According to the attachment theory, early in life newborns shape internal working models based on the extent to which caregivers are available and provide support in situation of distress (Bowlby, Reference Bowlby1969). Those models are defined as moderately stable mental representations of self and close relationships, which are heavily influenced by the first dyadic relationship experienced with the caregiver (Benetti et al., Reference Benetti, McCrory, Arulanantham, De Sanctis, McGuire and Mechelli2010).
The research method commonly used to describe the infant attachment style is called the ‘Strange Situation’ (Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978). This procedure is designed for infants aged 12–18 months and is based on the assumption that the separation of an infant from his/her attachment figure (i.e., the caregiver) in an unfamiliar setting, activates the infant's attachment system. Briefly, the strange situation consists of distress-evoking events, including the caregiver leaving the infant alone in a playroom and the entrance of a stranger into the playroom, which are followed by the reunion of the infant with the caregiver. Based on the infant's reaction when the caregiver returns, three attachment styles have been theorised: (i) secure, (ii) avoidant and (iii) anxious (Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978). Secure infants seek contact with the caregiver upon reunion and can be easily calmed by contact if distressed. Adults with a secure attachment style show positive and satisfying relationships, feel comfortable being intimate with others and without being worried about abandonment (Bowlby, Reference Bowlby1969; Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978). Avoidant infants seem undisturbed by the separation from the caregivers and actively avoid contact with them. Adults with the avoidant style of attachment tend to feel more comfortable being independent and alone often avoiding any attachment altogether. Avoidant individuals often do not care about close relationships, distancing themselves from other people and suppressing their emotions (Bowlby, Reference Bowlby1969; Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978). Anxious-ambivalent infants show exaggerated distress after the separation and exhibit proximity-seeking and anger towards the caregiver at the reunion. In adulthood, these people often express a generalised feeling of abandonment and rejection, insecurity about their close relationships and high emotional expressiveness and impulsiveness. This style of attachment often emerges in children that suffered abusive experiences (Ainsworth et al., Reference Ainsworth, Blehar, Waters and Wall1978; Hazan and Shaver, Reference Hazan and Shaver1987; McCarthy and Taylor, Reference McCarthy and Taylor1999). Other researchers proposed another style of attachment, i.e. disorganised, characterising infants who appeared to have an incoherent behavioural strategy to cope with separation and reunion (Main and Solomon, Reference Main, Solomon, Brazelton and Yogman1986). Fear-evoking and abusive parental behaviours seem to play an important role in the formation of disorganised attachment and a positive association has been shown between disorganised attachment style and personality disorders as well as other psychopathologies in adulthood (e.g. borderline personality disorder, bad stress management and dissociative behaviours) (Van Ijzendoorn et al., Reference Van Ijzendoorn, Schuengel and Bakermans-Kranenburg1999; Khoury et al., Reference Khoury, Zona, Bertha, Choi-Kain, Hennighausen and Lyons-Ruth2019).
A wide number of instruments have been developed to measure the style of attachment in both adults and children including self-reports, structured interviews and structured behavioural observations (Collins and Read, Reference Collins and Read1990; Griffin and Bartholomew, Reference Griffin and Bartholomew1994; Brennan et al., Reference Brennan, Clark and Shaver1998; Kerns et al., Reference Kerns, Aspelmeier, Gentzler and Grabill2001). The two main instruments for the measurement and study of attachment style are the Adult Attachment Interview (George et al., Reference George, Kaplan and Main1985) for adulthood and the Strange Situation for children but since they require a specific training and a complex procedure (George et al., Reference George, Kaplan and Main1985) are often replaced by other tools such as the Relationship Scale Questionnaire (RSQ; Griffin and Bartholomew, Reference Griffin and Bartholomew1994), the Adult Attachment Questionnaire (AAQ; Hazan and Shaver, Reference Hazan and Shaver1987) and the Experiences in Close Relationship questionnaire (ECR; Brennan et al., Reference Brennan, Clark and Shaver1998) which are self-reports investigating secure and insecure (i.e., anxious, avoidant) attachment styles. These questionnaires have been used also in research settings.
Indeed, a growing number of studies have explored the neurobiological correlates of attachment style in healthy subjects, showing mixed results (Quirin et al., Reference Quirin, Gillath, Pruessner and Eggert2009; Benetti et al., Reference Benetti, McCrory, Arulanantham, De Sanctis, McGuire and Mechelli2010; Zhang et al., Reference Zhang, Deng, Ran, Tang, Xu, Ma and Chen2018). In this review, we aimed to describe the latest evidence on brain structural and functional underpinnings of attachment style in healthy individuals.
The data search was conducted on the PUBMED database. The following key words were used for the search: ‘neuroimaging’ and ‘healthy controls’ and ‘attachment’. The inclusion criteria were: (i) original publication published in a peer-reviewed journal between 2008 and 2018; (ii) English language; (iii) inclusion of healthy adults and the use of validated tools to assess attachment style; (iv) application of structural or functional neuroimaging techniques. After title and abstract screening, 11 studies were identified and included in the review. Sample characteristics and magnetic resonance imaging (MRI) findings from each study are shown in Table 1. The first study on attachment ever conducted using structural MRI investigated whether differences in attachment styles were associated with specific grey matter (GM) volumes (Benetti et al., Reference Benetti, McCrory, Arulanantham, De Sanctis, McGuire and Mechelli2010). Authors showed that participants with high attachment-related anxiety had smaller anterior temporal pole and larger left lateral orbital gyrus. A more recent study by Acosta et al. (Reference Acosta, Jansen, Nuscheler and Kircher2018) also reported a positive association between attachment-related anxiety and left insula and left inferior frontal gyrus (IFG) (i.e., pars opercularis) volumes. Other studies by Zhang et al. (Reference Zhang, Deng, Ran, Tang, Xu, Ma and Chen2018) investigated the neuroanatomical correlates of attachment styles and the role of gender in healthy young adults. The authors found negative associations between attachment-related avoidance scores and GM volumes of the para-hippocampus and the middle temporal gyrus (MTG), conversely attachment-related anxiety scores were positively associated with greater anterior cingulate cortex (ACC) volumes. Of note, when analysing the role of gender, Zhang et al. (Reference Zhang, Deng, Ran, Tang, Xu, Ma and Chen2018) showed that attachment-related anxiety was negatively associated with the right middle occipital volume in women but positively in men.
Table 1. Neuroimaging studies of functional and structural neural correlates of attachment
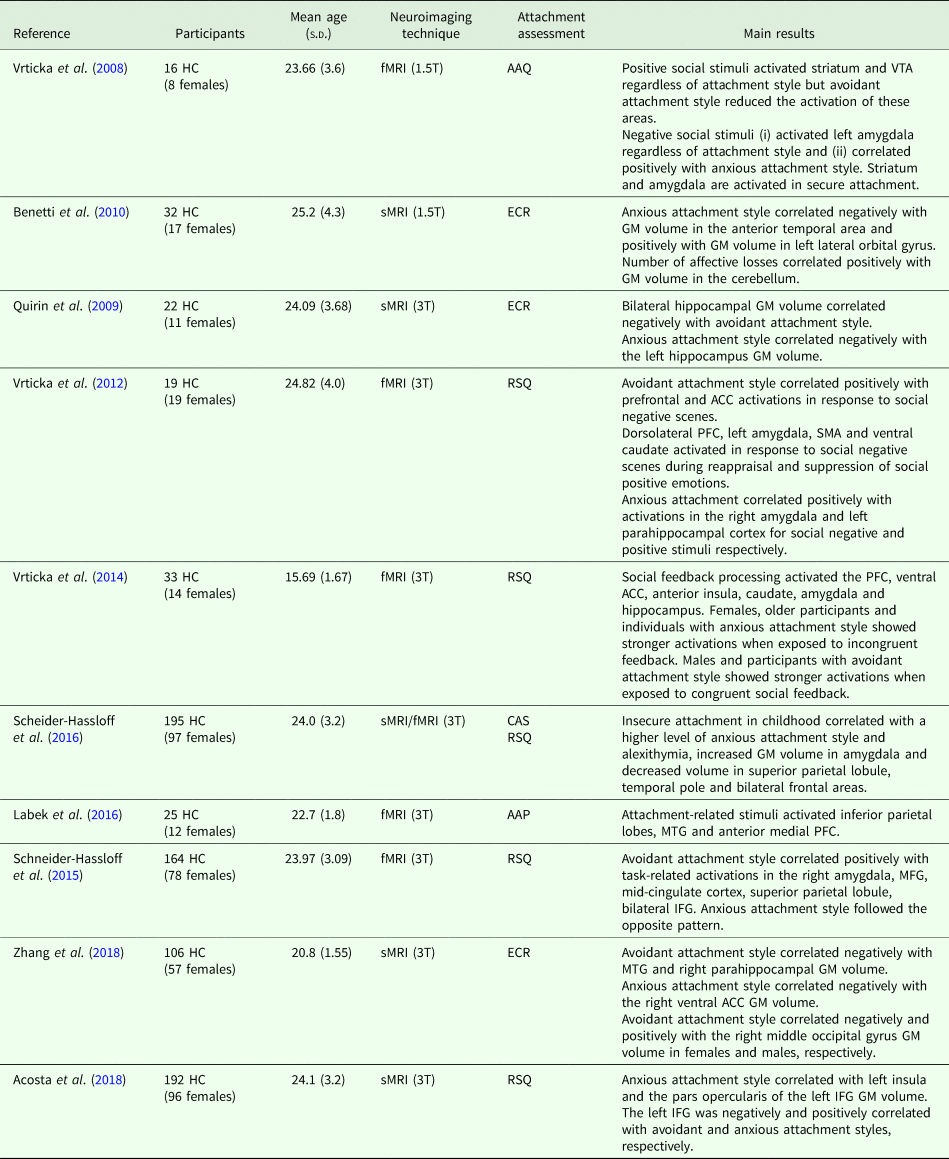
AAC, anterior cingulate cortex; AAP, Adult Attachment Projective Picture System (George and West, Reference George and West2012); AAQ, Adult Attachment Questionnaire (Hazan and Shaver, Reference Hazan and Shaver1987); CAS, Attachment Security in Childhood (Collins and Read, Reference Collins and Read1990); ECR, Experiences in Close Relationship questionnaire (Brennan et al., Reference Brennan, Clark and Shaver1998); fMRI, functional magnetic resonance imaging; GM, grey matter; HC, healthy controls; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; MTG, middle temporal gyrus; sMRI, structural magnetic resonance imaging; PFC, prefrontal cortex; RSQ, Relationship Scale Questionnaire (Griffin and Bartholomew, Reference Griffin and Bartholomew1994); SMA, supplementary motor area; VTA, ventral tegmental area.
Quirin et al. (Reference Quirin, Gillath, Pruessner and Eggert2009) evaluated the influence of attachment-related insecurity on the hippocampal GM volumes on young adults and found that left and bilateral reductions of hippocampal GM volume were associated with attachment-related avoidance and anxiety, respectively.
Lastly, two studies by Schneider-Hassloff et al. (Reference Schneider-Hassloff, Straube, Nuscheler, Wemken and Kircher2015, Reference Schneider-Hassloff, Straube, Jansen, Nuscheler, Wemken, Witt, Rietschel and Kircher2016) evaluated the neural correlates of attachment style with the use of a mentalising task, as mentalisation (i.e., the ability to understand one's state of mind and to have insight into what one is feeling) is considered an important coping skill used to regulate emotions influenced by attachment style (Huenefeldt et al., Reference Huenefeldt, Laghi, Ortu and Belardinelli2013). The mentalising task used in the two experiments consisted of an interactive version of the Prisoner's Dilemma Game in which two players simultaneously must decide whether to cooperate or to compete at the expense of the other participant by pressing the right or the left button. In the first study, specific attachment style-brain activations were detected: avoidance was positively and anxiety negatively correlated with the right amygdala, middle frontal gyrus, mid-cingulate cortex, IFG and parietal lobule activations (Schneider-Hassloff et al., Reference Schneider-Hassloff, Straube, Nuscheler, Wemken and Kircher2015). Schneider-Hassloff et al. (Reference Schneider-Hassloff, Straube, Jansen, Nuscheler, Wemken, Witt, Rietschel and Kircher2016) then expanded their previous study by studying the interaction between attachment style, genotype, brain structure and neural activations and found that insecure attachment style during childhood was associated with higher attachment-related anxiety during adulthood as well as larger amygdala volumes and lower volumes in the right superior parietal lobule, left temporal lobe and bilateral frontal regions. Other studies used functional MRI to explore the association between attachment styles and neural activations during tasks on, among others, social appraisal, emotion suppression and mentalisation. One of the earliest studies by Vrtička et al. (Reference Vrtička, Andersson, Grandjean, Sander and Vuilleumier2008) used a functional MRI paradigm to explore the influence of attachment styles on brain activation during appraisal of social cues (i.e., positive or negative stimuli conveying different types of feedbacks hostile v. friendly) presented after a performance-based task). Authors found that activations of the striatum and ventral tegmental area (VTA) were higher in a positive feedback condition but significantly reduced in participants with attachment-related avoidance. Left amygdala was shown to be involved in the processing of hostile stimuli (angry faces feedback) and positively correlated with attachment-related anxiety scores (Vrtička et al., Reference Vrtička, Andersson, Grandjean, Sander and Vuilleumier2008).
To further expand, Vrtička et al. (Reference Vrtička, Sander, Anderson, Badoud, Eliez and Debbané2014) also studied the effect of gender and age on brain activations while processing congruent and incongruent social cues: higher activity during the presentation of incongruent social feedback stimuli was seen only in women, older adolescents and individuals with high attachment-related anxiety. Conversely, congruent stimuli elicited higher activations in males and in participants with high attachment-related avoidance.
In another study, the same group investigated whether different attachment styles were associated with distinct brain activations during emotion recognition and suppression following the presentation of stimuli that could convey a social pleasant or unpleasant meaning (Vrtička et al., Reference Vrtička, Bondolfi, Sander and Vuilleumier2012). Results showed that participants with attachment-related anxiety had (i) increased prefrontal (PFC) and ACC activations in response to unpleasant scenes; (ii) a persistent increased activation in dorsolateral prefrontal cortex (PFC) and left amygdala for the same stimuli and (iii) supplementary motor area and ventral caudate activations during the suppression condition, whereas participants with attachment-related avoidance had activation increases in the right amygdala and in the left parahippocampal cortex when exposed to negative and positive social stimuli, respectively (Vrtička et al., Reference Vrtička, Bondolfi, Sander and Vuilleumier2012).
Another recent study implemented a different task aiming to elicit participants' attachment system while undergoing functional MRI scans (Labek et al., Reference Labek, Viviani, Gizewski, Verius and Buchheim2016). The authors used the Adult Attachment Projective Picture System (AAP, George and West, Reference George and West2012), a validated set consisting of attachment-related content to study the specific correlates of attachment. They found that the inferior parietal lobes, temporo-parietal junction (TPJ), MTG and anterior medial PFC activated when participants were exposed to attachment-related stimuli (Labek et al., Reference Labek, Viviani, Gizewski, Verius and Buchheim2016).
Discussion
In this review, we described the existing evidence from functional and structural MRI studies investigating the neurobiological correlates of attachment. Findings suggest that different attachment styles are associated with distinct functional and structural correlates in healthy individuals and that attachment-related stimuli can activate various regions thought to be the neural correlates of attachment. These regions are the TPJ, the MTG and the medial PFC (Labek et al., Reference Labek, Viviani, Gizewski, Verius and Buchheim2016). TPJ has been previously suggested to be the neural correlate of the theory of mind, involved in social cognition and other important cognitive functions (Carter and Huettel, Reference Carter and Huettel2013) and the MTG and the medial PFC seem to be active during various mentalising processes, a set of cognitive functions linked with attachment style (Nolte et al., Reference Nolte, Bolling, Hudac, Fonagy, Mayes and Pelphrey2013). Structural studies also showed that both avoidant and anxious attachment styles are correlated with specific GM volume alterations but share a common hippocampal and parahippocampal GM volume reduction with a different lateralisation effect (Quirin et al., Reference Quirin, Gillath, Pruessner and Eggert2009; Zhang et al., Reference Zhang, Deng, Ran, Tang, Xu, Ma and Chen2018). These findings may suggest, as others previously theorised, that specific attachment styles are associated with different stress-coping strategies and patterns of emotion regulation (Simpson and Rholes, Reference Simpson and Rholes2017) and that the hippocampus is involved not only in memory but also in emotions, conflicts processing and stress regulation (Herman et al., Reference Herman, Ostrander, Mueller and Figueiredo2005; O'Neil et al., Reference O'Neil, Newsome, Li, Thavabalasingam, Ito and Lee2015).
When it comes to evaluate the differences between anxious and avoidant attachment styles, a recent meta-analysis suggested that major differences reside in the IFG (deactivated in avoidant individuals) and in the amygdala (hyperactivated in anxious individuals) regions specifically during social processing tasks (Ran and Zhang, Reference Ran and Zhang2018). The correlation between amygdala hyperactivation and anxious attachment style suggests an increased vigilance to emotional stimuli in these individuals confirming the amygdala's role in the regulation of anxiety and social behaviour (Von Der Heide et al., Reference Von Der Heide, Vyas and Olson2014; Shackman and Fox, Reference Shackman and Fox2016). The positive correlation between anxious attachment style and ACC GM volume (Zhang et al., Reference Zhang, Deng, Ran, Tang, Xu, Ma and Chen2018) might also support this idea since the ACC has been implicated in various functions such as error detection, conflict monitoring, social evaluation and emotions (Etkin et al., Reference Etkin, Egner and Kalisch2010; Apps et al., Reference Apps, Rushworth and Chang2016).
The deactivations of frontal regions in avoidant individuals reported by Ran and Zhang could be explained by other studies showing the multiple roles of the IFG, a region involved in language comprehension and production, and behaviour inhibition (Aron et al., Reference Aron, Robbins and Poldrack2014). Recent findings on Broca's and pars opercularis areas also indicate that these frontal regions play a role in emotion and semantic processing co-activating with networks of sensory, motor and limbic structures and that could explain why these frontal areas are influenced by specific-attachment style during emotion processing tasks (Belyk et al., Reference Belyk, Brown, Lim and Kotz2017). The reported deactivations of the VTA and striatal regions during positive feedback tasks in avoidant-attached individuals seem to suggest an involvement of the limbic regions (Vrtička et al., Reference Vrtička, Andersson, Grandjean, Sander and Vuilleumier2008) and a different sensitivity to positive social feedbacks. These regions are in fact deeply involved in emotions, motivation and take part in the so-called reward system (Arias-Carrión et al., Reference Arias-Carrión, Stamelou, Murillo-Rodríguez, Menéndez-González and Pöppel2010; Schultz, Reference Schultz2016).
In conclusion, this review showed the involvement of different brain regions and their interaction with specific attachment styles during various social processing tasks ranging from the processing of social feedbacks to complex mentalisation tasks. We also tried to characterise the different attachment styles by disentangling the overlapping findings and analysing the most palpable differences in terms of neural activations and GM volumes showing an involvement of the amygdala, the IFG, the ACC and the hippocampus.
It is important to remember that some of the discussed articles showed how neural activations and brain structures can be influenced by the interaction between specific attachment styles, gender and lateralisation (Vrtička et al., Reference Vrtička, Sander, Anderson, Badoud, Eliez and Debbané2014; Zhang et al., Reference Zhang, Deng, Ran, Tang, Xu, Ma and Chen2018). Future research should thus investigate the exact implications of these and other factors considering their influence on neural structures in relation to specific attachment styles.
Author ORCIDs
Cinzia Perlini, 0000-0002-4281-0920.
Acknowledgements
This study was partially supported by grants from the Ministry of Health GR-2016-02361283 (CP).



