely begin during prenatal development. These processes pose a threat to the development of resilience by compromising the integrity of core neurobiology and skill-building, setting the stage for increasingly altered early development that constrains the learning processes and experiences essential for developing key adaptive systems (Feder et al., Reference Feder, Fred-Torres, Southwick and Charney2019; Patterson, Reference Patterson2002). Emerging developmental disorder that disrupts learning, socialization, or opportunities for experiences and adaptation to adversities could interfere with the development of resilience.
In addition to the significant challenges faced by those with ASD in the course of daily living due to symptoms of the disorder, core features of ASD also threaten the development of adaptive systems and processes that are fundamental to building lifelong human resilience capacity, particularly in the domains of relationships and social functioning. Individuals with ASD become tasked with navigating systems inextricably tied to (mostly unwritten) rules of the social world often without key relational resources of support. In this way, behaviors central to the ASD diagnosis may constrain core learning processes as well as the experiences essential for developing key lifelong adaptive systems of resilience. The dual challenges to resilience in the short and the long term posed by ASD-related disability or symptoms underscore the need to study the ways in which adaptive pathways can emerge early in life despite increased risk.
Charting ASD risk and adaptation through a resilience lens
Research on resilience requires operational criteria and measurement to capture three basic components essential to this framework: risk, adaptation, and resilience. These components are grounded in the following fundamental questions:
-
What are the challenges, liabilities, or conditions linked to the maladaptive phenotypes, outcomes, or pathways of interest?
-
Who is doing well among HR infant siblings (or within another specified risk context) and how do we chart positive development in relation to risk context?
-
What are the indicators or criteria for evaluating adaptation, either at a given point in time or as a pathway (i.e., how well the child is doing)?
-
What fosters adaptive success by these criteria in this context of risk (what are the cascading effects and protective or promotive processes that mitigate against risk effects or promote better adaptation)?
-
Do predictors of positive outcomes indicate lower risk, more assets, or the presence of moderators that protect positive development?
-
How do multilevel risk, promotive, and protective processes interact early in life to shape developmental trajectories?
-
How can a developmental systems resilience framework offer a new lens for thinking about existing findings and opening up new research directions?
Risk in ASD
By definition, resilience must be considered in the context of a designated risk, threat, or disturbance to development that occurs prior to the outcome of interest. Risk refers to the elevated probability of a negative outcome and can divide a population into high and low risk groups or place individuals on a risk gradient (Wright et al., Reference Wright, Masten, Narayan, Goldstein and Brooks2013; Kraemer et al., Reference Kraemer, Kazdin, Offord, Kessler, Jensen and Kupfer1997; Offord & Kraemer, Reference Offord and Kraemer2000). When defining risk, it is also important to delineate the negative outcome of reference. In the context of ASD, we define negative outcomes in terms of the functional, clinical impairment partially constitutive of the ASD diagnosis, as well as the many ASD-related disabilities and challenges that may persist over the life course for those with the disorder. Although, in some cases, risk is indicated by a discrete and measurable occurrence (e.g., a life event), risks often co-occur and they are often continuous, diffuse, and/or difficult to measure (e.g., socioeconomic disadvantage, family dysfunction, or disorders).
Similar to early resilience science investigations of those at heightened risk for schizophrenia (Garmezy, Reference Garmezy and Stevenson1985), quantifying risk for ASD is complex. Many investigators contend that, in most cases, ASD arises probabilistically as a result of multiple processes that may not be necessary or sufficient on their own for the emergence of the disorder, yielding both core features of ASD as well as marked phenotypic variability (Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014; Plomin et al., Reference Plomin, Haworth and Davis2009). For the purposes of this paper, we focus on familial risk as the indicator of risk for ASD and related difficulties, in alignment with the HR infant siblings program of research that prospectively studies the development of HR infants (who do and do not go on to receive ASD diagnoses) and LR infants with no familial history of the disorder. Importantly, we differentiate between risk context (i.e., familial risk for ASD by virtue of having a first-degree relative with the disorder) and risk marker, the term used in the field to reflect further evidence for manifested negative deviations from typical development (e.g., decreased attention to the eyes of others) that are often associated with a future ASD diagnosis.
Notably, in addition to multiple forms of phenotypic heterogeneity, ASD is characterized by etiological heterogeneity, or variability in the genetic and neurobiological processes that give rise to ASD in ways not fully understood as of yet (Betancur, Reference Betancur2011; Constantino, Reference Constantino2019; De Rubeis & Buxbaum, Reference De Rubeis and Buxbaum2015; Huguet & Bourgeron, Reference Huguet, Bourgeron, Sala and Verpelli2016; Satterstrom et al., Reference Satterstrom, Kosmicki, Wang, Breen, De Rubeis and An2020). Etiological heterogeneity of disorders and imperfect measures of risk are common to studies of resilience that seek to sharpen knowledge of etiology through iterative research on intertwining adaptive and risk processes. Although we focus on integrating a resilience perspective in the context of familial risk for ASD, it will be important to expand investigations to other risk contexts (e.g., polygenic risk scores, simplex vs. multiplex familial risk) to understand the roles of differing risk contexts in the manifestation of phenotypic pathways and outcomes.
Criteria for adaptive function in ASD
Studies of resilience also require researchers to establish criteria for adaptive function in order to evaluate who is doing well in development and identify positive pathways of adjustment. Although criteria for adaptive function vary over the course of development, there are common ways that investigators have defined and operationalized positive outcomes at given times in development, including indices of competence in age-salient developmental tasks, psychological wellbeing, or the absence of psychopathology (Masten & Barnes, Reference Masten and Barnes2018; Sroufe et al., Reference Sroufe, Egeland, Carlson and Collins2005; Werner & Smith, Reference Werner and Smith1982). These criteria may include positive evidence (i.e., achieving a particular developmental milestone) or negative evidence (i.e., failing to meet expectations or developing symptoms of maladjustment in a developmental task domain). It is also important to keep in mind that criteria for adaptive functioning are dynamic and varying with respect to development, individual differences, and changing contexts. Moreover, in addition to strategic criteria for assessing adaptation, investigators try to select developmentally strategic time points for measurement in order to maximize prediction of future adjustment. We also recognize the importance of studies charting development beyond the infancy and toddlerhood years.
Criteria for adaptive function are based on well-established hallmarks or milestones of typical development in a given developmental, cultural, and historical context. Some investigators have suggested that individuals with ASD must be included in this conversation to consider adaptive outcomes that may not be obvious to those not on the spectrum (e.g., Burgess & Gutstein, Reference Burgess and Gutstein2007). Further, establishing criteria for adaptive functioning will facilitate robust assays of who is doing well in the contexts that are relevant to the individual, perhaps helping resolve concerns about “shallow” compensation, or alternative processes that only appear adaptive but are easily subject to breakdown, outlined by Livingston and Happé (Reference Livingston and Happé2017).
Diagnosis-oriented criteria
In contrast to a developmental resilience approach that underscores the importance of positive markers of adaptive development, many risk studies, including early ASD research to date, utilize negative evidence to identify who does not meet criteria for disorder. For example, toddlers who do not meet clinical criteria for ASD in the third year of life using gold-standard clinical assessment tools are often considered to be doing relatively well. These criteria, the inverse of many commonly used outcomes in studies of ASD risk, serve as a benchmark but they do not explicitly index the presence of positive adaptive functioning or development. Importantly, not receiving an ASD diagnosis does not necessarily mean an infant is doing well from a broad developmental perspective or in regard to specific developmental tasks other than those used to make the diagnosis. HR infants who do not go onto develop ASD represent a heterogeneous group featuring varying levels and profiles of functioning.
While only about 1 in 5 HR infant siblings will develop ASD, HR unaffected siblings often differ from LR infants in potentially meaningful ways. For example, HR unaffected siblings have been found to have higher severity scores on the Autism Diagnostic Observation Schedule (ADOS) and lower verbal function than LR infants at age 3 (Georgiades et al., Reference Georgiades, Szatmari, Zwaigenbaum, Bryson, Brian, Roberts and Garon2013; Messinger et al., Reference Messinger, Young, Ozonoff, Dobkins, Carter, Zwaigenbaum and Sigman2013). They also have been found to have reduced surgency and effortful control (Clifford et al., Reference Clifford, Hudry, Elsabbagh, Charman and Johnson2013), seek out social interaction less (Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014), as well as exhibit fewer gestures (Mitchell et al., Reference Mitchell, Brian, Zwaigenbaum, Roberts, Szatmari, Smith and Bryson2006) than LR infants in the first 2 years of life. Messinger et al. (Reference Messinger, Young, Ozonoff, Dobkins, Carter, Zwaigenbaum and Sigman2013) used latent class analysis to identify two groups of HR siblings without ASD at age 3, one with high (but still subthreshold) ASD symptoms but with no language or cognitive delays and another with low levels of ASD symptoms but language and cognitive delays. Careful characterization of HR infants who do not develop ASD is important for charting adaptive development.
Dimensional criteria
Diagnostic measures may be best coupled with performance on more specific salient developmental tasks using measures designed for assessing positive outcomes. Positive development can be characterized dimensionally on multiple dimensions of growth or competence (e.g., adaptive functioning, language ability, social engagement). Yet to date, positive development is often characterized as the inverse of risk even when using dimensional measures, for example, by evidence a child has an ADOS score within the range of typical development. Often, HR toddlers who either outperform typically developing peers or are indistinguishable from them on a given measure at age of diagnostic assessment are considered to be doing well. Importantly, measures such as the ADOS were created primarily to determine social disability for the purposes of aiding clinicians in establishing a categorical ASD diagnosis; they were not designed to capture fine-grained variability or changes within more adaptive levels of social behavior. As a result, these measures may be limited in their ability to serve as dimensional markers for positive development, prompting alternative methodological approaches (e.g., Grzadzinski et al., Reference Grzadzinski, Carr, Colombi, McGuire, Dufek, Pickles and Lord2016). For example, adaptive outcomes could be defined by achieving good performance on multiple key developmental domains, such as dimensional assessments of social abilities, social communication, interpersonal synchrony, adaptive daily living skills, language, regulatory abilities, and executive functioning by two or 3 years of age. Criteria for positive outcomes ideally would tap core developmental competencies that predict continued positive development in early childhood and beyond. We suggest that it may be important to combine traditional diagnosis-oriented criteria in conjunction with dimensional metrics of positive development that are designed to measure variability across the spectrum of adaptive to maladaptive functioning.
Resilience in ASD: Predictors of positive development in the context of risk
Once the parameters of risk and adaptive functioning are defined, resilience research is typically focused on explaining positive variations in adaptive outcomes or pathways in the context of the risk conditions under study. In the initial stages of research, resilience investigators often attempt to identify predictors of better adaptation or positive changes over time among individuals in HR groups with the goal of uncovering processes implicated by these predictors (Masten, Reference Masten2007; Werner & Smith, 1992; Wright et al., Reference Wright, Masten, Narayan, Goldstein and Brooks2013). Investigators then turned their focus to understanding the processes through which predictors shape adaptive developmental outcomes.
Resilience researchers traditionally distinguish promotive factors associated with better adjustment across risk levels and protective factors or processes that have a greater or distinctive effect when risk levels are high (Masten, Reference Masten2018; Sameroff, Reference Sameroff2000; Rutter, Reference Rutter2012, Reference Rutter2013; Wright et al., Reference Wright, Masten, Narayan, Goldstein and Brooks2013). Figure 1 illustrates promotive and protective effects in relation to risk. Promotive and protective factors refer to functional effects of variables under study, with a specific risk context and in relation to a specific outcome and can operate at any level of analysis (e.g., brain, behavior, environmental context; Rutter, Reference Rutter2012, Reference Rutter2013). These effects can help illuminate mechanisms by which positive developmental functioning emerges from more general processes and may also be shaped by processes more specific to high-risk contexts. As noted by Rutter (Reference Rutter1987), the mediating mechanisms of protective or promotive factors may be in their ability to reduce the impact of risk by altering the risk factor itself, altering exposure to the risk, harnessing key turning points, and opening up opportunities. Many moderators are assessed on a continuum (e.g., parenting quality) where the negative end of the moderator could be construed as a vulnerability, exacerbating the effects of risk. Importantly, the protective effects for the high end of a moderator may operate developmentally through different processes from the negative effects of the same variable at the other end of a dimension (Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021; Rutter, Reference Rutter1987).

Figure 1. Figure 1 provides an illustration of how promotive and protective processes can interact with risk processes to influence development either directly or indirectly. Figure adapted from Masten (Reference Masten2015, Figure 11.1, p. 267) with permission of the author and Guilford Press.
Promotive processes in early ASD
In the context of risk for ASD, language and regulatory function may reflect promotive processes. Language deficits are not part of the core diagnostic criteria for ASD but those at HR and those with the disorder often struggle with language and functional communication. Language and nonverbal communication skills may represent core promotive processes. Language ability in the first 5 years of life is consistently one of the best predictors of future functioning for those with ASD across multiple domains of adaptation (e.g., Howlin et al., Reference Howlin, Goode, Hutton and Rutter2004; Landa et al., Reference Landa, Gross, Stuart and Bauman2012; Longard et al., Reference Longard, Brian, Zwaigenbaum, Duku, Moore, Smith and Bryson2017; Messinger et al., Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015; Rutter, Reference Rutter1970; Szatmari et al., Reference Szatmari, Bryson, Boyle, Streiner and Duku2003; Turner et al., Reference Turner, Stone, Pozdol and Coonrod2006). Infant sibling studies suggest developmental roots of language capacity may also be promotive. For example, Elsabbagh et al. (Reference Elsabbagh, Bedford, Senju, Charman, Pickles and Johnson2014) found that increased visual scanning of the mouth region during complex onscreen scenes at 7 months of age was associated with better expressive language at 36 months of age for all infants regardless of ASD risk or outcome. A similar finding of positive associations between infants fixating on their mother’s mouth during social interaction at 6 months and expressive language at 24 months of age was found by Young et al. (Reference Young, Merin, Rogers and Ozonoff2009). Further, abilities to respond to bids for joint attention, or sharing attention to an external object with another, at 14 months have also been found to be positively associated with future expressive and receptive language abilities for all risk and outcome groups (Sullivan et al., Reference Sullivan, Finelli, Marvin, Garrett-Mayer, Bauman and Landa2007). Others have found that the Early Learning Composite (including receptive and expressive language) of the Mullen Scales of Early Learning at 6 and 12 months predicts likelihood of being in a latent trajectory featuring high adaptive functioning from 12 to 36 months, regardless of ASD risk or diagnostic outcome (Sacrey et al., Reference Sacrey, Zwaigenbaum, Bryson, Brian, Smith, Raza, Roberts, Szatmari, Vaillancourt, Roncadin and Garon2019). Importantly, more studies specifically powered to detect any interactions with ASD risk and outcome are needed to build on these findings.
Executive functions and their regulatory precursors in development (e.g., executive attention; see Johnson et al., Reference Johnson, Charman, Pickles and Jones2021) provide another example of widely reported promotive effects in the context of risk for ASD (e.g., Johnson, Reference Johnson2012) as well as numerous other risk groups (Blair & Raver, Reference Blair and Raver2015; Happé et al., Reference Happé, Booth, Charlton and Hughes2006). Early regulatory functions may be another potential indicator of positive outcomes for both HR and LR infants, although more evidence is needed. For example, work by Bedford et al. (Reference Bedford, Gliga, Hendry, Jones, Pasco and Charman2019) identified emerging regulatory function, which is a precursor to later-emerging executive function abilities, at 14 months as a possible promotive factor for later ASD outcomes at 7 years of age, mitigating risk for ASD. They found a main effect such that regulatory function was negatively associated with later ASD traits for HR and LR infants (although the authors acknowledge the study was underpowered to test interactions with risk group). They also found that regulatory function moderated the association between early risk markers (i.e., scores on the Autism Observation Scale for Infants) and ASD traits at 7 years of age; early risk markers were predictive of later ASD traits only for infants with low regulatory function. This finding is consistent with the possibility that early emerging regulatory function functions as a promotive factor, possibly compensating for ASD-related atypicalities by supporting the flexible use of alternative social communication strategies.
Protective processes in early ASD
Protective factors may be either uniquely or especially predictive of positive outcomes for HR infants in comparison to LR infants, reflecting an interaction effect of the protective factor with risk group status. Some protective factors show effects only in HR contexts whereas others have promotive as well as protective effects; in the latter case, the factor is generally related to better outcomes on the criterion of interest for both HR and LR groups (a main effect), but also shows an additional benefit among the HR group (a protective interaction effect).
It should be noted that some moderators of risk function as vulnerability or liability factors (worsening the effects of risk exposure), while others have protective effects (buffering or mitigating the negative effects of risk, and some may work both ways. Neglectful parenting, for example, can exacerbate the risks of poverty and dangerous environments, whereas positive parenting can mitigate the effects of these environmental risks on development (Masten & Cicchetti, Reference Masten and Cicchetti2016; Rutter, Reference Rutter1987). Further, as noted above, effective parenting is one of the processes that can show protective as well as promotive effects. Good parenting is promotive because it is associated with strong outcomes in the general population (regardless of risk), but it may be particularly important in contexts of risk, such as for families experiencing homelessness or in a risk-laden context of dangerous neighborhoods with high levels of poverty, marginalization, and crime (Masten & Palmer, Reference Masten and Palmer2019).
Considering the female protective (and promotive) effect
Research investigating sex differences in ASD rates of diagnosis and phenotypic presentation reflects a clear pattern of reduced ASD-related disability for females, despite the presence of risk, with unknown underlying developmental processes. We suggest this line of research could benefit from a developmental resilience approach, illustrating the potential value of integrating resilience concepts in the study of ASD. Mounting evidence suggests that for reasons still not well understood, females may be categorically protected from ASD (Constantino, Reference Constantino2011, Reference Constantino2016, Reference Constantino2017; Werling, Reference Werling2016). It has been well-established that ASD, as it is currently defined and diagnosed, does not impact males and females equally. The ratio of males to females in ASD varies from about 3–4:1 for clinically referred samples (Constantino, Reference Constantino2016; Messinger et al., Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015), to approximately 2:1 in community samples (Constantino & Charman, Reference Constantino and Charman2012). A sex bias in ASD prevalence reveals that overall, fewer females than males meet clinical criteria for an ASD diagnosis (e.g., Constantino et al., Reference Constantino, Zhang, Frazier, Abbacchi and Law2010; Messinger et al., Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015; Ritvo et al., Reference Ritvo, Jorde, Mason-Brothers, Freeman, Pingree, Jones and Mo1989) leading to the proposal of a FPE (Robinson et al., Reference Robinson, Lichtenstein, Anckarsäter, Happé and Ronald2013).
It has been suggested that, if females are protected from ASD, it may require more extreme symptomology and greater accumulation of risk for them to reach the criteria for a diagnosis compared to males. Many studies find no overall sex differences in core ASD symptom severity for those diagnosed with the disorder (e.g., Carter et al., Reference Carter, Black, Tewani, Connolly, Kadlec and Tager-Flusberg2007; Constantino, Reference Constantino2017; Kopp & Gillberg, Reference Kopp and Gillberg2011; Lai et al., Reference Lai, Tseng, Hou and Guo2012; Messinger et al., Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015). However, existing evidence does suggest that more accumulated genetic and behavioral risk factors are required for females to receive an ASD diagnosis compared to males. Females with an ASD diagnosis are more likely than males to have lower IQ and other comorbid issues (e.g., Fombonne, Reference Fombonne2009; Gamsiz et al., Reference Gamsiz, Viscidi, Frederick, Nagpal, Sanders, Murtha and Morrow2013; Lord et al., Reference Lord, Schopler and Revicki1982; Ratto et al., Reference Ratto, Kenworthy, Yerys, Bascom, Wieckowski, White and Anthony2018; Volkmar et al., Reference Volkmar, Szatmari and Sparrow1993), suggesting that females may only be diagnosed when they have other accompanying symptoms. Further, females who are diagnosed with ASD have been found to carry greater genetic burden for ASD (De Rubeis et al., Reference De Rubeis, He, Goldberg, Poultney, Samocha and Ercument Cicek2014; Iossifov et al., Reference Iossifov, O’Roak, Sanders, Ronemus, Krumm and Levy2014; Jacquemont et al., Reference Jacquemont, Coe, Hersch, Duyzend, Krumm, Bergmann and Eichler2014; see also Sanders et al., Reference Sanders, Ercan-Sencicek, Hus, Luo, Murtha and Moreno-De-Luca2011) compared to males with the diagnosis. These lines of work point to potentially different thresholds for reaching an ASD diagnosis by sex such that a greater burden is required for females to make the “quantum leap” (Constantino, Reference Constantino2016; Werling & Geschwind, Reference Werling and Geschwind2013), crossing a threshold from ASD-like traits to a clinically impairing diagnosis (Reich et al., Reference Reich, Cloninger and Guze1975). In other words, it appears that in the absence of additional compounding cognitive and other deficits, females may be better able to adapt to ASD liability (Constantino & Charman, Reference Constantino and Charman2012).
Within HR infant sibling studies to date, evidence for sex differences is limited. Most studies have only included sex as a covariate in their models and thus were not designed or powered to measure sex differences. However, there are some exceptions. Zwaigenbaum et al. (Reference Zwaigenbaum, Bryson, Szatmari, Brian, Smith, Roberts and Roncadin2012) found small sex differences favoring females in fine motor skills as well as aspects of adaptive behavior at age 3 years for both HR and LR infants, consistent with a female promotive effect. Similarly, Messinger et al. (Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015) found sex differences in cognitive performance and repetitive behaviors in both HR and LR infants, reflecting more general population-level sex differences that are not specific to ASD. Further, other studies have found that among LR and non-diagnosed HR infants, males exhibited higher ASD symptom severity scores as well as lower levels of cognitive and adaptive functioning than females (Charman et al., Reference Charman, Young, Brian, Carter, Carver and Chawarska2017; Messinger et al., Reference Messinger, Young, Ozonoff, Dobkins, Carter, Zwaigenbaum and Sigman2013), further suggestive of male traits closer to those of clinical ASD. Of note, even large studies often face challenges establishing the requisite statistical power to test for three-way interactions that could reveal HR-specific gender moderating effects. Nonetheless, it could be the case that more general male delays and atypicalities compound and interact over the first years of life to confer a vulnerability that increases likelihood of reaching clinical criteria for ASD compared to females.
To date, there is little understanding of what specifically about being female is responsible for this sex bias and how these factors operate early in development to results in the FPE. From an integrative resilience perspective, this evidence suggests that there may be vulnerability, protective, and/or promotive factors spanning multiple levels of analysis underlying this sex bias. A developmental resilience framework may help to elucidate mechanisms that tend to differ by sex, perhaps both in the general population and uniquely in HR contexts that, on average, may steer female infant developmental systems away from, and male development toward, ASD-related disability early in life. Three major possibilities could account for the female advantage in the prevalence of ASD: compared to males, females may (1) have lower manifested ASD risk or less vulnerability (Rutter, Reference Rutter1987); (2) have more assets that counter or compensate for ASD risk processes; and/or (3) be protected by processes that alter the relationship between early risk or vulnerability and later ASD-related outcomes. While the first possible explanation of the FPE is consistent with a deficit-focused approach, the additional two possibilities are more congruent with a broader resilience-focused perspective. Evidence on each possibility is considered in the following section.
Lower ASD risk and neurodevelopmental vulnerability
It is possible that females may have lower risk or vulnerability for ASD than males. To date, genetic work suggests that ASD is likely not inherited through sex-specific mechanisms, as the vast majority of the heritable influence of ASD is attributable to autosomes, not sex chromosomes (Constantino, Reference Constantino2017) and no sex differences have been found in the autosomal loci known to confer risk (Mitra et al., Reference Mitra, Tsang, Ladd-Acosta, Croen, Aldinger, Hendren and Weiss2016). Moreover, others have deemed factors unique to male embryological development, such as circulating prenatal testosterone, unlikely to cause sex differences in ASD prevalence (Eriksson et al., Reference Eriksson, Lundström, Lichtenstein, Bejerot and Eriksson2016).
Alternatively, females could have less overall vulnerability than males to neurodevelopmental disorders (inclusive of ASD) in general. As other authors note, it is important to examine sexual dimorphisms in the greater population (Constantino, Reference Constantino2017) to understand how females might carry some added protection or males might be subject to increased vulnerability. Indeed, some evidence suggests that, in general, factors associated with being male confer added vulnerability to a wide range of neurodevelopmental and other hazards (McCarthy & Wright, Reference McCarthy and Wright2017; Rutter, Reference Rutter1987), especially early in development. In the general population, males are four to eight times more likely than females to have a neurodevelopmental disorder (Bale, Reference Bale2016). Sexually dimorphic processes in development may move males closer to neurodevelopmental vulnerability thresholds that might then be more susceptible to genetic or other perturbations (Constantino, Reference Constantino2017). For instance, the female distribution of autistic traits has been found to be more normative than that of males throughout the general adult population (Constantino & Charman, Reference Constantino and Charman2012; Hull et al., Reference Hull, Mandy and Petrides2017) and among siblings of those with ASD (Constantino, Reference Constantino2017). Additionally, neural evidence suggests that the white and gray matter neuroanatomy of ASD differs between adult males and females, the female presentation overlaps with areas that were sexually dimorphic in typical controls (Lai et al., Reference Lai, Lombardo, Suckling, Ruigrok, Chakrabarti, Ecker and Baron-Cohen2013), and that the male brain requires milder alterations to exhibit neurodevelopmental disorder than females (Jacquemont et al., Reference Jacquemont, Coe, Hersch, Duyzend, Krumm, Bergmann and Eichler2014). This evidence suggests a female promotive effect, or something associated with positive development (i.e., strong social abilities) that is characteristic of females in the general population could be partially responsible for the sex difference in ASD.
Developmental advantages or assets that counter or compensate for ASD risk
The FPE also could be explained by favorable characteristics or processes that are more likely to occur in females, or occur with more advantageous timing, that could counter risks or promote positive outcomes. Some of the attributes that promote better outcomes could be tangential to core ASD features, such as language skills, that serve to compensate for the negative impact of ASD symptoms via alternative routes to adaptive outcomes.
Being female could confer developmental advantages associated with outperforming males on key foundational abilities, including earlier achievement of developmental milestones directed related to ASD symptomology (e.g., social attention) or tangential to core ASD symptoms (e.g., language). Generally, females have been found to meet social and linguistic developmental milestones earlier than males (Eriksson et al., Reference Eriksson, Marschik, Tulviste, Almgren, Pérez Pereira, Wehberg and Gallego2012; Galsworthy et al., Reference Galsworthy, Dionne, Dale and Plomin2000; Gunnar & Donahue, Reference Gunnar and Donahue1980; see Lutchmaya & Baron-Cohen, Reference Lutchmaya and Baron-Cohen2002; Messinger et al., Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Szatmari, Brian, Smith, Roberts and Roncadin2012). It could be the case that small initial differences in the timing of achieving key socio-communicative developmental milestones are promotive for general development and possibly also protective for females in the context of ASD risk. Perhaps the normative, rich experience (especially in the social domain) gained during sensitive periods of development through meeting developmental milestones early may compensate for a particular ASD liability. These enriched experiences may open up critical opportunities for experience-dependent development, initiating positive cascades that increase likelihood of a more typical developmental trajectory (e.g., Bedford et al., Reference Bedford, Jones, Johnson, Pickles, Charman and Gliga2016), with particularly pronounced effects in domains relevant to ASD such as social communication. These positive cascades could occur through many neurobiological, behavioral, and environmental processes, beginning with sex differences in social predispositions present at birth (e.g., Connellan et al., Reference Connellan, Baron-Cohen, Wheelwright, Batki and Ahluwalia2000). Additionally, parents may interact with and interpret infant signals differently based on the infant’s sex and their own gender (Jaffe et al., Reference Jaffe, Beebe, Feldstein, Crown, Jasnow, Rochat and Stern2001; Johnson et al., Reference Johnson, Caskey, Rand, Tucker and Vohr2014; Weinberg et al., Reference Weinberg, Tronick, Cohn and Olson1999; Weinraub & Frankel, Reference Weinraub and Frankel1977) that may contribute to sex differences in early experience.
In support of this possibility, within the HR infant sibling design, Chawarska et al. (Reference Chawarska, Macari, Powell, DiNicola and Shic2016) found enhanced social attention in HR females between 6 and 12 months compared to males from both risk groups and LR females, which was in turn associated with less severe social impairment at 2 years. Importantly, these associations remained even after controlling for verbal, nonverbal, and social outcomes. The authors discuss how such a social orienting advantage during a sensitive period of development might confer initially small alterations in the type and timing of learning opportunities that could exponentially improve the quality of early social experience, with cascading long-term effects. They suggest that their findings could have roots in enhanced social motivation or heightened vigilance systems. This finding provides some evidence that atypically high social attention may confer an advantage to females and may uniquely counter ASD-related risks. It could be the case that atypically high social orienting in the first year is associated with increased caregiver engagement, more frequent and higher quality interactions, early achievement of joint attention, and early word learning, setting infants on a path of potentiated social development.
Buffering effects that modulate the effects of risk on outcomes
Resilience factors also may function to buffer or mitigate against the negative effects of risk on a given outcome by altering the functional relation between known risk markers and ASD-related outcomes, usually captured analytically by statistical interaction effects. For example, an oft-cited study suggests that a known SHANK1 (involved in formation and function of neuronal synapses) genetic mutation is associated with ASD outcomes for males and anxiety for females (Sato et al., Reference Sato, Lionel, Leblond, Prasad, Pinto and Walker2012). This finding suggested that something associated with females protects against the specific ASD-related liability conferred by this genetic mutation (and others, e.g., Willsey et al., Reference Willsey, Exner, Xu, Everitt, Sun, Wang and State2021) and is subject to continued scientific investigation.
Within the HR infant siblings design, a study by Bedford et al. (Reference Bedford, Jones, Johnson, Pickles, Charman and Gliga2016) examined sex differences in the association between key early risk markers – Autism Observation Scale for Infants scores, attention disengagement speeds, and gaze following– at age 14 months and future ASD traits at age 3 years. Although they found no sex differences in the risk markers themselves, they found sex differences in their functional relations. Their results suggest that the risk markers only predict later ASD traits for males (even when controlling for Mullen Scales of Early Learning scores that exhibited a female advantage) for both HR and LR infants. Although the risk markers were present in both sexes, they did not forecast ASD traits for females in the same way as they did for males. On their own, these results suggest unidentified moderating effects related to sex that could reflect male vulnerability processes, female promotive processes, or a combination of both. Of note, other studies have found sex differences in levels of risk markers, such as dimensions of cognitive function, but no interaction between sex and risk marker to predict outcomes (e.g., Messinger et al., Reference Messinger, Young, Webb, Ozonoff, Bryson, Carter and Zwaigenbaum2015; Zwaigenbaum et al., Reference Zwaigenbaum, Bryson, Szatmari, Brian, Smith, Roberts and Roncadin2012).
Future research for investigating female protection and promotion
These investigations of the FPE in ASD suggest there may be protective and promotive effects associated with being female, as well as vulnerabilities associated with being male. Promotive processes may include language or social milestones achieved earlier or at more skilled levels, as well as less vulnerability to neurodevelopmental disorders in general. Protective processes associated with being female may include atypically augmented attention to social features of the environment, specifically in the context of risk for ASD, affording early critical opportunities for social experience and learning that uniquely counter ASD risk. Studies also point to unidentified factors that may alter relations between known risk markers and ASD outcomes for females.
HR infant sibling studies are well poised to further investigate the protective and promotive processes underlying ASD-related sex differences. More work is needed to replicate and extend existing findings to uncover the more proximal systems driving these sex-specific differences, differential risk thresholds, and their relevant developmental timescales. It is likely that the sex bias in ASD prevalence arises through a combination of differential risk or vulnerability as well as promotive and protective processes, that interact with each other across biological and behavioral domains and exert influence at different time points in early development. It could be the case that early in development, normative female-favoring sex differences couple with male neurobiological liability for disorder to exacerbate both male vulnerabilities and female competence specifically within in the social communication domain. Females may meet social communication developmental milestones earlier than males, affording key promotive social experiences that set in motion positive developmental cascades, through which small differences build protection against ASD vulnerability. They may provide key ASD-specific assets (e.g., social engagement) and other compensatory skills (e.g., language), thereby boosting adaptation and altering the relationship between risk and future ASD-specific outcomes. Delays in key milestones coupled with degrees of more general brain-based neurodevelopmental vulnerabilities could compound at specific points in development, resulting in fewer and lower quality social learning experiences for males.
Future directions for HR infant sibling studies could include studies investigating promotive and protective processes through large longitudinal studies of HR and LR infants that are well-powered to detect sex differences, as illustrated in Figure 2. These studies should also include measures of potential candidate processes, such as language and social attention, to not only test for sex effects but also to attempt to explain these sex differences by their potential mechanisms at multiple levels of analysis (e.g., brain, behavior).
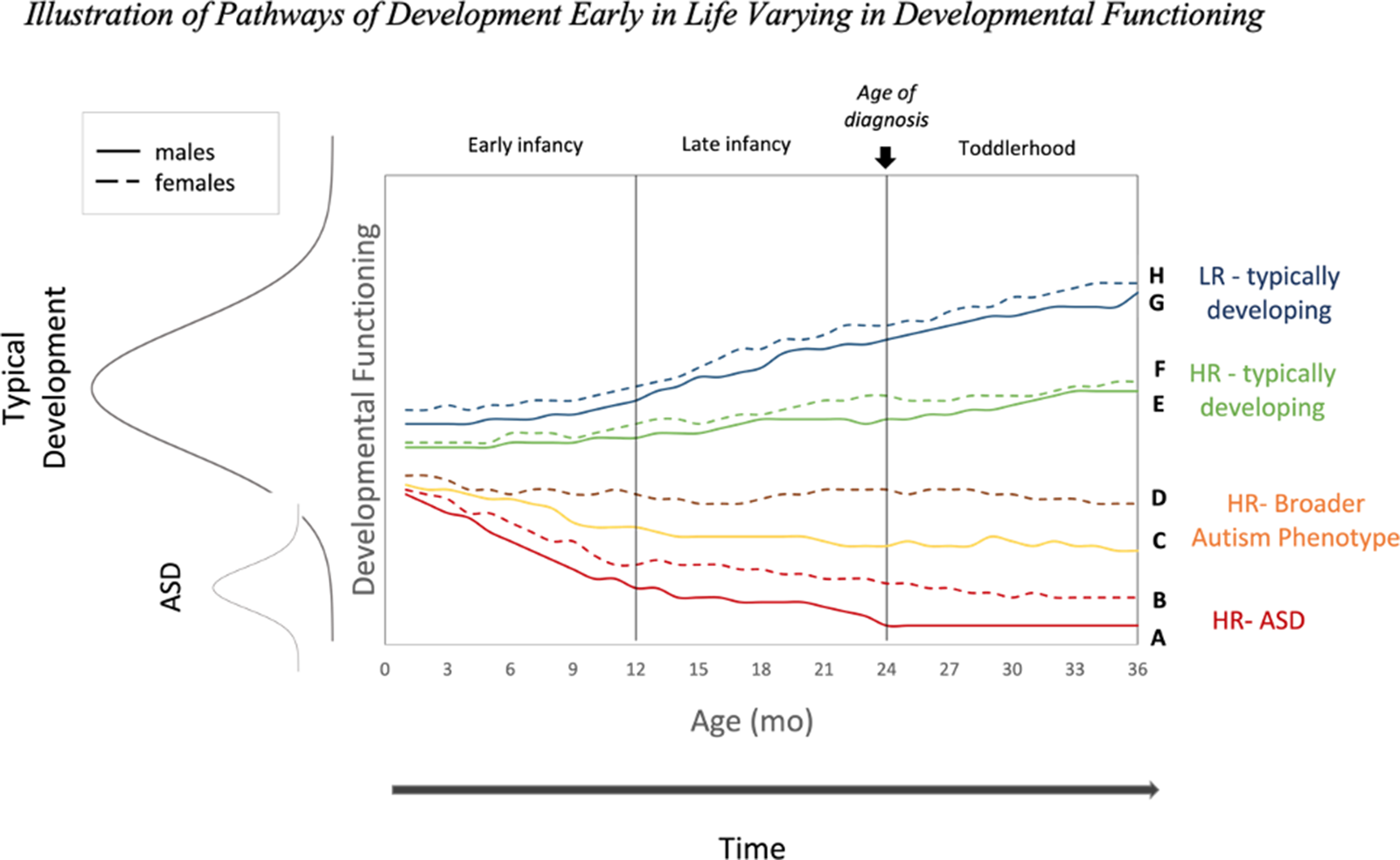
Figure 2. Illustration of pathways of development early in life for a given measure of ASD-related developmental functioning (e.g., social communication, adaptive function) for males (solid lines) and females (dashed lines). More ASD-like functioning is represented by lower levels of developmental functioning (Y-axis) in relation to the distribution of neurotypical functioning. This figure depicts a possible sex-related promotive factor, indicated by better female functioning relative to males for HR and LR infants. Pathways are colored by eventual diagnostic outcome (i.e., eventual ASD, BAP, or typically developing outcomes). Not all possible pathways are shown. The age of diagnosis marker is for illustration purposes, with the recognition that reliable diagnoses can sometimes be made earlier at 18 months and in practice children are often not diagnosed until 4–5 years of age.
We suggest that future studies capitalize on these group-level sex differences in ASD diagnosis rates to better understand individual differences, or those who deviate from their group averages. Much can be gleaned from the HR females who do develop ASD (Figure 2, path B) and HR males who do not (Figure 2, paths C and E), as these groups may reflect different processes at play. Sex differences and their mechanisms could be investigated by longitudinally charting the differential patterns by which male and female development might coalesce along different trajectories of relative (mal)adaptation early in life in HR and LR infants. For instance, examining how the factors and timescales associated with females who exhibit poorer social functioning early in development followed by improvement might differ from those of males who exhibit the same pattern (all of which could vary between HR and LR groups). The former could reveal ways in which positive cascades operate (and how promotive and protective processes may break down) while latter may demonstrate the emergence (and disruption) of a negative cascade. Studies of HR infant siblings are well poised to investigate some of these possibilities utilizing multiple measures and densely sampled longitudinal designs.
Additional evidence for potential protective and promotive processes in ASD
In addition to the FPE, evidence from studies of children who develop relatively well in the context of heightened ASD risk suggest there may be other potential protective and promotive processes at play. HR infant sibling programs of research seek to enrich their samples for ASD outcomes, yet many infants in their samples do not develop the disorder. Despite the many ways early risk markers accumulate, many infants in samples enriched for ASD risk (often including a small number who eventually gain an ASD diagnosis) tend to do well early in development and meet some appropriate milestones. Constantino and Charman (Reference Constantino and Charman2016) reflect on findings of striking group-level stability over time but also the presence of slow and steady “recovery,” and even improvement, in adaptive function for some HR infants. In the majority of infants, Elsabbagh and Johnson (Reference Elsabbagh and Johnson2010) have argued, it is likely that “well-described processes of brain adaptation may restore the developmental trajectory to a typical course” (p. 84).
A multitude of studies using group-based approaches to investigate clusters of infants based on their cognitive, social, and adaptive functioning profiles identify subgroups that exhibit strong stable or improving functioning in the first years of life, in addition to declining groups. Classification of young children from clinically referred samples consistently demonstrates groups with marked improvement, for up to a quarter of the sample, in trajectories of social communication (Kim et al., Reference Kim, Bal, Benrey, Choi, Guthrie, Colombi and Lord2018; Lord et al., Reference Lord, Luyster, Guthrie and Pickles2012) as well as language and non-verbal IQ (Visser et al., Reference Visser, Rommelse, Lappenschaar, Servatius-Oosterling, Greven and Buitelaar2017). Authors often note the presence of cross domain divergences; that nonverbal and verbal communication improve for most children with ASD despite worsening trajectories of core ASD symptoms. HR infant sibling studies have also identified relatively more adaptive subgroups marked by high-scoring stable trajectories (compared to decreasing groups), significant increases (compared to decreasing or steady groups), or relatively higher rates of growth (compared to groups with lower growth rates). These more adaptive subgroups consistently include HR infants who do and do not develop ASD, for cognitive development (16% of infants who developed ASD, 52% of unaffected siblings; Brian et al., Reference Brian, Roncadin, Duku, Bryson, Smith, Roberts and Zwaigenbaum2014), expressive and receptive language (4%–13% of infants who developed ASD, 14%–40% HR unaffected infants; Franchini et al., Reference Franchini, Duku, Armstrong, Brian, Bryson, Garon and Smith2018; Longard et al., Reference Longard, Brian, Zwaigenbaum, Duku, Moore, Smith and Bryson2017), gesture production (10% of those who developed ASD, 22% of HR unaffected; Franchini et al., Reference Franchini, Duku, Armstrong, Brian, Bryson, Garon and Smith2018), and adaptive functioning (5%–9% of infants who developed ASD, 5% of HR unaffected infants; Bussu et al., 2019; Sacrey et al., Reference Sacrey, Zwaigenbaum, Bryson, Brian, Smith, Raza, Roberts, Szatmari, Vaillancourt, Roncadin and Garon2019).
Together, these findings highlight the heterogeneity of prodromal phenotypes, the presence of unknown potential subgroups with uneven profiles featuring both strengths and difficulties, and the consistent presence of marked improvement or stabilization of strong functioning in some HR infants (including some of those who go on develop ASD). Although designed to elucidate risk markers for ASD-related disability, these studies may provide clues to potential interacting systems of resilient adaptation and their relevant developmental timescales.
Future directions for integrated risk-and-resilience studies of early ASD
Many prospective HR infant sibling studies aim to chart the development of HR infant siblings in order to compare the trajectories of those who develop ASD, those who do not, and LR typically developing infants. A developmental resilience approach calls for establishing consensus for charting who is doing well, ascertaining markers of positive functioning (e.g., social communication skills, social synchrony with others, adaptive behavior), and assessing promotive and protective factors at multiple levels of analysis and their relevant timescales. In addition, it will be important to leverage densely sampled prospective longitudinal designs and mixed person- and variable-centered methods to investigate pathways of adaptive functioning, including the presence of dynamic and potentially uneven profiles, and the factors that shape these trajectories in the first years of life. We highlight some potential future directions below.
Establishing key multilevel markers and processes of positive functioning
Many reviews on HR infant siblings research detail an extensive matrix of behavioral and brain markers in the first 2 years of life that are associated with familial risk, future acquisition of an ASD diagnosis, and severity of ASD symptoms (e.g., Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016; Gliga et al., Reference Gliga, Jones, Bedford, Charman and Johnson2014; Johnson et al., Reference Johnson, Gliga, Jones and Charman2015a; Jones et al., Reference Jones, Gliga, Bedford, Charman and Johnson2014; Varcin & Jeste, Reference Varcin and Jeste2017). These risk markers are often operationalized as delays and atypicalities in key developmental milestones compared to both LR typically developing infants and HR infants who did not develop the disorder and span multiple domains. Many of these risk markers have been found to differentiate HR from LR infants, as well as those who will eventually develop ASD (e.g., Miller et al., Reference Miller, Iosif, Hill, Young, Schwichtenberg and Ozonoff2017; Stallworthy et al., Reference Stallworthy, Lasch, Berry, Wolff, Pruett and Marrus2021; Sullivan et al., Reference Sullivan, Finelli, Marvin, Garrett-Mayer, Bauman and Landa2007). Importantly, the majority of these studies were designed and powered to interrogate the manifestations of risk, or negative deviations from typical development; they chart how risk markers differ between diagnostic groups, forecast a categorical ASD diagnosis, or predict ASD symptomology.
Although those are important goals, studies crafted for the study of risk with measures created primarily for diagnostic categorization markers cannot fully elucidate the relevant markers and processes through which positive development emerges. For example, a study demonstrating that responding to joint attention abilities are impaired in HR infants who develop ASD compared to other HR and LR infants cannot illuminate the processes associated with better-than-expected development of effective joint attention in spite of risk. High levels of responding to joint attention may interact with other competencies to exhibit potential positive developmental cascades, alter relations between risk markers and negative outcomes, and these processes may differ by risk group.
Investigating developmental pathways, sensitive periods, and cascades
The first few years of life mark a period of unparalleled development of brain and behavior, limiting the predictive and explanatory power of markers at any given point in time. Arguably, more important than the markers themselves is how they function in early development and in relation to other levels of analysis (Rutter, Reference Rutter1987). Further, as noted by Livingston and Happé (Reference Livingston and Happé2017), single markers of developmental functioning likely index the interactive manifestation of both risk and adaptive processes at any given point in time. A developmental resilience framework underscores the inextricable role of development in these research efforts. The effects of a given factor or influence on the pathways toward or away from ASD-related disability may vary by age, and growth may interact by sex, risk indicator, and other processes. In their recent work, Johnson et al. (Reference Johnson, Charman, Pickles and Jones2021) posit that neurocognitive modifier systems based in anterior frontal cortex exert compounding or compensating effects on early emerging sensorimotor deviations associated with ASD, and “push individual developmental trajectories towards particular common behavioral phenotypes” (p. 618). These modifiers are thought to represent key compensatory processes in development and illuminate an important area of future study at the neural level from a resilience perspective. Accordingly, a developmental resilience systems approach suggests a focus on multisystem pathways of development over time, leading up to and following common diagnostic time frames (i.e., the first 3 years of life), in order to better understand markers of risk and resilience processes, how they interact across psychophysiological systems and time, and function to steer development to and away from ASD-related disability.
Researchers of early risk markers have recognized the importance of densely sampled longitudinal designs (e.g., Klin & Jones, Reference Klin and Jones2015; Shen & Piven, Reference Shen and Piven2017) for better understanding within- and between-person divergences from typical infant development. Within the first 3 years of life, trajectories of behavior –such as looking to the eyes (Jones & Klin, Reference Jones and Klin2013) and faces (Ozonoff et al., Reference Ozonoff, Iosif, Baguio, Cook, Hill, Hutman and Young2010) of others, speed and flexibility of visual orienting (Elsabbagh et al., Reference Elsabbagh, Fernandes, Jane Webb, Dawson, Charman and Johnson2013), temperament (del Rosario et al., Reference del Rosario, Gillespie-Lynch, Johnson, Sigman and Hutman2014), repetitive behaviors (Wolff et al., Reference Wolff, Botteron, Dager, Elison, Estes, Gu and Piven2014), gestures (Landa et al., Reference Landa, Holman and Garrett-Mayer2007), shared smiles, and vocalizations (Ozonoff et al., Reference Ozonoff, Iosif, Baguio, Cook, Hill, Hutman and Young2010), joint attention (Ibañez et al., Reference Ibañez, Grantz and Messinger2013; Nyström et al., Reference Nyström, Thorup, Bölte and Falck-Ytter2019; Yoder et al., Reference Yoder, Stone, Walden and Malesa2009), cognitive abilities (McDonald et al., Reference McDonald, Senturk, Scheffler, Brian, Carver, Charman and Jeste2020) –as well as brain –such as hyper-expansion of cortical surface area (Hazlett et al., Reference Hazlett, Gu, Munsell, Kim, Styner and Wolff2017), brain overgrowth (Hazlett et al., Reference Hazlett, Gu, Munsell, Kim, Styner and Wolff2017), EEG (Gabard-Durnam et al., Reference Gabard-Durnam, Wilkinson, Kapur, Tager-Flusberg, Levin and Nelson2019) –differentiate HR infants who will go on to develop ASD from those who do not. Studies within this framework that are powered and designed to test for moderation could be useful for clarifying for whom these relation hold and the processes that explain adaptive individual variation despite risk.
Similar studies conducted within a resilience framework for understanding pathways toward positive developmental outcomes can illuminate the developmental course, inflection points, and multisystem pathways that diverge toward adaptive outcomes, away from ASD-related disability. Further, mixed person- and variable approaches, such as clustering techniques (e.g., Brian et al., Reference Brian, Roncadin, Duku, Bryson, Smith, Roberts and Zwaigenbaum2014; Bussu et al., Reference Bussu, Jones, Charman, Johnson, Buitelaar and Baron-Cohen2018; Franchini et al., Reference Franchini, Duku, Armstrong, Brian, Bryson, Garon and Smith2018; Hendry et al., Reference Hendry, Jones, Bedford, Andersson Konke, Begum Ali, Bölte, Brocki, Demurie, Johnson, Pijl, Roeyers, Charman, Achermann, Agyapong, Astenvald, Axelson, Bazelmans, Blommers, Bontinck and van Wielink2020; Sacrey et al., Reference Sacrey, Zwaigenbaum, Bryson, Brian, Smith, Raza, Roberts, Szatmari, Vaillancourt, Roncadin and Garon2019), as well as network-based approaches (e.g., Ioannidis et al., Reference Ioannidis, Askelund, Kievit and van Harmelen2020; Kalisch et al., Reference Kalisch, Cramer, Binder, Fritz, Leertouwer, Lunansky and van Harmelen2019) have the potential to shed light on ways adaptive capacities hang together or exhibit uneven profiles and model inter-related causal configurations of risk and adaptive processes. These studies could help identify possible critical windows in early development, or the time frames during which early abilities demonstrate the most variability, exhibit rapid change, are most tightly interwoven with other domains, and are most predictive of future adaptive outcomes.
The rapid increase in early intervention work seen in recent years (Siller & Morgan, Reference Siller, Morgan, Siller and Morgan2018) illustrates a powerful test of resilience models. The scientific study of resilience in children at risk has had a transformative effect on how intervention and prevention efforts are conceptualized, as models in developmental psychopathology shifted from a deficit-oriented approach to an emphasis on strengths and resources in the person, their relationships, and the broader context (Masten, Reference Masten2011). Early interventions for ASD aim to modify the quality and quantity of experiences in order to alter cortical organization, enhance learning, and improve early development during sensitive periods (Bradshaw et al., Reference Bradshaw, Steiner, Gengoux and Koegel2015). The targets (e.g., joint engagement), approaches (e.g., parent-mediated; Oono et al., Reference Oono, Honey and McConachie2016), and timing (e.g., in the first year of life) of successful early intervention can help to identify malleable protective and promotive factors and reveal how they may influence developmental task achievement and core ASD symptoms and clarify the nature of cascades during sensitive periods. Early intervention work can help inform risk and resilience science by helping address questions such as the following: What key systems are involved in promoting positive development? Do early interventions succeed by alleviating core ASD deficiencies (e.g., social communication difficulties) or strengthening separate compensatory processes (e.g., language abilities)? Are they similar to early interventions for other HR groups? How do parent-mediated interventions function? Is there evidence of developmental cascades or more pronounced and long-lasting effects during specific developmental time windows?
Reframing existing findings
In addition to catalyzing new research efforts, a developmental systems resilience perspective can also reframe existing work in informative ways. In addition to reconsidering the FPE in greater depth, it could be useful for interrogating the persistent findings of very few overt behavioral risk markers for ASD in the first year of life. In other words, HR infants who will go on to develop ASD-related disability can often appear indistinguishable from their typically developing peers on key developmental milestones. This puzzle of the first year suggests that the earliest roots of ASD-related development may be more difficult to detect, possibly more subtle, general, widespread, or later emerging (Elsabbagh & Johnson, Reference Elsabbagh and Johnson2016). During this first year of life, when HR infants who go on to develop ASD often appear indistinguishable from their LR counterparts, it is possible that risk has not yet manifested, or various compensatory and protective processes are transiently present but require more sensitive instruments to measure. A developmental resilience framework also invites a refocus on the finding that not all infants who go on to eventually receive a diagnosis exhibit the same deficits at the earliest time of diagnosis. While the stability of an ASD diagnosis at 18 and 24 months has been found to be high, many children who receive a diagnosis at 36 months were undetected at early time points (Ozonoff et al., Reference Ozonoff, Young, Landa, Brian, Bryson, Charman and Iosif2015): 38% of those diagnosed at 3 years had not met clinical criteria at either of those earlier time points. The authors note that infants diagnosed as negative for ASD at early time points and diagnosed with the disorder at 36 months exhibited higher developmental functioning at the time, displaying fewer ASD symptoms and higher verbal and nonverbal abilities (Rogers & Talbott, Reference Rogers, Talbott, Hodapp and Fidler2016). For both lines of inquiry, we might ask questions including: is the apparent more adaptive functioning during these periods indicative of early protective or promotive processes, obfuscated by studies focused on risk, that then break down for some infants? Are these examples of risk that has not yet manifested, if so why? Are these examples of insufficient measurement precision?
Varying ASD risk context
The study of resilience processes necessitates attention to risk context and benefits from studies that take different approaches to risk. Importantly, by definition, risk is probabilistic rather than deterministic –risk indices serve as indicators of increased likelihood of the occurrence of a threat to development. In reality, it is likely that multiple psychophysiological processes interact at different timescales, that are not fully known, within specific environments to produce disorder. Nonetheless, as the scientific knowledge and measurement of risk contexts sharpens, it can advance our understanding of both disorder and adaptation within those risk contexts.
Most of the examples incorporated in this paper draw on the classic HR infant sibling research design that utilizes familial risk, or the presence of a first-degree relative with ASD, as a putative marker of increased risk for ASD. Despite ongoing efforts to explicate the specific genetic processes thought to underly the disorder, there is growing consensus that ASD is heterogenous in etiology. For example, common allele variation, single gene de novo mutations, and copy number variants all likely contribute to genetic risk for ASD (De Rubeis & Buxbaum, Reference De Rubeis and Buxbaum2015; Huguet & Bourgeron, Reference Huguet, Bourgeron, Sala and Verpelli2016; Satterstrom et al., Reference Satterstrom, Kosmicki, Wang, Breen, De Rubeis and An2020). Further, more specifically within familial risk contexts, evidence suggests that the number of family members with the disorder (McDonald et al., Reference McDonald, Senturk, Scheffler, Brian, Carver, Charman and Jeste2020) further confer increased likelihood of ASD. We suggest that in addition to classic HR infant sibling models, it will be important for studies to incorporate diverse metrics of ASD risk context (see Elsabbagh, Reference Elsabbagh2020) including emerging multilevel risk biomarkers (e.g., Molnar-Szakacs et al., Reference Molnar-Szakacs, Kupis and Uddin2021) and risk contexts established within community samples to examine the extent to which resilience processes (and their interactions with risk markers) are similar or differ by how risk is measured. Through this work, integrated risk and resilience perspectives may help push forward knowledge about distinct etiological pathways and their corresponding phenotypic pathways to and away from impairment. Additionally, throughout the lifespan more work could consider the processes through which children and adults attain adaptive achievements despite an ASD diagnosis.
ASD-specific versus general processes
Lastly, it will be important to understand the risk and resilience processes that are unique to ASD-related disability (forecasted by diverse definitions of risk context) and shared within other contexts of risk for neurodevelopmental and other disorders. Intervention science can also help answer questions about the extent to which targets and mediators of successful intervention are specific to boosting abilities in the context of ASD risk or may be more similar to other interventions (e.g., other commonly used parenting or parent-mediated interventions) and could be more broadly promotive of positive development for other high-risk groups of children. Given marked comorbidity and potential overlap in the biological processes underlying many neurodevelopmental disorders (Thapar et al., Reference Thapar, Cooper and Rutter2017), there are likely general protective and promotive processes that extend across disorders and risk contexts (e.g., preterm birth, developmental delay). Conversely, work in this vein could also further investigate potential tradeoffs or “costs” to certain alternative pathways of development. For example, it is possible that certain adaptive capacities (e.g., strong language skills) could obscure the assessment of functional impairment and its detection by clinicians, delaying access to intervention and services. Additionally, more effortful compensatory social strategies employed by those with ASD may be beneficial for some areas of functioning but are associated with increased anxiety or depression (Livingston et al., Reference Livingston, Colvert, Bolton and Happé2019a, Reference Livingston, Shah and Happé2019b). These notions underscore the importance of a full understanding of the complexity of interrelated risk, adaptation, and outcome factors to ensure that young children and adults receive appropriate clinical attention and resources.
Conclusions
To date, a deficit model has predominated in HR infant sibling research on the early development of ASD. However, there is emerging interest in the potential of integrating a resilience framework into this body of work. In this paper, we propose that a developmental resilience framework can enrich our understanding of the processes involved in pathways of development pertinent to understanding the early emergence of ASD and heterogeneity observed among children at risk for the disorder, informing basic and translational science and opening new directions for research.
In this article, we articulate steps toward integrating a developmental resilience framework into research on the early development of ASD. Adding this lens, the HR infant siblings research program could elucidate pathways of positive development early in life by interrogating questions of who might do well in the context of risk for ASD-related disability, how, and through what processes. Drawing on findings from HR infant siblings research, we illustrated both diagnostic and continuous approaches to establishing criteria for adaptive outcomes; some candidate risk, protective, and promotive factors; and potential early life mechanisms to consider. We discussed how considering the role of resilience processes during sensitive periods of early development may result in a better understanding of the protective and possibly promotive processes that result in a lower likelihood of ASD-related disability for females, and ways HR infant sibling designs can further this inquiry. Lastly, we highlighted future directions for studying markers and trajectories of positive development within multiple systems using densely sampled longitudinal designs, the possibility of reframing existing findings in informative ways, extending research to employ diverse measures of ASD risk, and interrogating ASD-specific versus more general processes. Considering ASD through a resilience lens underscores an appreciation for the variation in human development in spite of risk for negative life outcomes, windows of opportunity afforded by the adaptive capacities of complex human systems and periods of neural plasticity, and the power of interventions that are well targeted and timed to redirect the course of development.
Funding statement
Preparation of this paper was supported in part by a National Science Foundation Graduate Research Fellowship (Stallworthy; 00074041), as well as Regents and Irving B. Harris Professorships in Child Development from the University of Minnesota (Masten).
Conflicts of interest
None.





