Implications
Calcium is essential in multiple physiological processes. Under normal physiological conditions, calcium is tightly regulated by parathyroid hormone, calcitriol and calcitonin. Dairy cows have been selected for high milk production. At the onset of lactation, the high demand of calcium for milk production often causes decreased calcium concentrations in blood, which leads to subclinical or clinical hypocalcemia. Besides the impact on performance and health, hypocalcemia increases the risk of suffering from further metabolic diseases. This review describes factors associated to calcium regulation around parturition as well as established and novel strategies to reduce hypocalcemia incidence in dairy cows.
Introduction
Calcium is the most abundant mineral in all vertebrate animals. Most of the body’s calcium (99%) is stored in the bones and teeth, with only 1% stored in blood and other tissues. Calcium is a critical regulator of multiple physiological processes, including blood coagulation, nerve conduction, membrane permeability, muscle contraction, enzyme activity and hormone release. Based on the multiple important functions of calcium, maintenance of calcium concentrations in the normal physiological range is tightly regulated in all organisms (Brown, 1991). The classic calciotropic hormones – parathyroid hormone (PTH), calcitriol and calcitonin (CT) – regulate calcium homeostasis under normal physiological conditions in mammals by coordinating the availability of calcium in the blood at the level of the bones, intestine and kidneys (Brommage and DeLuca, 1985; Hoenderop et al., 2005b).
Lactation is part of the reproductive strategy employed by mammals to improve the survival of their offspring. Calcium is the most abundant mineral present in milk. Because calcium is involved in many functional systems of the organism, and despite the enormous loss of calcium into milk, calcium homeostasis is crucial for the survival of the lactating animal. Therefore, calcium homeostasis is differentially regulated during pregnancy and lactation compared to non-pregnant and non-lactating physiological states (Bauman and Currie, 1980; Salari and Abdollahi, Reference Salari and Abdollahi2014). The calcium uptake by the mammary gland for milk production decreases circulating calcium concentrations, which triggers the homeostatic mechanisms to restore calcium concentrations in blood. The homeostatic regulation of calcium in lactating rodents and humans is known to be largely regulated by parathyroid hormone-related protein (PTHrP) (Kovacs, Reference Kovacs2011), rather than PTH, which is crucial in non-lactating physiological states. Unlike other mammalian species, dairy cows have been genetically selected for high milk production beyond neonatal requirements (Table 1). Overall, the produced amount of milk does usually not exceed the nutritional requirements of the offspring. As shown in Table 1, calcium concentrations are higher in both colostrum and milk from dairy cows than those for humans. Therefore, the enormous demand of the mammary gland for calcium around parturition makes dairy cows unique to study different physiological processes involved in calcium homeostasis at the onset of lactation.
Table 1. Calcium dynamics during lactation in human, rat and dairy cow species1
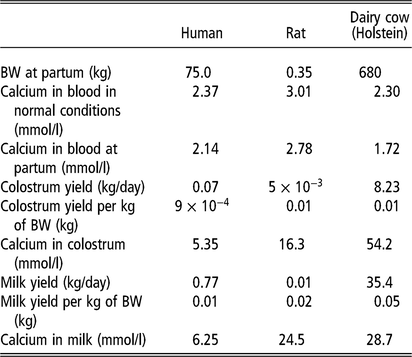
1 Data obtained from Keen et al., 1981; Saint et al., 1984; Neville et al., 1988; Goldstein, 1990; Thorsdottir and Birgisdottir, 1998; LaBorde et al., 1999; VandeHaar and St-Pierre, 2006; Tsioulpas et al., 2007; Capper and Cady, 2012; Hernández-Castellano et al., 2017b).
This review describes diverse endocrine systems and other factors associated with the regulation of calcium homeostasis in mammalian species at the onset of lactation, focusing on dairy cows. Additionally, we review traditional strategies such as those based on the manipulation of the mineral balance, the dietary availability of calcium and the administration of either calcitriol or its precursors to reduce the incidence of hypocalcemia (HC) in parturient dairy cows. We also review novel players in calcium homeostasis such as serotonin and prolactin, which may be implemented in future strategies to prevent HC in dairy cows.
Endocrine regulation of calcium during pregnancy and lactation
Calcium concentrations in blood are tightly maintained by the regulation of intestinal calcium absorption, renal calcium reabsorption and bone calcium resorption. Calcium fluxes in these three organs are primarily regulated by the hormones PTH, calcitriol and CT. Decreased calcium concentrations activate calcium-sensing receptors located on the parathyroid chief cells, which cause PTH release into the bloodstream (Kumar and Thompson, 2011). Under normal physiological conditions, PTH signals via its receptor on the osteoblast membrane, which increases osteoclast proliferation, increasing bone resorption activity. In addition to osteoclastic bone resorption, osteocytes seem to contribute to calcium transferred from bones through a mechanism known as osteocytic osteolysis (Wysolmerski, Reference Wysolmerski2012). Osteocytes are able to remodel bone matrix contained in the lacunae, releasing calcium into the bloodstream through the osteocyte canalicular network. Increased PTH levels stimulate osteocytes to resorb perilacunar mineral (mainly calcium) and collagen, which increases the size of the lacunar area (Belanger and Migicovsky, 1963; Tazawa et al., 2004; van Bezooijen et al., Reference van Bezooijen, Roelen, Visser, van der Wee-Pals, de Wilt, Karperien, Hamersma, Papapoulos, ten Dijke and Lowik2004). Wysolmerski (Reference Wysolmerski2012) described increased lacunar area in lactating rats compared to virgin rats. However, these differences were not evidenced in rats with osteocyte-specific disruption of the PTH receptor 1 gene. These results suggest that osteocytic osteolysis could play an important role in calcium homeostasis during lactation; however, further studies will be necessary to know how quantitatively important is osteocytic bone remodeling during lactation. During bone resorption, collagen is degraded by osteoclasts and osteocytes, and factors such as pyridinoline (PYD), deoxypyridinoline (DPD) and collagen type I C telopeptide (CTX) are released into the bloodstream (Urena et al., Reference Urena, Ferreira, Kung, Morieux, Simon, Ang, Souberbielle, Segre, Drueke and Devernejoul1995). Therefore, PYD, DPD and CTX are often used as markers for calcium release from the bone to the bloodstream not only in humans (Urena et al., Reference Urena, Ferreira, Kung, Morieux, Simon, Ang, Souberbielle, Segre, Drueke and Devernejoul1995; Eastell et al., 1997) but also in dairy ruminants (Liesegang et al., 1998; Liesegang, 2000; Holtenius and Ekelund, 2005; Wilkens et al., Reference Wilkens, Liesegang, Richter, Fraser, Breves and Schroder2014; Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez, Sauerwein and Bruckmaier2017a).
In addition to increased bone resorption, PTH also stimulates the secretion of the renal 1-α-hydroxylase, the enzyme that catalyzes the hydroxylation of calcidiol to calcitriol. During pregnancy and lactation, other endocrine factors such as prolactin, estradiol and placental lactogen are also able to stimulate the activity of 1-α-hydroxylase (Kovacs and Kronenberg, Reference Kovacs and Kronenberg1997), contributing to increased calcitriol concentrations. Although similar studies do not exist in ruminants, several authors have described increased calcitriol concentrations in cows (Özçelik et al., Reference Özçelik, Bruckmaier and Hernández-Castellano2017), goats (Starke et al., 2016) and sheep (Klinger et al., 2016) during late pregnancy and early lactation. Therefore, it seems that the regulation of calcitriol is expanded during this period due to the increased demand of calcium. Calcitriol, also known as the active form of vitamin D (1,25-(OH)2D3), increases calcium concentrations in blood by increasing both intestinal calcium absorption and renal calcium reabsorption (Horst et al., Reference Horst, Goff and Reinhardt1994). In the intestine, calcitriol regulates calcium absorption mainly through the active transcellular pathway. Intestinal transcellular transport consists of calcium entry through specific calcium channels such as transient receptor potential vanilloid type 5 (TRPV5) and transient receptor potential vanilloid type 6 (TRPV6) present in membranes of the brush border, followed by intracellular transport mediated by the calcium-binding protein, calbindin-D9k. Calcium is then actively transported to the bloodstream by the plasma membrane calcium ATPase (PMCA1b) (Lieben et al., Reference Lieben, Carmeliet and Masuyama2011). Calcitriol also increases the efficiency of passive paracellular transport of calcium by increasing the expression of claudin-2 and claudin-12 in the gut (Lieben et al., Reference Lieben, Carmeliet and Masuyama2011); however, this mechanism is still not fully understood. In the kidney, calcitriol regulates calcium reabsorption which is mediated by active transcellular transport in the distal nephron (Hoenderop et al., 2005a). Calcium enters the kidney through TRPV5, binding to calbindin-D9k and calbindin-D28k. Then, calcium enters the bloodstream through the Na+/Ca2+ exchanger (NCX1) and PMCA1b (Hoenderop et al., 2005a). In the intestine and the kidney, calcitriol increases the expression of calcium transporters (i.e., calbindin-D9k, calbindin-D28k, PMCA1b, TRPV5 and TRPV6) (Fleet and Wood, 1999; Li et al., 2001; Lieben et al., Reference Lieben, Carmeliet and Masuyama2011). In humans, Ardawi et al. (Reference Ardawi, Nasrat and BA'Aqueel1997) showed how calcitriol concentrations are increased two- to threefold during pregnancy (333 pmol/l) and lactation (267 pmol/l) compared to normal physiological conditions (93 pmol/l). However, in rodents, the role of calcitriol in calcium homeostasis during lactation seems to be secondary, as mice and rats deficient in the calcitriol receptor are able to lactate and provide adequate milk for their pups (Fudge and Kovacs, 2010; Kovacs, Reference Kovacs2012).
When calcium concentrations increase above normal physiological levels, increased cytoplasmic calcium concentrations in the parafollicular cells of the thyroid (C cells) cause CT release from C cells into the bloodstream (Garrett et al., 1995). Calcitonin primarily acts on the osteoclasts, reducing calcium resorption from bones. Specifically, CT promotes dephosphorylation of Pyk2 and phosphorylation of focal adhesion kinase, which reduce osteoclast adhesion. Additionally, CT also disrupts the cytoskeletal organization of the osteoclasts, reducing osteoclast mobility and causing cell retraction (Zhang et al., 2002; Takahashi et al., 2007; Del Fattore et al., 2008). As observed in a study in humans, CT concentrations increase during pregnancy (20 pmol/l) and early lactation (25 pmol/l) compared to non-pregnant physiological states (9.2 pmol/l) (Ardawi et al., Reference Ardawi, Nasrat and BA'Aqueel1997). These data suggest that CT may have an important role protecting the maternal skeleton from excessive calcium resorption. Similarly, mice deficient for the CT gene lose twice as much bone mineral content (−53.6%) compared to wild-type mice (−23.6%) during the first 21 days of lactation (Woodrow et al., 2006), suggesting again the protective role of CT on bones during the periods of high calcium demand, such as pregnancy and lactation.
Prolactin and its role in calcium homeostasis
As described above, calcium homeostasis is regulated by other endocrine factors in addition to PTH, calcitriol and CT. Prolactin is secreted by lactotrophs in the anterior pituitary (Lamberts and Macleod, 1990). This hormone has a predominant role in milk secretion during lactogenesis, and it has also been demonstrated to regulate calcium homeostasis. At the onset of lactation, prolactin concentrations are increased about 5- to 20-fold in humans, rats and cows compared to non-lactating concentrations (Pepperell et al., 1977; Johke, 1979; Jahn et al., 1993; Mazor et al., 1996; Tong et al., 2018). In addition to the role of prolactin in lactogenesis, it has also been demonstrated to be critical for intestinal calcium transport in mice, rats and humans (Pahuja and DeLuca, 1981; Ajibade et al., Reference Ajibade, Dhawan, Fechner, Meyer, Pike and Christakos2010; Christakos et al., 2011), regulating diverse calcium transport proteins such as TRPV6 and calbindin-D9k (Ajibade et al., Reference Ajibade, Dhawan, Fechner, Meyer, Pike and Christakos2010). As demonstrated by Wongdee et al. (2016), prolactin increases intestinal calcium absorption in rats by increasing the absorptive surface area and enhancing the expression of other calcium transporters (PMCA1b and NCX1) in addition to TRPV6. Less research is available regarding the role of prolactin on renal calcium reabsorption. Lotinun et al. (1998) observed that pregnant rats treated with prolactin reduced urinary calcium excretion (5 µmol/kg of BW per day) compared to control pregnant rats (10 µmol/kg of BW per day). Additionally, Robinson et al. (1982) suggested that prolactin indirectly reduces renal calcium excretion by increasing the activity of 1-α-hydroxylase, which increases calcitriol concentrations.
In addition to the effects of prolactin on the intestine and kidneys, prolactin increases calcium resorption from bones and reduces renal calcium excretion in humans and rats during pregnancy and lactation (Mainoya, 1975; Ritchie et al., 1998; Lotinun et al., Reference Lotinun, Limlomwongse, Sirikulchayanonta and Krishnamra2003; Charoenphandhu and Krishnamra, Reference Charoenphandhu and Krishnamra2007). Prolactin’s effects on bone calcium metabolism are predominantly on the primary trabecular sites (Puntheeranurak et al., 2006; Charoenphandhu and Krishnamra, Reference Charoenphandhu and Krishnamra2007). Prolactin acts by decreasing mRNA expression of runt-related transcription factor (Runx) 2 (Charoenphandhu et al., Reference Charoenphandhu, Teerapornpuntakit, Methawasin, Wongdee, Thongchote and Krishnamra2008), which is essential for osteoblast maturation and differentiation (Komori et al., 1997; Xiao et al., 2005). When osteoblast maturation and formation are suppressed, calcium absorption from blood into bones is reduced in favor of increased bone resorption from bones.
Serotonin and its role in calcium homeostasis
Serotonin (5-Hydroxytryptamine; 5-HT), a biogenic amine and tryptophan derivative, is synthesized in the CNS and regulates physiological processes related to mood or appetite (Jenkins et al., 2016). However, 98% of 5-HT is synthesized in organs such as the intestine, lung, pancreas, prostate, salivary gland, thyroid, liver and the mammary gland (Lauder, 2004; Hernandez et al., Reference Hernandez, Limesand, Collier, Horseman and Collier2009; Pai and Marshall, 2011). Serotonin has been demonstrated to contribute to calcium homeostasis in humans (Modder et al., 2010), mice (Laporta et al., Reference Laporta, Keil, Vezina and Hernandez2014) and dairy cows (Laporta et al., Reference Laporta, Moore, Peters, Peters and Hernandez2013). During lactation, prolactin stimulates the expression of genes essential for 5-HT biosynthesis (tryptophan hydroxylase, TPH; and aromatic amine decarboxylase, AADC) by mammary epithelial cells in response to filling of the mammary gland (Matsuda et al., Reference Matsuda, Imaoka, Vomachka, Gudelsky, Hou, Mistry, Bailey, Nieport, Walther, Bader and Horseman2004). Consequently, mammary epithelial cells increase the secretion of 5-HT into the bloodstream, contributing to an elevation in circulating 5-HT concentrations (Matsuda et al., Reference Matsuda, Imaoka, Vomachka, Gudelsky, Hou, Mistry, Bailey, Nieport, Walther, Bader and Horseman2004; Hernandez et al., Reference Hernandez, Gregerson and Horseman2012; Laporta et al., Reference Laporta, Keil, Vezina and Hernandez2014). Additionally, 5-HT has been demonstrated to stimulate the production of mammary-derived PTHrP (Hernandez et al., Reference Hernandez, Gregerson and Horseman2012). As reviewed by Cowin and Wysolmerski (Reference Cowin and Wysolmerski2010), PTHrP is necessary for full differentiation of the mammary mesenchyme and regulates the expression of at least 40 molecules in the mammary gland. During lactation, the mammary gland secretes PTHrP into milk at concentrations exceeding 10 000 times the amount of PTHrP present in the blood (Riond et al., 1995; Kovacs, Reference Kovacs2011). Despites the function of PTHrP in milk remains unknown, the concentration of this peptide in milk is correlated to circulating calcium concentrations in cows (Kocabagli et al., 1995). At the onset of lactation, PTHrP concentrations in the circulation increase to detectable levels in humans, rats and cows (Thiede and Rodan, 1988; Onda et al., 2006; Kovacs, Reference Kovacs2011), for the purpose of coordinating calcium metabolism during lactation (Rakopoulos et al., 1992; Onda et al., 2006). It has been demonstrated that PTHrP utilizes the PTH receptor, suggesting that it has the same biological activity as PTH, although it does not regulate calcitriol (Philbrick et al., 1996; Strewler, 2000). Therefore, the interaction of 5-HT with PTHrP implicates the possibility of 5-HT in regulating calcium homeostasis during lactation (Hernandez, Reference Hernandez2017). Schilling et al. (1993) demonstrated that PTHrP does not stimulate renal 1-hydroxylase activity in patients with humoral hypercalcemia of malignancy. This is in contrast to the action of PTH during primary hyperparathyroidism in which PTH stimulates renal 1-hydroxylase activity and consequently increases calcitriol concentrations. Although PTH increases intestinal calcium absorption and decreases renal calcium excretion through increased calcitriol concentrations, the effect of PTHrP on these mechanisms remains unclear. Therefore, it is possible that PTHrP may only act on the bone to regulate calcium metabolism.
Calcium regulation in dairy cows and hypocalcemia during the periparturient period
The periparturient period involves considerable metabolic adaptations in mammals. In high-yielding dairy cows, the sudden high demand of nutrients for milk production at the onset of lactation and the associated metabolic load required for the production of this copious amount of milk increasingly exceeds the adaptive capacity of the animal, leading to increased incidence of several metabolic diseases (Paudyal et al., 2018), HC being one of the most relevant (Figure 1). There are a variety of factors that can modulate and affect the metabolic adaptation around parturition.
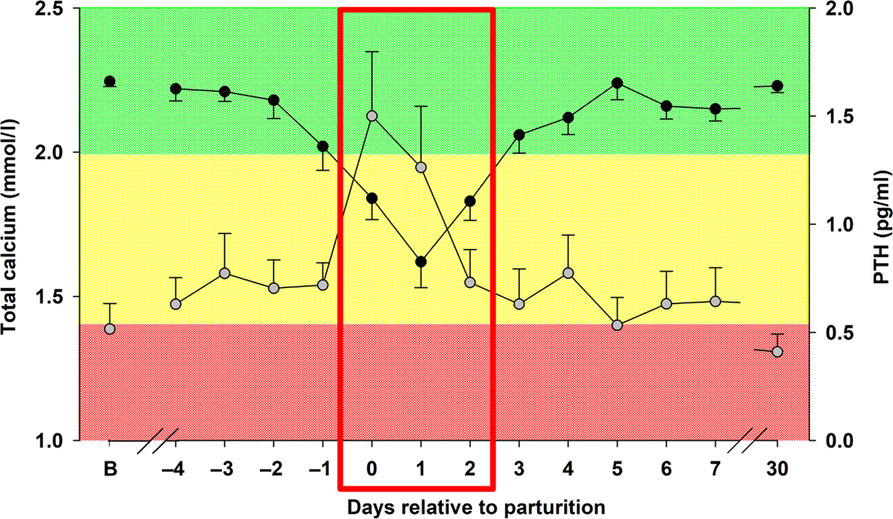
Figure 1 Total calcium (![]() ) and parathyroid hormone (PTH;
) and parathyroid hormone (PTH; ![]() ) concentrations in dairy cows around parturition. Total calcium was measured in blood serum. PTH was measured in blood plasma. The red box shows time around parturition, when circulating calcium concentrations abruptly drop, causing an increase in PTH concentrations. B, baseline obtained 2 weeks before parturition. Cows with calcium concentrations within the green area are within the normal circulating calcium levels. Cows with calcium concentrations within the yellow area are under subclinical hypocalcemia. Cows with calcium concentrations within the red area are under clinical hypocalcemia. Adapted from Hernández-Castellano et al. (Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez, Sauerwein and Bruckmaier2017a and Reference Hernández-Castellano, Weaver, Hernandez and Bruckmaier2017b).
) concentrations in dairy cows around parturition. Total calcium was measured in blood serum. PTH was measured in blood plasma. The red box shows time around parturition, when circulating calcium concentrations abruptly drop, causing an increase in PTH concentrations. B, baseline obtained 2 weeks before parturition. Cows with calcium concentrations within the green area are within the normal circulating calcium levels. Cows with calcium concentrations within the yellow area are under subclinical hypocalcemia. Cows with calcium concentrations within the red area are under clinical hypocalcemia. Adapted from Hernández-Castellano et al. (Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez, Sauerwein and Bruckmaier2017a and Reference Hernández-Castellano, Weaver, Hernandez and Bruckmaier2017b).
Lactation, breed and milk yield
Lactation number is a critical factor in the etiology of HC. Reinhardt et al. (Reference Reinhardt, Lippolis, McCluskey, Goff and Horst2011) described that plasma calcium concentrations decreased much less during the first 48 h postpartum in primiparous cows compared to multiparous cows (2.15 v. 1.85 mmol/l, respectively). These data suggest that the mechanisms for calcium homeostasis become less effective as the cow undergoes subsequent lactations and may contribute to greater or prolonged HC in older animals. In agreement with this observation, Fleischer et al. (2001) and Caixeta et al. (2017) described a positive correlation between HC and lactation number. In primiparous cows, remodeling processes in the bones are very active, and therefore calcium resorption from the bones is rapid and efficient compared to multiparous cows (Iwama et al., 2004). Aging also results in a decline in the active transport of calcium in the intestine and impaired production of calcitriol (Woodrow et al., 2006), affecting the capacity of the animal to rapidly modulate imbalances in blood calcium concentrations.
In addition to lactation number, breed also contributes to HC susceptibility in dairy cows. Henderson (1938) reported significant differences in HC incidence between cow breeds (15.3% in Brown Swiss, 13.3% in Shorthorn, 8.6% in Guernsey and 6.0% in Ayrshire). Lean et al. (2006) demonstrated that HC incidence is 2.25 times higher in Jersey cows than in Holstein cows. Similar results were reported by Chiwome et al. (Reference Chiwome, Kandiwa, Mushonga, Sajeni and Habarugira2017) on HC incidence in Jersey and Holsteins cows (14.78% and 4.82%, respectively). Despite several studies having described HC incidence among breeds, the mechanism involved in these differences remains unclear. Goff et al. (Reference Goff, Reinhardt, Beitz and Horst1995) determined that Jersey cows have fewer intestinal calcitriol receptors than Holstein cows (95 v. 142 fmol/mg protein, respectively). The relative amount of intestinal calcitriol receptors in Jersey cows compared to Holsteins cows may affect the amount of calcium that can be absorbed in the intestine, which could be a potential explanation for the differences in HC susceptibility between breeds. It has also been suggested that differences in HC incidence between Jerseys and Holsteins may be related to the amount of milk produced by kilogram of BW (Blake et al., 1986; Chiwome et al., Reference Chiwome, Kandiwa, Mushonga, Sajeni and Habarugira2017), which is higher in Jerseys (average BW = 454 kg; average milk production = 31.7 kg/day) compared to Holsteins (average BW = 680 kg; average milk production = 35 kg/day) (Capper and Cady, 2012). However, not only the relation between milk yield and BW, but milk yield alone appears to be positively correlated with HC incidence in dairy cows. Jawor et al. (2012) demonstrated that cows suffering HC during the first 48 h postpartum are those that produced approximately 6.4 kg of milk more per day compared to cows without HC. Similarly, Gild et al. (2015) described that cows with subclinical HC produced 3.17, 2.71 and 1.90 kg more milk compared to normocalcemic cows on the first, second and third days of lactation, respectively. It is assumed that cows with greater capacity for milk production may also be prone to greater net loss of calcium into the milk shortly after parturition (Jawor et al., 2012), exceeding the already saturated adaptive capacity of the animal.
Mineral balance
Adequate mineral balance is crucial for the normal function of the metabolic and physiological processes in animal systems. Deficiencies or imbalances in the mineral content can lead to abnormal nerve function, muscle contraction, immune response, the blood coagulation cascade and other physiological processes. Minerals present in organisms in large amounts are calcium, phosphorus, magnesium, sodium, potassium, chloride and sulfur. In the specific case of calcium, several studies have detected a correlation between low circulating magnesium concentrations and low circulating calcium concentrations in humans (Classen et al., 1986; Paunier, 1992), mice (Zimmermann et al., 2000) and dairy cows (Kronqvist et al., Reference Kronqvist, Emanuelson, Sporndly and Holtenius2011 and Reference Kronqvist, Emanuelson, Traven, Sporndly and Holtenius2012). Low magnesium concentrations have been shown to reduce the secretion of PTH (Suh et al., 1973), as well as decrease tissue responsiveness to PTH (Reddy et al., 1973; Rude, 1998). Phosphorous status also affect cow’s ability to regulate calcium concentrations in blood around parturition. Indeed, Cohrs et al. (2018) showed how reduced dietary phosphorus intake in late pregnancy increased the calcium resorption from bones, which in turn increase circulating calcium concentrations around parturition.
Based on these facts, it can be assumed that blood mineral status (i.e., magnesium and phosphorous) directly affects the efficiency of the mechanisms involved in calcium homeostasis during the periparturient period in cows, which may result in increased susceptibility to HC.
Hypocalcemia: Traditional treatments and new alternatives
At the onset of lactation, the demand for calcium by the mammary gland often causes decreased circulating calcium concentrations in dairy cows between 12 and 24 h postpartum (Goff, Reference Goff2008) (Figure 1). As described by Reinhardt et al. (Reference Reinhardt, Lippolis, McCluskey, Goff and Horst2011), 50% of dairy cows are affected by subclinical HC (blood calcium concentrations within 1.4 to 2.0 mmol/l) postpartum. Furthermore, it was determined that 5% to 7% of dairy cows suffer clinical HC (calcium concentration <1.4 mmol/l) (Roche and Berry, Reference Roche and Berry2006). In most of the cases, cows with clinical HC are not able to restore normal calcium concentrations postpartum. If the cow is not treated rapidly, clinical HC can lead to the death of the animal (Oetzel, Reference Oetzel1988). Therefore, cows with clinical HC are often treated with intravenous infusions of calcium gluconate (23%, 500 ml = 10.8 g of calcium) (Oetzel, Reference Oetzel1988). Alternatively, the administration of a ruminal calcium bolus (approximately 43 g of calcium) can be used to restore blood calcium concentrations; however, calcium absorption is slower compared to intravenous administration of calcium gluconate. When cows undergo clinical and subclinical HC, they are more susceptible to other metabolic and infectious diseases (Compton et al., 2007). Therefore, most strategies for HC prevention focus on the compensation of circulating calcium deficiencies during the first 24 to 48 h postpartum. Prevention of HC is crucial in order to increase the health and welfare in dairy cows as well as the economic benefit for dairy farmers.
Traditional management practices for the prevention of hypocalcemia
The use of diets formulated with a negative dietary cation-anion difference (DCAD) prior to parturition has long been established to increase calcium concentrations around parturition (Ender et al., Reference Ender, Dishington, Helge-Bostad and Martinsons1962; Dishington, Reference Dishington1975; Block, 1988). Shohl and Sato (1923) were the first to hypothesize that mineral interrelationships were related to acid–base status. Then, Shohl (1939) proposed that the body excretes dietary cations and anions excess in order to maintain normal acid–base balance. Based on these findings, Dishington (Reference Dishington1975) established that the net acid intake in dairy cows, based on dietary difference between cations and anions, was mostly influenced by sodium, potassium, chloride and sulfur. Therefore, DCAD diets for dairy cows are calculated based on the following formula: DCAD = (sodium + potassium) – (chloride + sulfur) mEq/kg of DM (Charbonneau et al., 2006). Negative DCAD diets aim to reduce the charge ion difference in blood, which decreases blood and urine pH, resulting in strong ion metabolic acidosis. This metabolic acidosis is compensated in part by the bone accepting hydrogen ions (H+) in exchange for calcium (Lemann et al., 2003). Thus, the metabolic acidosis created by feeding negative DCAD diets inhibits renal calcium reabsorption prepartum, increasing urinary calcium excretion. This fact triggers the homeostatic mechanisms that increase calcium influx (Gaynor et al., 1989; Phillippo et al., 1994; DeGaris and Lean, Reference DeGaris and Lean2008) and target tissue responsiveness to parathyroid hormone (Horst et al., 1997). In addition, feeding negative DCAD diets (i.e., −59 mEq/kg DM) results in increased calcitriol concentrations in blood compared to cows being fed positive DCAD diets (68 v. 40 pmol/l on day −3 relative to parturition, respectively) (Phillippo et al., 1994). However, decreased palatability of anionic salts is thought to be a limitation of this strategy, which can dramatically affect the already reduced feed intake in dairy cows around parturition (Moore et al., 2000). More recently, the combination of diets with reduced basal cations in combination with more palatable anion sources have been used to increase circulating calcium concentrations around parturition without negative effects on feed intake (DeGroot et al., Reference DeGroot, Block and French2010; Weich et al., 2013; Weiss et al., Reference Weiss, Azem, Steinberg and Reinhardt2015). However, the relation between decreased feed intake and DCAD composition is still controversial. According to a recent publication by Zimpel et al. (2018), reduced feed intake in cows fed negative DCAD diets prepartum (−114 and −113 mEq/kg DM) seems to be a direct consequence of the metabolic acidosis status of the animal and not a consequence of the addition of acidogenic products or salts containing chloride in the DCAD diet. The number of days that cows need to be fed with DCAD diets is another factor that may influence the capacity of these diets to regulate calcium homeostasis at parturition. Lopera et al. (2018) fed two different negative DCAD diets (−70 and −180 mEq/kg DM) to dairy cows for either 42 or 21 days prepartum. They showed that cows fed for 21 days prepartum showed a similar metabolic acidosis at calving as those fed for 42 days postpartum. Therefore, it seems that feeding cows with a negative DCAD for 21 days prepartum is enough to cause a metabolic acidosis at calving and prevent or reduce HC incidence. Metabolic acidosis level prepartum can be easily monitored by measuring either urinary calcium concentrations or urinary pH (Martin-Tereso and Martens, 2014). As described by Leno et al. (Reference Leno, Ryan, Stokol, Kirk, Zanzalari, Chapman and Overton2017) cows fed a negative DCAD diet prepartum (−74 mEq/kg DM) increased urinary calcium excretion (8.2 v. 0.7 g/day, in negative DCAD diet and control cows, respectively) and maintained urine pH between 5.5 and 6.0 compared to the control cows (urine pH 7.89). Additionally, circulating calcium concentrations (2.27 v. 2.16 mmol/l), feed intake (3.07% v. 2.88% of BW) and milk yield (43.9 v. 40.8 kg/day) increased postpartum in cows fed a negative DCAD diet compared to control cows. Similar results have been recently shown by Diehl et al. (2018) using a negative DCAD diet (−222 mEq/kg DM) during the last 21 days prepartum.
As an alternative to negative DCAD, feeding low calcium diets prepartum have also been used to induce homeostatic adaptation of calcium metabolism in dairy cows. Calcium requirements for dry cows are minimal (22 g/day for a 500 kg cow; NRC, 2001), being necessary only for maintenance and fetal skeletal development. Feeding <20 g of calcium per day during the dry period decreases calcium concentrations in blood, which activates calcium homeostatic mechanisms involving calcium mobilization from bones, intestinal calcium absorption and renal calcium resorption (Kichura et al., 1982). This prepartum feeding strategy results in increased calcium mobilization from bones into the bloodstream and contributes to compensate calcium requested for milk production (Thilsing-Hansen et al., Reference Thilsing-Hansen, Jorgensen, Enemark and Larsen2002a). Additionally, cows fed low calcium diets before parturition increase intestinal efficiency for calcium absorption at parturition. In order to activate calcium homeostatic mechanisms before parturition, low calcium diets should provide <20 g of calcium per day. Therefore, high calcium forages such as alfalfa (12 g of calcium/kg DM) need to be exchanged by low calcium forages such as corn silage (1.7 g of calcium/kg DM) and barley silage (3.4 g of calcium/kg DM) in dry cow diets in order to reduce calcium intake. However, this is difficult to achieve in some places of Europe and North America, due to the predominant forages available for dairy cows. Therefore, an alternative strategy was explored in instances where calcium content of forages is relatively high, which is the inclusion of rumen-protected rice bran in the diet (Martin-Tereso et al., Reference Martin-Tereso, Martens, Deiner, van Laar, den Hartog and Verstegen2016). The low calcium content of this product (5.5 g/kg DM) and the calcium-binding capacity of the phytic acid present in rice bran (61.5 g of phytic acid/kg DM) reduce the dietary calcium available for intestinal absorption, activating the homeostatic calcium mechanisms. Indeed, Martin-Tereso et al. (Reference Martin-Tereso, Martens, Deiner, van Laar, den Hartog and Verstegen2016) showed that cows receiving 140 g of rumen-protected rice bran/kg DM prepartum exhibited higher calcium concentrations compared to controls at parturition (1.98 v. 1.87 mmol/l, respectively), 6 h postpartum (2.05 v. 1.85 mmol/l, respectively) and 12 h postpartum (2.15 v. 1.92 mmol/l, respectively). As described above, these extraordinary physiological conditions for efficient calcium absorption can be used in cows after parturition by feeding them a diet without zeolite and with high calcium content. Large amounts of calcium absorbed in the intestine will contribute to the required amount of calcium for milk production and will maintain circulating calcium concentration constant during the first days after parturition. An additional strategy was developed by Thilsing-Hansen et al. (Reference Thilsing-Hansen, Jorgensen and Ostergaard2002b) to maintain low calcium concentrations in dairy cow diets prepartum. These authors aimed to increase the efficiency the intestinal calcium absorption by feeding dietary zeolite (sodium aluminum silicate) during the last 2 weeks prepartum. Similar to rice bran, zeolite acts by binding dietary calcium, reducing the amount of calcium available for intestinal absorption. Therefore, the functional calcium deficiency prepartum caused by the dietary zeolite stimulates the intestine to increase calcium absorption efficiency at parturition. The use of zeolite appears to be effective in the prevention of HC as cows supplemented with zeolite prepartum had higher calcium concentrations compared to the controls around parturition (2.35 v. 2.05 mmol/l, respectively) (Thilsing-Hansen et al., Reference Thilsing-Hansen, Jorgensen and Ostergaard2002b). Similar results were shown by Khachlouf et al. (Reference Khachlouf, Hamed, Gdoura and Gargouri2019) in cows supplemented with 200 g of sodium aluminum silicate per day during the last 40 days prepartum. In this study, supplemented cows showed higher circulating calcium concentrations at calving than the control cows (~2.34 v. 2.23 mmol/l, respectively).
Another strategy for the prevention of HC consists on the administration of exogenous calcitriol or its precursors in order to increase calcitriol concentrations around parturition, resulting in increased calcium concentrations in blood. Cholecalciferol is one of the precursors of calcitriol. Cholecalciferol is converted to calcidiol, the substrate for 1-α-hydroxylase and renal synthesis of calcitriol. Consequently, the role of cholecalciferol in the prevention of HC has been thoroughly investigated. Hibbs et al. (1947) demonstrated that supplementation with 1 to 5 million IU of cholecalciferol per day during 2 to 4 weeks before parturition was not sufficient to prevent HC in Jersey cows. In subsequent studies, Hibbs and Pouden (Reference Hibbs and Pounden1955) demonstrated that daily supplementation with 30 million IU of cholecalciferol between 8 and 3 days prevented HC in Jersey cows with previous HC problems. However, several studies have shown that the amount of dietary cholecalciferol required for increasing calcium concentrations is close to the toxic dose (Swan, 1951; Capen et al., 1966; Julien et al., 1977), causing marked anorexia, loss of BW, dyspnea, tachycardia, recumbency and severe cardiovascular calcifications (Thilsing-Hansen et al., Reference Thilsing-Hansen, Jorgensen, Enemark and Larsen2002a). According to nutrient requirements for dairy cattle published by the NRC (2001), approximately 20 000 IU of cholecalciferol per day are necessary to support calcium and phosphorus homeostasis during lactation. In another study, a single subcutaneous injection of calcitriol (300 µg) within the first 6 h postpartum successfully increased calcitriol concentrations from 58.5 to 1271 pmol/l 24 h, resulting in increased total calcium concentrations from 2.52 to 3.02 mmol/l (Vieira-Neto et al., Reference Vieira-Neto, Lima, Lopes, Lopera, Zimpel, Sinedino, Jeong, Galvao, Thatcher, Nelson and Santos2017). Additionally, the combination of DCAD diets together with dietary supplementation of either cholecalciferol or calcidiol prepartum in dairy cows has been explored (Martinez et al., Reference Martinez, Rodney, Block, Hernandez, Nelson, Lean and Santos2018). Feeding prepartum cows a diet containing a negative DCAD diet (−130 mEq/kg DM) regardless of the source of vitamin D resulted in increased calcium concentrations around parturition (2.18 mmol/l) compared to cows fed a positive DCAD (+130 mEq/kg DM) diet (1.96 mmol/l). This suggests that the source of vitamin D supplementation does not matter prepartum as long as it is in combination with a negative DCAD diet.
Novel treatments for the prevention of hypocalcemia
In the last decade, the knowledge surrounding the endocrine regulation of calcium during lactation has increased. Based on this new knowledge, factors such as 5-HT have been manipulated to develop novel and potentially more efficient strategies to prevent HC in parturient dairy cows.
In addition to the ability of the mammary gland to synthesize 5-HT, the mammary gland is able to respond to 5-HT as the bovine mammary epithelium expresses multiple 5-HT receptors such as 5-HT-1B, 5-HT-2A, 5-HT-2B, 5-HT-4 and 5-HT-7 (Hernandez et al., Reference Hernandez, Limesand, Collier, Horseman and Collier2009). At the level of the mammary gland, 5-HT is thought to increase PTHrP production (Hernandez et al., Reference Hernandez, Gregerson and Horseman2012); however, it is not known how 5-HT induces the production of PTHrP in the mammary gland. Based on these findings in rodents, research has been performed to evaluate the role of 5-HT on calcium homeostasis in dairy cows (Weaver et al., Reference Weaver, Prichard, Endres, Newhouse, Peters, Crump, Akins, Crenshaw, Bruckmaier and Hernandez2016; Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez, Sauerwein and Bruckmaier2017a and Reference Hernández-Castellano, Weaver, Hernandez and Bruckmaier2017c; Weaver et al., Reference Weaver, Prichard, Maerz, Prichard, Endres, Hernandez-Castellano, Akins, Bruckmaier and Hernandez2017). In these studies, 5-hydroxy-l-tryptophan (5-HTP), a hydroxylated derivative of the amino acid tryptophan, has been intravenously infused to promote the synthesis of 5-HT by the peripheral tissues in dairy cows prior to parturition. Furthermore, 5-HTP has been used as an oral supplement to increase 5-HT concentrations in dairy calves (Hernández-Castellano et al., Reference Hernández-Castellano, Özçelik, Hernandez and Bruckmaier2017b). In Holstein cows, increased 5-HT concentrations in the prepartum period resulted in a less dramatic decrease in calcium concentrations compared to control cows on day 1 (1.93 v. 1.62 mmol/l, respectively) and day 2 postpartum (2.07 v. 1.83 mmol/l, respectively) (Hernández-Castellano et al., Reference Hernández-Castellano, Weaver, Hernandez and Bruckmaier2017c). Similar results were observed in Holstein cows in Weaver et al. (Reference Weaver, Prichard, Endres, Newhouse, Peters, Crump, Akins, Crenshaw, Bruckmaier and Hernandez2016). However, these authors observed that the administration of 5-HTP prepartum resulted in decreased calcium concentrations in Jersey cows before parturition (2.60, 2.25, 2.15 and 2.10 mmol/l from day 4 to 1 prior to parturition, respectively). Jersey cows treated with 5-HTP prepartum increased circulating calcium concentrations earlier postpartum compared to controls (2.25 v. 2.00 mmol/l on day 1 postpartum, respectively). Therefore, it appears that Holstein and Jersey cows differentially respond to 5-HTP administration prepartum. It is possible that in Jersey cows, decreased circulating calcium concentrations prepartum may trigger an earlier recovery of circulating calcium concentrations at parturition. The intravenous infusion of 5-HTP prepartum also resulted in an increase of PYD concentrations in blood on day 1 postpartum (Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez, Sauerwein and Bruckmaier2017a). These results suggest that cows with increased 5-HT concentrations prepartum potentially mobilized calcium from bone tissue prior to control cows. Furthermore, the administration of 5-HTP did not result in a change of either PTH (Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez, Sauerwein and Bruckmaier2017a) or calcitriol concentrations (54 and 48 pmol/l in 5-HTP and control groups, respectively; Hernández-Castellano and Bruckmaier, unpublished data). Therefore, the current data suggest that 5-HT possibly increases calcium resorption from the bones through a different mechanism than PTH, potentially via PTHrP secretion by the mammary gland. The administration of 5-HTP prepartum also resulted in increased 5-HT concentrations in colostrum (37 v. 24 nmol/l in 5-HTP and control cows, respectively) (Hernández-Castellano et al., Reference Hernández-Castellano, Weaver, Hernandez and Bruckmaier2017c). As 5-HTP infusions ceased at parturition, no differences in milk 5-HT concentrations were detected between groups on day 7 postpartum (12 nmol/l). Additionally, Slater et al. (Reference Slater, Endres, Weaver, Cheng, Lauber, Endres, Olstad, DeBruin, Crump, Block and Hernandez2018) combined the use of negative DCAD (−55 v. +14 mEq/kg DM) with the administration of 5-HTP (1 mg 5-HTP/kg BW) prior to parturition, resulting in further increases of both ionized and total calcium concentrations compared to negative DCAD or 5-HTP treatment alone.
Concluding remarks
The present work summarizes the endocrine mechanisms involved in the regulation of calcium homeostasis in mammalian species during lactation, with a special focus on dairy cows. Furthermore, it describes novel research on calcium homeostasis as well as new strategies for the prevention of HC at parturition in dairy cows. Research and knowledge present in this review may be used in the near future to reduce HC incidence. Reduced HC incidence will not only increase farmer´s benefit but also improve animal welfare and reduce the risk of other metabolic diseases during the transition period.
L. E. Hernández-Castellano 0000-0003-2729-0434
Declaration of interest
The authors declare that there are no conflicts of interest.
Ethics statement
None.
Software and data repository resources
None of the data were deposited in an official repository.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731119001605




