Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a relatively common disorder, characterized by inattention, hyperactivity, and impulsivity.Reference Matthews, Nigg and Fair1 In U.S. adolescents, the 12-month prevalence of ADHD is 6.5% (standard error, 0.5),Reference Kessler, Avenevoli and Costello2 with 76% of patients experiencing moderate to severe ADHD.Reference Kessler, Avenevoli and Costello3 In many cases, pediatric ADHD will persist into adulthood: 60% of patients display symptoms throughout their lives,Reference Gajria, Lu and Sikirica4 with the severity and treatment of pediatric ADHD acting as significant predictors of persistence in adulthood.Reference Caye, Spadini and Karam5 In cases of “adult-onset” ADHD, in which adult patients did not have a prior medical history of ADHD, patients were more likely to have evidence of psychopathology in childhood, suggesting that they may have displayed symptoms of ADHD below the diagnostic threshold.Reference Franke, Michelini and Asherson6 Recently, the prevalence of adult ADHD diagnoses in U.S. adults has increased from 2.20 per 1000 patients in 1999 to 10.57 in 2010. Despite this increase, approximately half of patients with adult ADHD are untreated, suggesting that this is an underserved patient population.Reference Zhu, Liu, Li, Wang and Winterstein7 Approximately 66% of adult patients with ADHD have comorbid psychiatric disorders, including substance use disorders (SUDs, 39.2%), anxiety disorders (23%), and mood disorders (18.1%).Reference Pineiro-Dieguez, Balanza-Martinez, Garcia-Garcia and Soler-Lopez8 However, neither an ADHD diagnosisReference Vitiello, Perez Algorta, Arnold, Howard, Stehli and Molina9 nor treatment with stimulants such as methylphenidateReference Hollis, Chen and Chang10 increase the risk of psychotic disorders. Similarly, psychostimulants have not been found to increase the risk for SUDsReference Humphreys, Eng and Lee11 and have been shown to reduce the risk of smoking.Reference Schoenfelder, Faraone and Kollins12
In adults, untreated ADHD is associated with impaired quality of life (QoL), impaired relationships, reduced employment, impaired driving safety, premature death from accidents, and vulnerability to addiction, depression, and anxiety.Reference Geffen and Forster13 Disease trajectory is highly variable, and comorbidities such as SUD, antisocial personality disorder, and sleep disorder may emerge in adults with ADHD.Reference Franke, Michelini and Asherson6 Notably, treatment of ADHD has demonstrated a protective benefit against delinquency in adults, with a large epidemiologic study demonstrating a significant reduction in the rate of criminality during periods in which patients received ADHD treatment compared with nonmedication periods (men, −32%; women, −41%)Reference Lichtenstein, Halldner and Zetterqvist14; these findings are consistent with an analysis of recently released prisoners in Sweden, which found a 43% decrease in violent re-offenses in individuals receiving psychostimulants.Reference Chang, Lichtenstein, Långström, Larsson and Fazel15 Despite its increasing prevalence and demonstrated response to medication, adult ADHD has only recently been recognized, and understanding of treatment in adults is hampered by a dearth of long-term data.Reference Billin, Honeycutt and McDougal16 Treatment for ADHD may include stimulants, such as amphetamine and methylphenidate,Reference Huss, Duhan, Gandhi, Chen, Spannhuth and Kumar17, Reference De Crescenzo, Cortese, Adamo and Janiri18 or other medications, such as atomoxetine (a presynaptic inhibitor of the norepinephrine transporter)Reference Schwartz and Correll19 and clonidine and guanfacine (α-2 adrenergic agonists).Reference Hirota, Schwartz and Correll20 Numerous studies have compared the efficacy of these regimens in children, adolescents, and adults. A meta-analysis of 18 trials found that, compared with placebo, treatment with methylphenidate resulted in a moderate improvement in ADHD symptoms in adults.Reference Castells, Ramos-Quiroga and Rigau21 Additionally, a large analysis of 133 studies (51 of which focused on adults) found that amphetamines, methylphenidate, bupropion, and atomoxetine improved ADHD symptoms relative to placebo in adults; based on the findings, the authors recommended amphetamine as the primary short-term treatment option in adult patients but noted that additional research into long-term treatment was needed.Reference Cortese, Adamo and Del Giovane22
Methylphenidate is available as an oral medication with a number of formulations, including immediate-release tablets, extended-release (ER) tablets, sustained-release tablets, oral liquid suspension, chewable tablets, and orally disintegrating tablets.Reference Cortese, D’Acunto, Konofal, Masi and Vitiello23 The methylphenidate transdermal system (MTS) is approved in the United States for the treatment of children and adolescents with ADHD. Transdermal patches offer a number of benefits over oral formulations, including improved adherence, personalization of wear times, minimization of hepatic side effects and first-pass metabolism, and reduced gastrointestinal adverse events (AEs).Reference Findling and Dinh24 MTS is the only transdermal treatment approved for ADHD, and it has demonstrated significant improvement in ADHD symptoms in children and adolescents compared with placebo. Additionally, safety studies have shown that the majority of AEs were mild or moderate, and only 9% of patients discontinued due to AEs.Reference Findling and Dinh24 Unique to MTS is the variable duration of action based on the wear time of the patch. Significant improvement in ADHD symptoms from 2 to 12 hours after applying MTS with a 9-hour wear time was observed in clinical trials. Moreover, MTS can be removed before 9 hours if a shorter duration of effect is desired.Reference Wilens, Boellner and Lopez25
While the persistence of ADHD into adulthood has become increasingly appreciated, few studies have examined optimal treatment options in adults or how to manage the transition of treatment from childhood into adulthood.Reference Ginsberg, Beusterien, Amos, Jousselin and Asherson26 Adults with ADHD have reported long delays in diagnosis, and access to treatments for adults is limited because of both a lack of dedicated adult services and an unwillingness of psychiatrists to prescribe stimulants to adults.Reference Ginsberg, Beusterien, Amos, Jousselin and Asherson26 Long-term studies of efficacy and safety in adults with ADHD are also limited, potentially contributing to the under-treatment of adults.Reference Fredriksen, Halmoy, Faraone and Haavik27 Of particular importance is the rate of nonadherence among adult patients. Overall rates of nonadherence have been found to range from 13.2% to 64.0% of patients with ADHD, but predictors of nonadherence included older age and later-onset ADHD, suggesting adherence may be a greater challenge in adult patients.Reference McCarthy28 This result is supported by findings from long-term studies of adult ADHD, which found a high rate of nonadherence to ADHD medications, with up to 50% of adults discontinuing treatment after 2 years.Reference Fredriksen, Halmoy, Faraone and Haavik27 This trend is observed in ADHD medications of all types, including long-acting stimulants (discontinuation rate, 19.1% across all ages), short-acting stimulants (99% beyond 12 months in 6-12-year-old patients), and atomoxetine (26.0%-38.3% across all ages).Reference Gajria, Lu and Sikirica4 Additionally, it has been demonstrated that patients aged 15 to 21 years are the most likely to discontinue treatment, just as they transition treatment from pediatric to adult services, which is often provided by general practitioners.Reference Zetterqvist, Asherson, Halldner, Langstrom and Larsson29 Several strategies to improve adherence have been proposed, including increasing discussion between patients and physicians regarding the importance of adherence, implementing self-monitoring, addressing AEs, and simplifying dosage and regimens.Reference McCarthy28 Long-acting transdermal formulations can reduce the dosing frequency compared with that required for other formulations. In addition, multiple studies in different patient populations have demonstrated improved adherence with transdermal formulations.Reference Findling and Dinh24 To this end, the use of transdermal formulations in adults with ADHD has the potential to improve adherence in this underserved patient population. This literature review was conducted to evaluate the safety and efficacy of transdermal treatment options in children, adolescents, and adults, with a focus on the potential for transdermal systems in the treatment of adults with ADHD.
Methods
Search strategy
Because of the relative lack of studies in adults with ADHD, a broad and comprehensive search strategy was designed in order to identify clinical trials of transdermal treatments in patients with ADHD, regardless of patient age or date of publication. On December 4, 2019, a search of the MEDLINE, Embase, BIOSIS, and SCOPUS databases was conducted to identify clinical trials of transdermal treatment conducted in patients with ADHD, using the following search string: TITLE-ABS-KEY(transdermal OR dermal OR *cutaneous) AND TITLE-ABS-KEY(“adhd” OR “attention deficit” OR “addh” OR “minimal brain dysfunction” OR “hyperkinetic syndrome”) AND TITLE-ABS-KEY(randomized OR randomised OR “equivalence trial” OR “clinical trial” OR “clinical trials” OR “equivalence trials” OR “non inferiority trial” OR “non inferiority trials” OR “noninferiority trial” OR “noninferiority trials” OR “superiority trial” OR “superiority trials” OR “intention to treat analysis” OR “controlled trial” OR “controlled trials” OR “pragmatic trial” OR “pragmatic trials” OR “equivalence design” OR “non inferiority design” OR “noninferiority design” OR “superiority design”) AND NOT INDEX(medline).
Results were limited to English language, and the following categories of publications were excluded: Review, Conference Paper, Conference Abstract, Note, Editorial, Letter, Literature Review, Short Survey, Meeting Abstract, Conference Review, and Systematic Review. The search was not restricted by publication date. Eligibility criteria were applied, and a review of the selected hits was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.Reference Moher, Liberati, Tetzlaff and Altman30 After removal of duplicates, 145 unique hits were identified (Figure 1). Of these, 107 articles were excluded at title and abstract level. Altogether, 38 full text articles were reviewed, and a further 15 were excluded because they were off topic (n = 11; not transdermal focused, 5; nicotine analog, 1; endpoint not relevant in adults, 1; genetics, 1; methodology, 1; pharmacokinetic/pharmacodynamic [without safety or efficacy findings], 1; scale validation, 1), commentary (n = 1), drug profile (n = 1), meta-analysis (n = 1), or a narrative review (n = 1). Efficacy, safety, adherence, abuse, cost efficacy, and health-related quality of life (HRQoL) data were extracted from the remaining articles and used in the present qualitative synthesis.
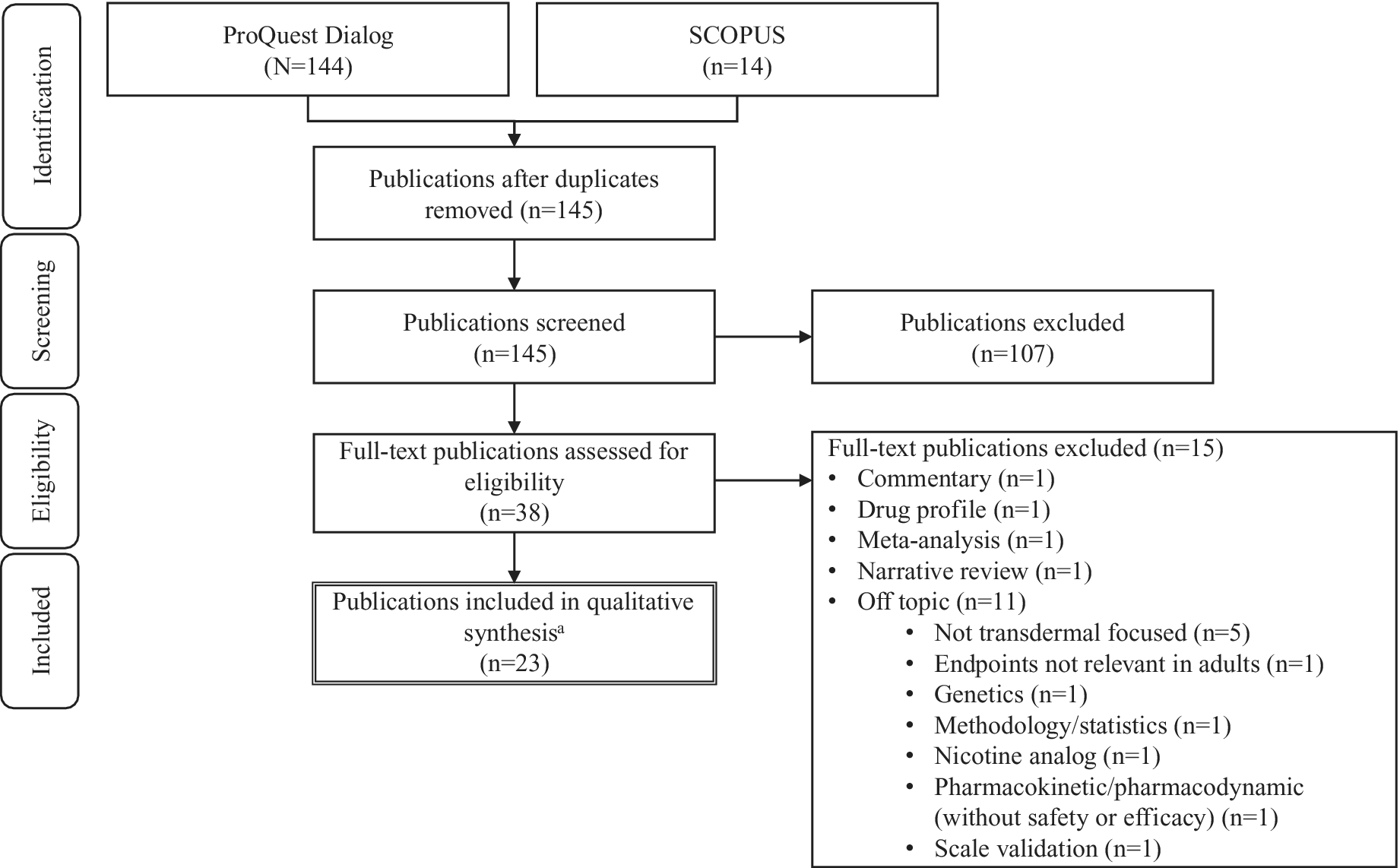
Figure 1. PRISMA flow diagram. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. aRepresenting 19 individual studies.
Results
Summary of studies
After applying exclusion criteria, 23 articles were identified by the systematic search (Table 1). These articles represented 19 unique clinical trials: Arnold et alReference Arnold, Bozzolo and Hodgkins31 and Bukstein et alReference Bukstein, Arnold, Landgraf and Hodgkins32 concerned the same patient population, as did Wilens et al,Reference Wilens, Boellner and Lopez25 Manos et al,Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42 and Frazier et al.Reference Frazier, Weiss, Hodgkins, Manos, Landgraf and Gibbins41 Findling et alReference Findling, Katic, Rubin, Moon, Civil and Li40 described an open-label extension of Findling et al,Reference Findling, Turnbow, Burnside, Melmed, Civil and Li39 and Findling et alReference Findling, Wigal and Bukstein37 reported an open-label extension of four previous trials, two of which were also captured in this search (McGough et alReference McGough, Wigal, Abikoff, Turnbow, Posner and Moon38 and Findling et alReference Findling, Bukstein and Melmed36). Altogether, 15 publications described randomized trials (double-blind, n = 10; open-label, n = 4; blinding not specified, n = 1; parallel group = 4, cross-over = 11), and 6 reported nonrandomized open-label studies (extension studies of a randomized trial = 2). All studies included MTS as the transdermal treatment; 12 studies focused on children (N = 1418, range 23-305), 1 on adolescents (N = 217), 1 on both children and adolescents (N = 64), and 5 on adults (N = 274, range 14-90). Patients from manuscripts concerning the same initial trial (eg, post hoc analyses or open-label extensions) were counted only once; for Findling et al,Reference Findling, Wigal and Bukstein37 only patients not included in McGough et alReference McGough, Wigal, Abikoff, Turnbow, Posner and Moon38 and Findling et alReference Findling, Bukstein and Melmed36 were included in the n-value (N = 33). The impact of treatment on severity of ADHD symptoms was assessed in 17 publications (children/adolescents, n = 12; adults, n = 5); the 6 papers that did not evaluate ADHD symptoms focused on sleep,Reference Faraone, Glatt, Bukstein, Lopez, Arnold and Findling35 effect of patch placement on pharmacokinetics/pharmacodynamics,Reference Gonzalez, Campbell and Rubin43 effect of formulation on pharmacokinetics/pharmacodynamics,Reference Pierce, Katic, Buckwalter and Webster49 and HRQoL improvements.Reference Bukstein, Arnold, Landgraf and Hodgkins32, Reference Frazier, Weiss, Hodgkins, Manos, Landgraf and Gibbins41, Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42 In children/adolescents, the most common efficacy measure used was the Attention Deficit Hyperactivity Disorder Rating Scale-IV (ADHD-RS-IV, 12/18), which evaluates symptom severity as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria. Among the publications related to adults with ADHD, 4 of 5 studies used the Wender–Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), which is based on the Utah Criteria for ADHD in adults and measures ADHD symptom severity in the following seven domains: attention difficulties, hyperactivity/restlessness, temper, affective lability, emotional over-reactivity, disorganization, and impulsivity.
Table 1. Characteristics of Reviewed Articles
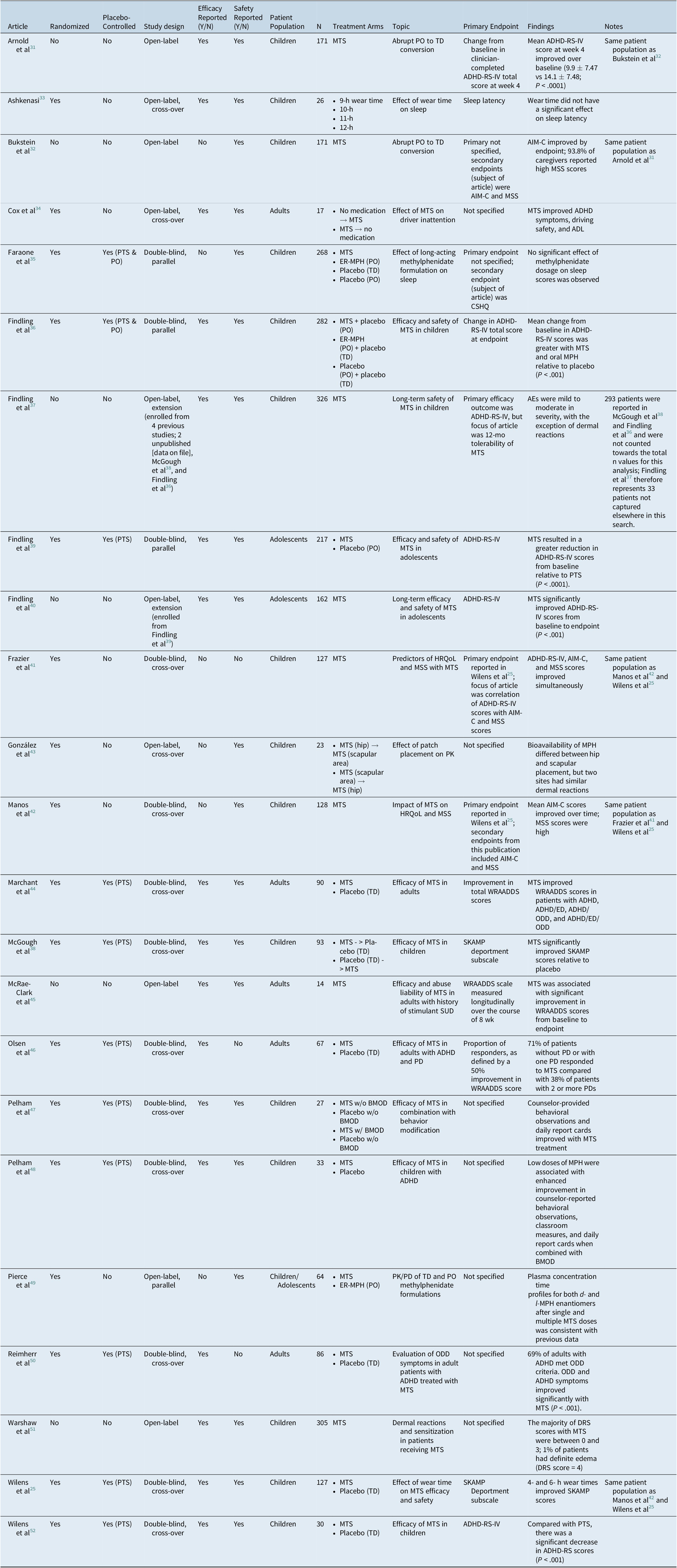
Abbreviations: ADHD-RS-IV, Attention Deficit Hyperactivity Disorder Rating Scale-IV; ADL, activities of daily living; AIM-C, ADHD Impact Module-Child; CSHQ, Children’s Sleep Habits Questionnaire; ED, emotional dysregulation; ER-MPH, extended-release methylphenidate; HR-QoL, health-related quality of life; MSS, Medication Satisfaction Survey; MTS, methylphenidate transdermal system; ODD, oppositional-defiant disorder; PO, oral administration; PTS, placebo transdermal system; SKAMP, Swanson, Kotkin, Agler, M-Flynn, and Pelham; TD, transdermal; WRAADDS, Wender-Reimherr Adult Attention Deficit Disorder Scale.
Efficacy: children and adolescents
A total of 12 publications reported on efficacy outcomes in children and adolescents (children, n = 10; adolescents, n = 2). In children and adolescents, treatment with MTS generally improved ADHD symptoms across all rating scales. Of the seven publications that evaluated change in ADHD-RS-IV scale from baseline, all reported a significantly improvement with MTS.Reference Wilens, Boellner and Lopez25, Reference Ashkenasi33, Reference Findling, Wigal and Bukstein37, Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42, Reference Warshaw, Squires, Li, Civil and Paller51, Reference Wilens, Hammerness, Martelon, Brodziak, Utzinger and Wong52 In publications on placebo-controlled trials reporting efficacy findings (n = 6), MTS consistently showed significant improvement over the placebo transdermal system (PTS).Reference Findling, Bukstein and Melmed36, Reference McGough, Wigal, Abikoff, Turnbow, Posner and Moon38, Reference Findling, Turnbow, Burnside, Melmed, Civil and Li39, Reference Pelham, Burrows-Maclean and Gnagy47, Reference Pelham, Manos and Ezzell48 A placebo-controlled study of oral and transdermal methylphenidate found that MTS (mean total score at endpoint, 18.8) and oral methylphenidate (21.8) significantly improved ADHD-RS-IV scores relative to placebo (placebo, 32.1; P < .0001), whereas there was no significant difference in efficacy between the oral and transdermal methylphenidate formulations (P = .2192).Reference Findling, Bukstein and Melmed36 An open-label extension of a randomized MTS vs PTS trial demonstrated that ADHD-RS-IV scores significantly improved from baseline of the antecedent study (mean change in score, −23.0; P < .001).Reference Findling, Katic, Rubin, Moon, Civil and Li40 Importantly, patients abruptly transitioning from oral extended-release methylphenidate (ER-MPH) to MTS reported improved ADHD-RS-IV scores with MTS (mean score [standard deviation, SD], 9.9 [7.47]) over baseline scores with ER-MPH (14.1 [7.48]; P < .0001).Reference Arnold, Bozzolo and Hodgkins31
Efficacy: adults
All five studies of adults with ADHD reported efficacy findings. A double-blind, placebo-controlled, randomized, cross-over trial of MTS and PTS in adults found that, compared with placebo, MTS significantly improved total WRAADDS (mean score [SD]; MTS, 11.2 [7.2]; placebo, 17.9 [6.6]), Clinical Global Impression-Improvement scale (CGI-I; proportion moderately improved; MTS, 65%; placebo, 15%; χ 2 = 26.9, df = 1, P = .001), and Clinical Global Impression-Severity scale (CGI-S) scores (χ 2 = 24.5, df = 1, P = .001).Reference Marchant, Reimherr, Robison, Olsen and Kondo44 Importantly, two publications reported on the comorbidity of adult ADHD with one or more personality disorders (PDs), such oppositional defiant disorder (ODD), emotional dysregulation (ED), generalized anxiety disorder, and major depression.Reference Marchant, Reimherr, Robison, Olsen and Kondo44, Reference Olsen, Reimherr, Marchant, Wender and Robison46 Reimherr et alReference Reimherr, Marchant, Olsen, Wender and Robison50 demonstrated that 42% of adult patients with ADHD in their study met diagnostic criteria for ODD and that a further 27% reported childhood ODD that had previously resolved. The proportion of responders, defined as patients who demonstrated a 50% improvement in the self-reported WRAADDS-ODD scale, significantly improved with MTS relative to placebo (MTS, 66%; placebo, 33%). Using the same criteria to define responders, an additional trial of adults with ADHD with or without PD found that 71% of patients without PD or with one PD responded to MTS (P < .001). However, this effect was not significant in patients with more than one comorbid PD (37%, P = .24).Reference Olsen, Reimherr, Marchant, Wender and Robison46 Another study found that, relative to placebo, MTS improved both ADHD and ODD symptoms in adult patients, regardless of underlying ODD or ED.Reference Marchant, Reimherr, Robison, Olsen and Kondo44
MTS has also been demonstrated to improve other important related dimensions of ADHD. In a study of young adults (mean age, 20.82 years; SD, 2.40), patients treated with MTS self-reported fewer total ADHD (P < .04) and inattentive symptoms (P = .014).Reference Cox, Davis, Mikami, Singh, Merkel and Burket34 Risky driving behaviors (P = .059) and collisions (P < .005) were also significantly reduced during periods in which patients took MTS compared with periods in which patients took no medication.Reference Cox, Davis, Mikami, Singh, Merkel and Burket34
Adherence
Adherence parameters were reported in six publications (children, n = 5; adults, n = 1), as summarized in Table 2. Two papers assessed adherence based on the return of unused study medication and defined compliance as use of between 80% and 100% of dispensed medication. In general, adherence was high among pediatric patients treated with MTS, ranging from 97% to 99% in the two aforementioned studies.Reference Findling, Bukstein and Melmed36, Reference McGough, Wigal, Abikoff, Turnbow, Posner and Moon38 The study by Cox et al,Reference Cox, Davis, Mikami, Singh, Merkel and Burket34 which included the use of MemsCaps, showed that, during the MTS condition, the MemsCap was opened on 56% of the days medication was to be taken, ranging from 4% to 91% across participants. Bukstein et alReference Bukstein, Arnold, Landgraf and Hodgkins32 found that the percentage of patients with missed doses decreased numerically when patients switched from oral formulations (28.6%) to MTS (23.6%). Furthermore, data reported by Manos et alReference Manos, Frazier, Landgraf, Weiss and Hodgkins42 showed that the score for the ADHD Impact Module-Children (AIM-C) missed-doses items decreased over the study period (proportion missing ≥4 doses: baseline, 17.2%; endpoint, 0), suggesting that compliance increased as patients became more familiar with the transdermal treatment.
Table 2. Summary of Adherence Parameters
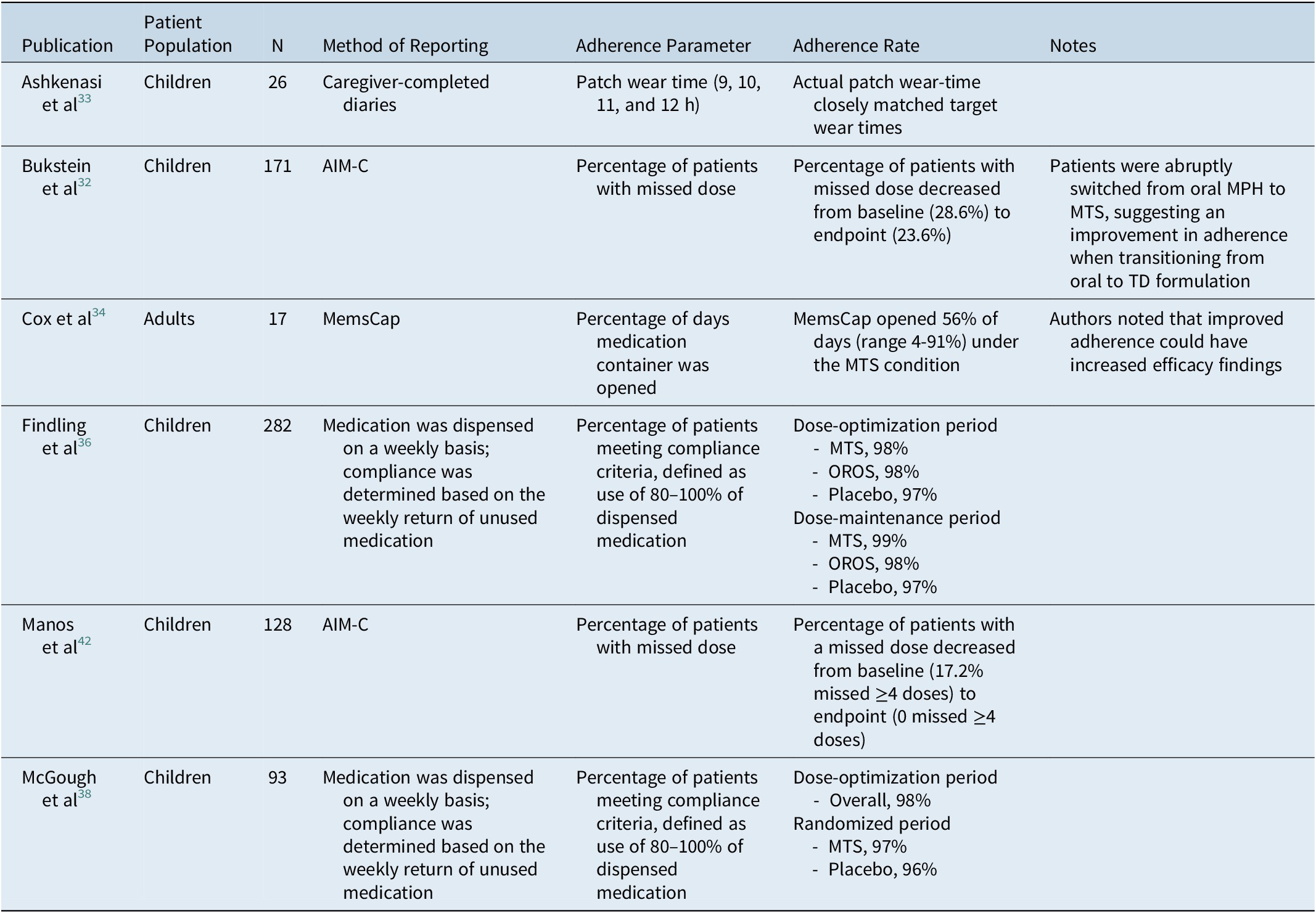
Abbreviations: AIM-C, ADHD Impact Module-Child; MPH, methylphenidate; MTS, methylphenidate transdermal system; OROS, osmotic-release oral system; TD, transdermal.
Safety
Altogether, 16 publications reported specific treatment-emergent adverse events (TEAEs), 14 in children and adolescents and 2 in adults (Table 3). The proportion of MTS-treated patients experiencing TEAEs ranged from 22% to 81.3% (median, 67%) in the 11 publications reporting this endpoint.Reference Arnold, Bozzolo and Hodgkins31, Reference Bukstein, Arnold, Landgraf and Hodgkins32, Reference Findling, Bukstein and Melmed36–Reference Findling, Katic, Rubin, Moon, Civil and Li40, Reference Gonzalez, Campbell and Rubin43–Reference McRae-Clark, Brady, Hartwell, White and Carter45, Reference Warshaw, Squires, Li, Civil and Paller51 In publications concerning children and adolescents, the median proportion of patients experiencing TEAEs was 73.5% (range 22%-81.3%); in adults, two publications reported the proportion of patients with TEAEs (57% and 67%).Reference Marchant, Reimherr, Robison, Olsen and Kondo44, Reference McRae-Clark, Brady, Hartwell, White and Carter45 The most commonly reported TEAEs were headache (13 of 16 studies; range of proportion of patients affected, 3.8%-28.6%), decreased appetite (13/16; 4.0%-29.8%), insomnia (13/16; 3.5%-47.2%), abdominal pain (9/16; 2.7%-30.0%), nausea (8/16; 3.7%-12.5%), nasopharyngitis (5/16, 1.3%-7.4%), decreased weight (5/16, 3.0%-10.1%), and tics/abnormal jaw movement (5/16; 2.8%-7.4%; Table 3). The proportion of patients reporting specific TEAEs other than skin reactions was largely comparable between children and adults.
Table 3. Percentage of Specific Treatment-Related Adverse Events in MTS-Treated Patients Reported in 4 or More Studiesa
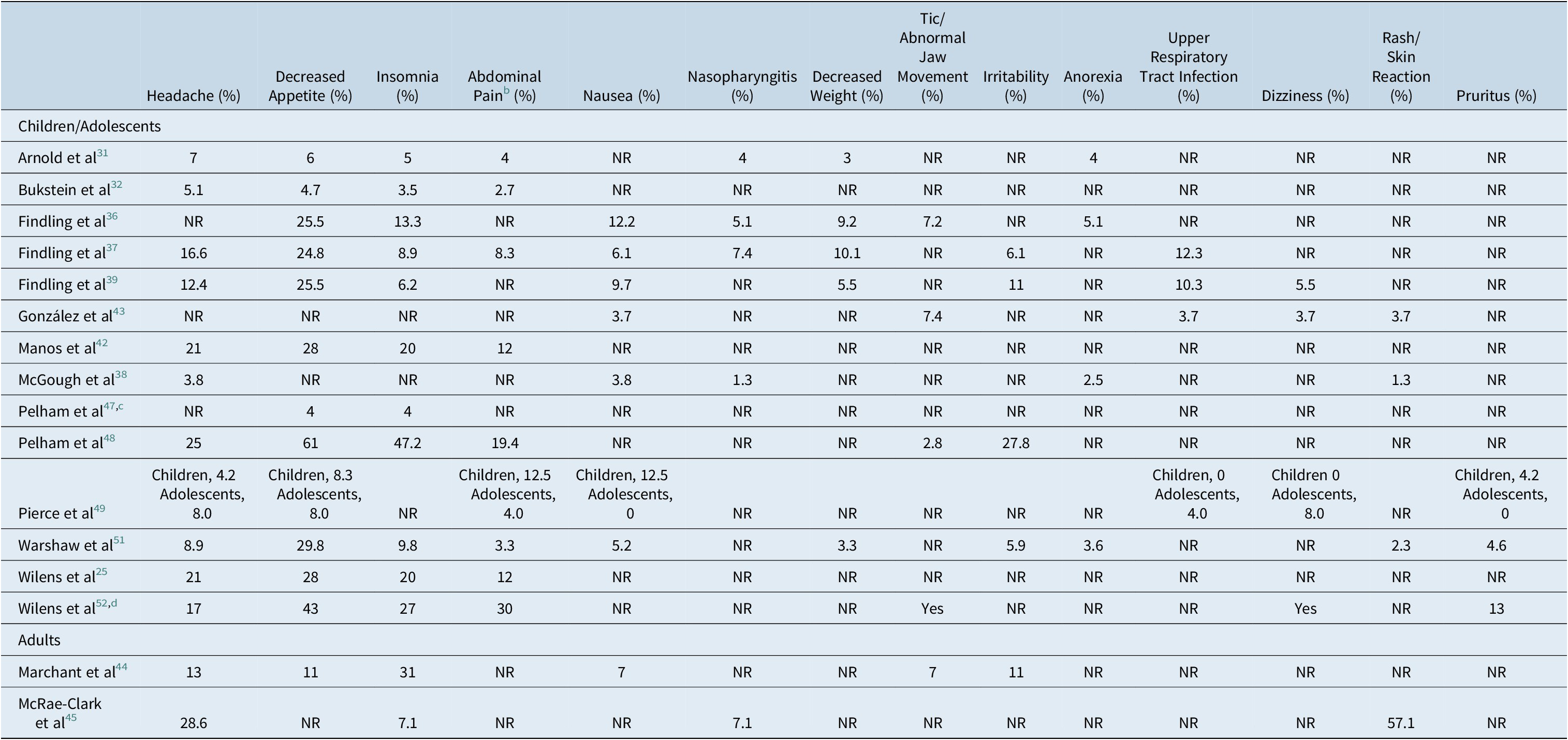
Abbreviation: NR, not reported.
a Ashkenasi,Reference Ashkenasi33 Cox et al,Reference Cox, Davis, Mikami, Singh, Merkel and Burket34 Faraone et al,Reference Faraone, Glatt, Bukstein, Lopez, Arnold and Findling35 Findling et al,Reference Findling, Katic, Rubin, Moon, Civil and Li40 Frazier et al,Reference Frazier, Weiss, Hodgkins, Manos, Landgraf and Gibbins41 Olsen et al,Reference Olsen, Reimherr, Marchant, Wender and Robison46 and Reimherr et alReference Reimherr, Marchant, Olsen, Wender and Robison50 did not report the incidence of specific AEs.
b Including general, lower, and stomachache.
c Reporting only parent-reported adverse events.
d The frequency of some adverse events were not reported; these have been captured as “yes.”
A total of 13 publications reported on TEAE severity; in these 13 publications, the majority (>92%) of TEAEs were mild to moderate in severity. Across 16 publications reporting specific adverse events, only 2 patients in 2 separate trials experienced a serious adverse event that was considered related or possibly related to study treatment: 1 patient in Bukstein et alReference Bukstein, Arnold, Landgraf and Hodgkins32 experienced acute depression and suicide attempt, and 1 patient in Findling et alReference Findling, Turnbow, Burnside, Melmed, Civil and Li39 experienced two episodes of syncope.
Studies in the present analysis reported that while dermal reactions tended to be worse with MTS compared with placebo,Reference Wilens, Boellner and Lopez25, Reference Marchant, Reimherr, Robison, Olsen and Kondo44 the majority of erythema cases were mild and transient. In McRae-Clark et al,Reference McRae-Clark, Brady, Hartwell, White and Carter45 57.1% of adult patients reported a skin reaction, a greater proportion of study subjects than was observed in any of the pediatric studies (n = 3, 1.3%-3.7%).Reference McGough, Wigal, Abikoff, Turnbow, Posner and Moon38, Reference Gonzalez, Campbell and Rubin43, Reference Warshaw, Squires, Li, Civil and Paller51 This difference may be partially related to small sample size, as McRae-Clark et alReference McRae-Clark, Brady, Hartwell, White and Carter45 included only 14 patients. In other studies, patients generally had low skin reaction and dermal response scores.Reference Wilens, Boellner and Lopez25, Reference Arnold, Bozzolo and Hodgkins31, Reference Ashkenasi33, Reference Findling, Bukstein and Melmed36–Reference Findling, Katic, Rubin, Moon, Civil and Li40, Reference Gonzalez, Campbell and Rubin43, Reference Marchant, Reimherr, Robison, Olsen and Kondo44, Reference Pelham, Burrows-Maclean and Gnagy47, Reference Pierce, Katic, Buckwalter and Webster49, Reference Warshaw, Squires, Li, Civil and Paller51 Furthermore, Warshaw et alReference Warshaw, Squires, Li, Civil and Paller51 (N = 305) demonstrated that >90% of patients experienced either mild or no discomfort resulting from skin reactions and that their severity diminished quickly over time.
Even though most skin responses were mild, 10 studies had at least 1 patient discontinue treatment due to application site reactions.Reference Wilens, Boellner and Lopez25, Reference Arnold, Bozzolo and Hodgkins31, Reference Bukstein, Arnold, Landgraf and Hodgkins32, Reference Findling, Bukstein and Melmed36, Reference Findling, Wigal and Bukstein37, Reference Findling, Turnbow, Burnside, Melmed, Civil and Li39, Reference Findling, Katic, Rubin, Moon, Civil and Li40, Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42, Reference Gonzalez, Campbell and Rubin43, Reference McRae-Clark, Brady, Hartwell, White and Carter45, Reference Pelham, Manos and Ezzell48, Reference Warshaw, Squires, Li, Civil and Paller51 The proportion of patients discontinuing due to skin reactions ranged from <1% to 7.1% in McRae-Clark et al.Reference McRae-Clark, Brady, Hartwell, White and Carter45 However, due to small sample size, the latter percentage reflected the discontinuation of only one patient.Reference McRae-Clark, Brady, Hartwell, White and Carter45 One trial of 64 children and adolescents did not report any discontinuations due to adverse events, including application site reactions.Reference Pierce, Katic, Buckwalter and Webster49
Sleep
Insomnia was reported as a TEAE in 13 of 16 studies reporting specific TEAEs, including 2 publications in adults and 11 in children/adolescents (Table 3).Reference Wilens, Boellner and Lopez25, Reference Arnold, Bozzolo and Hodgkins31, Reference Bukstein, Arnold, Landgraf and Hodgkins32, Reference Findling, Bukstein and Melmed36, Reference Findling, Wigal and Bukstein37, Reference Findling, Turnbow, Burnside, Melmed, Civil and Li39, Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42, Reference Marchant, Reimherr, Robison, Olsen and Kondo44, Reference McRae-Clark, Brady, Hartwell, White and Carter45, Reference Pelham, Burrows-Maclean and Gnagy47, Reference Pelham, Manos and Ezzell48, Reference Warshaw, Squires, Li, Civil and Paller51, Reference Wilens, Hammerness, Martelon, Brodziak, Utzinger and Wong52 The proportion of patients experiencing insomnia varied widely between studies, and the reported values ranged from 7.1% to 31.0% in adults and 3.5% to 47.2% in child/adolescent patients (Table 3).
Four pediatric studies evaluated sleep dysfunction; none reported a change in sleep latency or total sleep time in patients using MTS.Reference Faraone, Glatt, Bukstein, Lopez, Arnold and Findling35–Reference Findling, Wigal and Bukstein37, Reference Findling, Katic, Rubin, Moon, Civil and Li40 Rather, improvements in certain sleep parameters from baseline to endpoint were noted, with an increased proportion of patients reporting sleeping through the night (baseline, 51.5%; endpoint, 66.7%) and a shorter time spent awake in those who woke during the night (baseline, 13.2 minutes; endpoint, 7.5 minutes).Reference Findling, Katic, Rubin, Moon, Civil and Li40 AshkenasiReference Ashkenasi33 found no association between sleep latency or sleep time and patch wear time and reported a trend toward improved sleep quality with longer wear times.
Health-related quality of life
While the studies of adult ADHD in this analysis did not address QoL, four publications on MTS trials in children and adolescents universally reported improved QoL scores across several assessments. AIM-C and family HRQoL scores improved in all domains in patients initiating MTS treatment.Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42 In children switching from oral ER-MPH to MTS, AIM-C scores improved, including child and family impact, worry, behavior, and missed-dose items.Reference Bukstein, Arnold, Landgraf and Hodgkins32 An open-label extension trial of MTS in adolescents found that scores in the four perceptual domains (self, relationship, environment, and general quality of life) of the Youth Quality of Life-Research instrument improved from baseline of the antecedent study.Reference Findling, Katic, Rubin, Moon, Civil and Li40 Medication Satisfaction Survey scores were generally high with MTS, with the majority of caregivers expressing satisfaction with the medication.Reference Bukstein, Arnold, Landgraf and Hodgkins32, Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42 In a time course analysis of children initiating MTS treatment, medication satisfaction and AIM-C child HRQoL increased with dosage.Reference Frazier, Weiss, Hodgkins, Manos, Landgraf and Gibbins41 This analysis also found that improvements in symptomology and HRQoL occurred simultaneously, rather than HRQoL “lagging behind” improved symptoms.
Abuse liability
In the only open-label study of MTS reporting on abuse liability, WRAADDS and CGI-S scores of 14 adult ADHD patients with a history of SUD significantly improved across all domains measured, and urinalysis throughout the trial period indicated that all patients were negative for stimulants.Reference McRae-Clark, Brady, Hartwell, White and Carter45 One patient self-reported abuse of oral stimulants, but no other indications of stimulant abuse were identified in the study.
Cost efficacy
In two studies conducted in children, the AIM-C economic impact items improved with MTS treatment. The proportion of patients reporting parental missed days of work and extra tutoring, nursing, or other home healthcare decreased with MTS treatment.Reference Bukstein, Arnold, Landgraf and Hodgkins32, Reference Manos, Frazier, Landgraf, Weiss and Hodgkins42
Discussion
Despite being an increasingly recognized subset of the ADHD patient population, few trials have been conducted to examine ADHD in adult patients, and fewer still have focused on treatment with MTS in the adult population. The current analysis identified 23 manuscripts concerning the treatment of ADHD in adult and pediatric patients using MTS.
While more publications focused on children and adolescents than adults (18 vs 5), efficacy and safety parameters were largely comparable between adult and pediatric publications. In pediatric papers, all studies measuring change from baseline in ADHD-RS-IV score reported that MTS significantly improved symptoms of ADHD. In publications concerning adult trials, ADHD symptomology also improved with MTS treatment, as evidenced by improved WRAADDS, CGI-I, and CDI-S scores. While direct comparison of improvements in ADHD-RS-IV in children and adolescents and WRAADDS scores in adults is complicated by the difference in the underlying diagnostic criteria used for the evaluations (DMS-IV and the Utah Criteria, respectively),Reference Marchant, Reimherr, Wender and Gift53, Reference Pappas54 criteria largely overlapped, and the directionality of the improvements was nonetheless consistent. Additionally, other domains improved in patients treated with MTS, such as dangerous driving in young adultsReference Cox, Davis, Mikami, Singh, Merkel and Burket34 and improved classroom scores in children.Reference McGough, Wigal, Abikoff, Turnbow, Posner and Moon38 These findings are comparable to a large meta-analysis conducted in children and adults, which found that methylphenidate was superior to placebo in both age groups.Reference Cortese, Adamo and Del Giovane22
Although two publications on ADHD in adults included evaluation of comorbid ODD, this is not the most common comorbid disorder typically observed with adults with ADHD. More commonly observed psychopathologies include mood and anxiety disorders, SUDs, and PDs.Reference Katzman, Bilkey, Chokka, Fallu and Klassen55 Therefore, while interesting, the impact of MTS on comorbid ODD needs to be interpreted with caution and does not reflect effects on the most relevant comorbidities observed in adults with ADHD.
While fewer studies in adults reported on the proportion of patients experiencing TEAEs (n = 2, range 57%-67%), the range was comparable to the median proportion of patients experiencing TEAEs in children and adolescents (n = 9; median, 73.5%). The majority of articles detailing specific TEAEs reported headache, decreased appetite, insomnia, abdominal pain, and nausea (Table 3); however, it is difficult to compare the occurrence of specific TEAEs between pediatric and adult populations due to the small number of studies available in adults.
Insomnia, which was identified as a TEAE in the majority of studies that provided detailed TEAE data, varied widely in incidence between publications, occurring at a rate of 7.1% to 31.0% of adults and 3.5% to 47.2% of pediatric patients (Table 3). However, despite this, four studies of the effect of MTS on sleep dysfunction in pediatric patients did not demonstrate a significant effect on sleep latency or total sleep time.Reference Faraone, Glatt, Bukstein, Lopez, Arnold and Findling35–Reference Findling, Wigal and Bukstein37, Reference Findling, Katic, Rubin, Moon, Civil and Li40 Therefore, while stimulants have the potential to increase insomnia and sleep latency, the extent to which stimulants contribute to sleep dysfunction in patients with ADHD is unclear.Reference Ashkenasi33
In one trial evaluating abuse liability in adults with a prior history of SUD, there was no evidence of abuse of MTS.Reference McRae-Clark, Brady, Hartwell, White and Carter45 However, the potential for abuse is an important factor to consider when treating adult ADHD with stimulants, especially as adult ADHD is known to be associated with SUD,Reference Olsen, Reimherr, Marchant, Wender and Robison46 and patients with ADHD and comorbid ODD may be more likely to have a SUD,Reference Reimherr, Marchant, Olsen, Wender and Robison50 although this trend was not consistently observed.Reference Olsen, Reimherr, Marchant, Wender and Robison46 More studies in larger populations of adults are necessary to critically evaluate the risk of abuse liability of methylphenidate and other ADHD treatment options, including by formulation type.
While MTS was generally associated with worse skin reactions than placebo, these reactions were largely mild and transient. Ten studies identified skin reactions as a reason for treatment discontinuation in at least one patient, including one study of adults. The percentage of patients discontinuing treatment was relatively small across all studies, ranging from 0% to 7.1%.Reference Pierce, Katic, Buckwalter and Webster49
Among the articles reporting on adults, three papers assessed MTS in adult ADHD patients with comorbid psychiatric disorders.Reference Marchant, Reimherr, Robison, Olsen and Kondo44, Reference Olsen, Reimherr, Marchant, Wender and Robison46, Reference Reimherr, Marchant, Olsen, Wender and Robison50 These studies provide a unique opportunity to compare findings in adults and pediatric studies; previous findings have indicated that, in the pediatric population, comorbid ODD is associated with greater functional impairment. However, treatment with stimulants and nonstimulants can improve symptoms even in patients with very poor baseline functioning scores and can preclude the need for additional medications to control symptoms.Reference Blader, Pliszka, Jensen, Schooler and Kafantaris56, Reference Coghill, Werner-Kiechle, Farahbakhshian, Bliss, Robertson and Huss57 These findings are corroborated by articles identified in the present search, in which MTS improved symptoms of ADHD, PD, ODD, and ED in adult patients.Reference Marchant, Reimherr, Robison, Olsen and Kondo44, Reference Olsen, Reimherr, Marchant, Wender and Robison46, Reference Reimherr, Marchant, Olsen, Wender and Robison50
While some studies of MTS have included adults, amphetamine patch data are missing. Recently, a large meta-analysis recommended the use of amphetamines as the primary oral short-term treatment option in adult ADHD.Reference Cortese, Adamo and Del Giovane22 Given the benefits observed with MTS in both children and adults, transdermal application of amphetamines may serve to increase adherence while providing comparable safety and efficacy outcomes, as observed with oral amphetamines. It should be noted that in adults with a history of SUD, MTS treatment was not associated with stimulant abuse.Reference McRae-Clark, Brady, Hartwell, White and Carter45 While this finding would have to be confirmed in a similar population with a transdermal amphetamine preparation, the lack of an abuse signal is encouraging. As amphetamines have been recommended for adults over other ADHD treatments, including methylphenidate, this suggests a role for transdermal amphetamine formulation in this underserved population. However, such potential benefits will need to be confirmed by trials in adult patients with ADHD.
This study has several limitations. First, only five studies reported on transdermal stimulants in adults, limiting the extent to which findings in adults and children/adolescents could be compared. This low number was especially limiting in studies reporting safety outcomes, as only two articles discussed TEAEs in adults. Furthermore, in-depth studies of sleep disturbance were not identified in adults, and there were limited data reported for domains such as adherence, HR-QoL, and HRU. Future studies of adult ADHD should include these important domains.
Second, variability in endpoint reporting between studies complicated direct comparison between trials. Only two trials, both in adults, reported responder rates.Reference Olsen, Reimherr, Marchant, Wender and Robison46, Reference Reimherr, Marchant, Olsen, Wender and Robison50 Similarly, while multiple studies reported on skin reactions, variability in the scales and definitions used precluded direct and simple cross-comparison of results. Standardization of endpoint reporting would improve future trials, facilitating comparison between various studies and populations.
Third, although the search was not limited by transdermal treatment type, all studies identified for this analysis evaluated transdermal formulations of methylphenidate; studies that focused on alternative transdermal treatments, such as amphetamines, were not found. This result is despite evidence of the efficacy of amphetamines in ADHD.Reference Cortese, Adamo and Del Giovane22 As there are currently no approved transdermal amphetamine formulations, their development represents an important unmet need.
Finally, due to the heterogeneous nature of the data, only a qualitative/semiquantitative summary and synthesis were possible. Results in pediatric vs adult samples could only be broadly extrapolated for some key efficacy and safety outcomes. Nevertheless, to our knowledge, this is the first systematic review of transdermal treatments for patients with ADHD across all ages. These data should inform future studies of MTS and transdermal amphetamine treatment options in adults, and such studies should provide results from a comprehensive battery of symptomatic, functional, and adverse effect outcomes.
In summary, MTS has demonstrated considerable efficacy with a tolerable safety profile in both children and adults. However, adult ADHD remains an understudied area with few treatment options. This analysis supports the applicability of transdermal treatment options in adults and provides a strong rationale for the development of other transdermal systems, such as transdermal amphetamine, in this underserved patient population.
Acknowledgments
The authors thank Meghan Sullivan, PhD, of PharmaWrite, LLC, for medical writing and editorial assistance, which were sponsored by Noven Pharmaceuticals, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP3 Guidelines.”
Funding
The authors did not receive funding for the preparation of this manuscript. Noven Pharmaceuticals, Inc., provided funding to PharmaWrite, LLC, for medical writing and editorial support for this manuscript.
Disclosures
Dr. Correll has been a consultant and/or advisor to or has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma.
Dr. Starling is employed by Noven Pharmaceuticals, Inc and reports nonfinancial support from Hisamitsu Pharmaceutical Co, Inc., during the conduct of the study.
Dr. Huss has been a consultant and/or advisor to or has received honoraria from Eli Lilly, Engelhard Arzneimittel, Janssen/J&J, Lundbeck, MEDICE, Novartis, Shire, Steiner Arzneimittel, and Takeda. He has provided expert testimony for MEDICE and Shire. He received grant support from Eli Lilly, Engelhardt Arzneimittel, MEDICE, and Steiner Arzneimittel.






