Bipolar disorder is a highly prevalent, recurrent and disabling mental illness worldwide, and is often accompanied by high mortality rates, comorbity and economic burdens resulting from suicide and other related medical illnesses. Reference Dutta, Boydell, Kennedy, van Os, Fearon and Murray1,Reference Lloyd, Kennedy, Fearon, Kirkbride, Mallett and Leff2 Although biological research on bipolar disorder has expanded significantly, there has been no precise understanding as to its pathophysiology. Furthermore, distinguishing between major depressive disorder (MDD) and bipolar disorder with depressive episodes remains a diagnostic challenge for clinicians, especially when a patient presents during their first major depressive episode.
Finding novel biomarkers for bipolar disorder, however, has been repeatedly considered to be a crucial breakthrough in combating these challenges, so much so that the development of a reliable and robust diagnostic test has been thought of as the longstanding ‘Holy Grail’ in psychiatry. Reference Le-Niculescu, Kurian, Yehyawi, Dike, Patel and Edenberg3 Finding novel biomarkers is critical to understanding the underlying pathophysiology of bipolar disorder and developing effective psychopharmacological treatment. Furthermore, finding these biomarkers capable of identifying patients with bipolar disorder early in their first depressive episode would allow for early intervention and thereby improve the outcome of the disorder and therapeutic treatments.
In the search for potential bipolar disorder biomarkers, an increasing number of studies strongly suggest that a neurobiological basis may underlie the aetiology of bipolar disorder. In particular, brain-derived neurotrophic factor (BDNF) has attracted attention for its key role in mediating neuronal survival, growth, plasticity and connectivity as well as synaptic efficacy, all of which are thought to be involved in the pathophysiology of bipolar disorder. Reference Duman, Malberg, Nakagawa and D'Sa4,Reference Einat and Manji5 Growing evidence has shown that patients with bipolar disorder displayed lower BDNF levels in both peripheral and central nervous system tissues compared with healthy controls. Reference Dunham, Deakin, Miyajima, Payton and Toro6,Reference Rajkowska7 Moreover, a meta-analysis demonstrated that BDNF levels decreased in patients with bipolar disorder during manic or depressive phases, Reference Fernandes, Gama, Ceresér, Yatham, Fries and Colpo8 and another study noted that mood-stabilising drugs such as lithium and valproate - the most commonly used treatment options for bipolar disorder - were previously shown to increase BDNF levels in hippocampal tissues. Reference Fukumoto, Morinobu, Okamoto, Kagaya and Yamawaki9,Reference Frey, Andreazza, Cereser, Martins, Valvassori and Réus10 Interestingly, however, down-regulated BDNF levels were also reported in patients with MDD. Reference Brunoni, Lopes and Fregni11-Reference Kelleher, Govindarajan and Tonegawa13 It remains to be seen whether or not BDNF levels can differentiate between MDD and bipolar disorder in the first depressive episode or whether BDNF levels can predict bipolar disorder in patients with a first major depressive episode.
In the present study, patients who were drug-naive and in their first major depressive episode were recruited and followed for 3 years to identify which individuals were diagnosed with bipolar disorder and MDD. Presuming that BDNF mRNA and/or plasma BDNF levels on admission could differentiate two patient groups (patients with either MDD or bipolar disorder in their first depressive episode), we re-examined blood samples taken from the patients before they were diagnosed with either bipolar disorder or MDD, to see whether or not BDNF levels provided any clues for establishing a reliable predictor.
Method
Participants
Participants were recruited from the Shanghai Mental Health Center between January 2007 and January 2009. The parameters and methodologies of the study were reviewed and approved by the Institutional Review Board of Shanghai Mental Health Center, and all protocols related to human experiments were conducted in accordance with the Declaration of Helsinki. We ensured that all participants were given an adequate understanding of the study and written informed consent was obtained from all individuals prior to their inclusion in the study.
All participants underwent the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P), Reference First, Spitzer, Gibbon and Williams14 following which, demographic data on age, gender, body mass index (BMI), the duration of major depressive episode, history of smoking, alcohol and other drug use was collected. Assessments of the Hamilton Rating Scale for Depression-17 (HRSD-17) Reference Hamilton15 and Young Mania Rating Scale (YMRS) Reference Young, Biggs, Ziegler and Meyer16 were conducted independently by two experienced psychiatrists (interrater reliability, kappa = 0.84 and kappa = 0.81 respectively) on admission.
For the purposes of this study, patients with a first depressive episode, who were drug-naive (i.e. never taken any psychotropic medication), aged 19-50 years old and who met DSM-IV 17 criteria for a major depressive episode, with an HRSD-17 score of ⩾17, were recruited. Patients with other comorbid Axis I psychiatric disorders were excluded, including those with anxiety disorder, schizophrenia, nicotine dependence, alcohol dependence and substance dependence. Patients with a history or current use of alcohol, nicotine and other substances were also excluded (in this study, smoking and/or alcohol use refers to smoking and/or alcohol harmful use or misuse, excluding recreational use). Patients with severe medical illness (e.g. cancer, diabetes), organic brain disease and those who were pregnant were excluded to ensure clarity.
Age- and gender-matched healthy controls were recruited by advertisement, and those whose HRSD-17 score was <7 were enrolled. Individuals with any major Axis I disorder (including substance dependence, psychotic disorder, mood disorder and anxiety disorder), family history of mental disorder or severe physical diseases (e.g. hypertension, diabetes, cancer) were excluded. In total, 203 patients with a mean age of 31.1 years (s.d. = 4.8) (47 males and 156 females) and 167 healthy controls with a mean age of 30.9 years (s.d. = 4.5) (35 males and 132 females) were included on admission.
RNA and plasma preparation
On admission, 20 ml peripheral venous blood of fasting patients and healthy controls were collected between 07.00 h and 09.00 h. Total RNA was extracted from 10 ml peripheral blood samples using the QIAamp RNA blood Mini Kit (Qiagen, Chatsworth, California, USA) and then treated with DNase (Qiagen, Chatsworth, California, USA). The complementary DNA (cDNA) was synthesised by incubating DNase-treated total RNA (1.0 μg) with omniscript reverse transcription reagents (Qiagen, Chatsworth, California, USA) and a random primer according to the manufacturer’s protocols. Plasma samples were separated from 10 ml peripheral venous blood and centrifuged at 3500 rpm at 4°C for 20 min. All plasma samples were then frozen to –80°C.
Gene relative expression levels analysis by quantitative reverse transcription polymerase chain reaction (RT-PCR)
BDNF mRNA expression levels were measured by quantitative RT-PCR using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, California, USA) with a 384-well format. For the RNA internal control, we used glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Applied Biosystems, California, USA) mRNA expression to normalise the target gene expression levels. TaqMan Universal PCR MasterMix and TaqMan probes/primers were obtained from Applied Biosystems. Quantitative RT-PCR reaction was carried out as follow: 50°C for 2 min and 95°C for 10 min, then 95°C for 50 cycles of 10 s, 59°C for 1 min. Experiments were performed in triplicate for each sample.
Results of the real-time PCR data were represented as cycle threshold (Ct) value, defined as the threshold cycle of PCR at which a significant increase in the fluorescence signal was first detected. Data were collected and analysed with Sequence Detector Software 2.1 (Applied Biosystems, California, USA). The comparative Ct value (ΔCt) was used for relative expression in target gene product, and 2–ΔCt represents the relative expression level. The ΔCt value of each sample (patients and controls) was obtained by subtracting the average GAPDH Ct value of each sample from the average target gene Ct value of each sample.
Plasma levels analysis by enzyme-linked immunosorbent assay (ELISA)
Each plasma sample was measured in duplicate by the ELISA method according to the manufacturer’s protocols (R&D Systems, Minneapolis, Minnesota, USA). Several sample measurements were repeated to confirm reproducibility of the assay and the inter-assay coefficient of variation was 4.18%. Researchers were masked to the clinical data of all participants.
Naturalistic follow-up
During the naturalistic follow-up, patients could present to the out-patient department for treatment as needed. However, they were asked to be interviewed and assessed by two experienced psychiatrists from our research team every 6 months, using the SCID-I/P, YMRS and HRSD-17. Patients diagnosed with bipolar disorder or those who had a manic episode during the 3-year study period were identified as having bipolar disorder. The primary end-point was the occurrence of a hypomanic or manic episode.
Data analysis and statistical tests
Demographic data were analysed using chi-squared, t-test or ANOVA (one-way) as appropriate. Data were examined for normality using the Kolmogorov-Smirnov test. As the plasma BDNF levels were not normally distributed, they were transformed into normal distribution using natural logarithms prior to statistical analysis. ANOVA (one-way) followed by Bonferroni multiple comparison test was used to analyse the difference of BDNF expression levels or plasma levels among patients with MDD (MDD group), patients with bipolar disorder (BPD group) and healthy controls on admission. Because several previous studies demonstrated that age, gender and BMI might affect BDNF levels, Reference Narisawa-Saito and Nawa18,Reference Pillai, Bruno, Sarreal, Hernando, Saint-Louis and Nierenberg19 linear regression models were used to analyse the correlation between BDNF levels and HRSD-17 in the MDD and BPD groups on admission. Confounding factors such as age, gender, illness duration and BMI were also evaluated in a linear regression model to balance their effects. To determine the best model for differentiating bipolar disorder from MDD at the first depressive episode, the discriminatory capacity of each model (gene expression level of BDNF, plasma BDNF level, and the combination thereof) was analysed by calculating the area under the receiver operating characteristic (ROC) curve using logistic regression and a decision tree. Decision trees are predictive models mapping observations about an item to a conclusion on its target value. Reference Zhang and Singer20 Classification and regression (CRT) algorithms were used to build this classification model. In these tree structures, leaves represent classifications and branches represent conjunctions of features that cause those classifications. A tenfold cross-validation was applied to detect the efficiency of this technique. Reference Breiman, Friedman, Olshen and Stone21
A value of 0.5 indicated that the model is equivalent to pure chance, whereas a value of 1 indicated perfect discrimination; concordance statistics between 0.7 and 0.8 were generally considered acceptable. Reference Hosmer and Lemeshow22 The optimal cut-off value was defined by ROC. Throughout all analyses, a level of 0.05 was assumed to be significant. Statistical analyses were conducted using SAS 9.2 for Windows (SAS Institute, Cary, North Carolina, USA).
Results
Naturalistic follow-up
A total of 203 patients with a major depressive episode were recruited into the study. After 3 years’ follow-up, 39 patients dropped out of the study prior to the primary end-point as we were unable to contact them, leaving 164 patients that completed the study. Demographic data collected at the beginning of the study showed there was no difference between the 39 patients who dropped out and the 164 patients who completed the study in terms of age (t = 0.51, P = 0.61), gender (χ2 = 2.90, P = 0.09), BMI (t = 0.86, P = 0.39), the duration of the depressive episode (t = 0.48, P = 0.63) and HRSD-17 scores (t = 0.74, P = 0.46). Among the 164 patients who completed the study, 21 patients were identified as having bipolar disorder (type I n = 6, type II n = 15) and 143 patients were diagnosed as having MDD (Fig. 1). Demographic data collected during the baseline visit from the two patient groups and the healthy controls (including age, gender, BMI, duration of depressive episode and HRSD-17 scores) were comparable, as shown in Table 1.
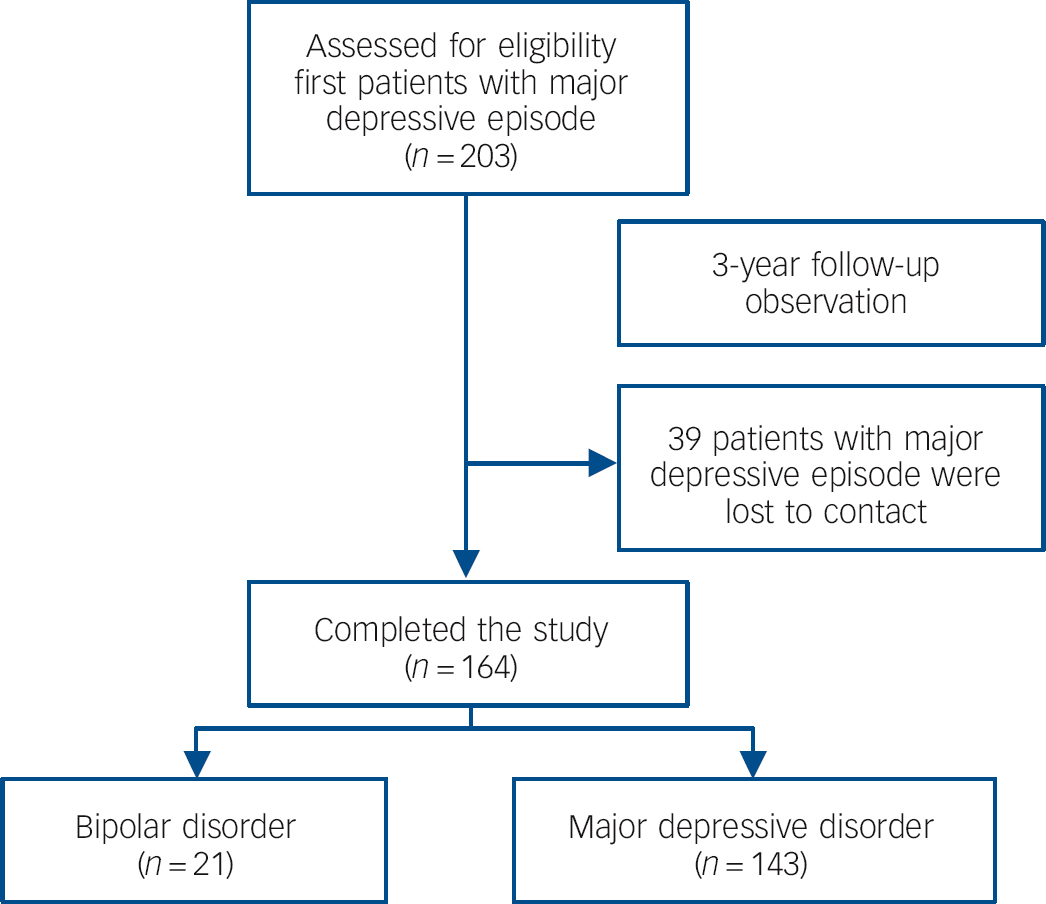
Fig. 1 Cohort diagram.
Table 1 Demographic data for the three groups

| MDD group (n = 143) |
BPD group (n = 21) |
Healthy controls (n = 167) |
t, F
or χ2 |
P | |
|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 31.0 (5.1) | 32.2 (4.1) | 30.9 (4.5) | 0.72 | 0.49 |
| Male, n (%) | 36 (25.2) | 6 (28.6) | 35 (21.0) | 1.12 | 0.57 |
| Body mass index, mean (s.d.) | 21.7 (2.3) | 21.4 (3.5) | 21.6 (2.6) | 0.16 | 0.85 |
| Duration of depressive episode, months: mean (s.d.) | 2.8 (1.0) | 2.8 (0.8) | - | 0.06 | 0.95 |
| Family history of mood disorder, n (%) | 10 (7.0) | 2 (9.5) | - | 0.17 | 0.68 |
| HRSD-17 score on admission, mean (s.d.) | 22.4 (1.6) | 22.2 (1.7) | - | 0.43 | 0.67 |
MDD, major depressive disorder; BPD, bipolar disorder; HRSD-17, 17-item Hamilton Rating Scale for Depression.
Relative expression levels of BDNF mRNA in the BPD group, MDD group and healthy controls on admission
We noted a significant difference in BDNF expression levels among the BPD group (0.0064, s.d. = 0.0023), the MDD group (0.0081, s.d. = 0.0022) and healthy controls (0.0088, s.d.= 0.0022) (F = 12.08, d.f. = 2, P<0.001). ANOVA (one-way) followed by Bonferroni multiple comparison test showed that BDNF levels were decreased in both the BPD group and the MDD group compared with the healthy controls (P<0.001 and P = 0.02 respectively), but that BDNF levels in the BPD group were even lower than those in the MDD group (P = 0.004) (Fig. 2(a)). There was no observed difference in BDNF levels between patients with bipolar I and bipolar II disorder (t = 1.10, d.f. = 19, P = 0.28).
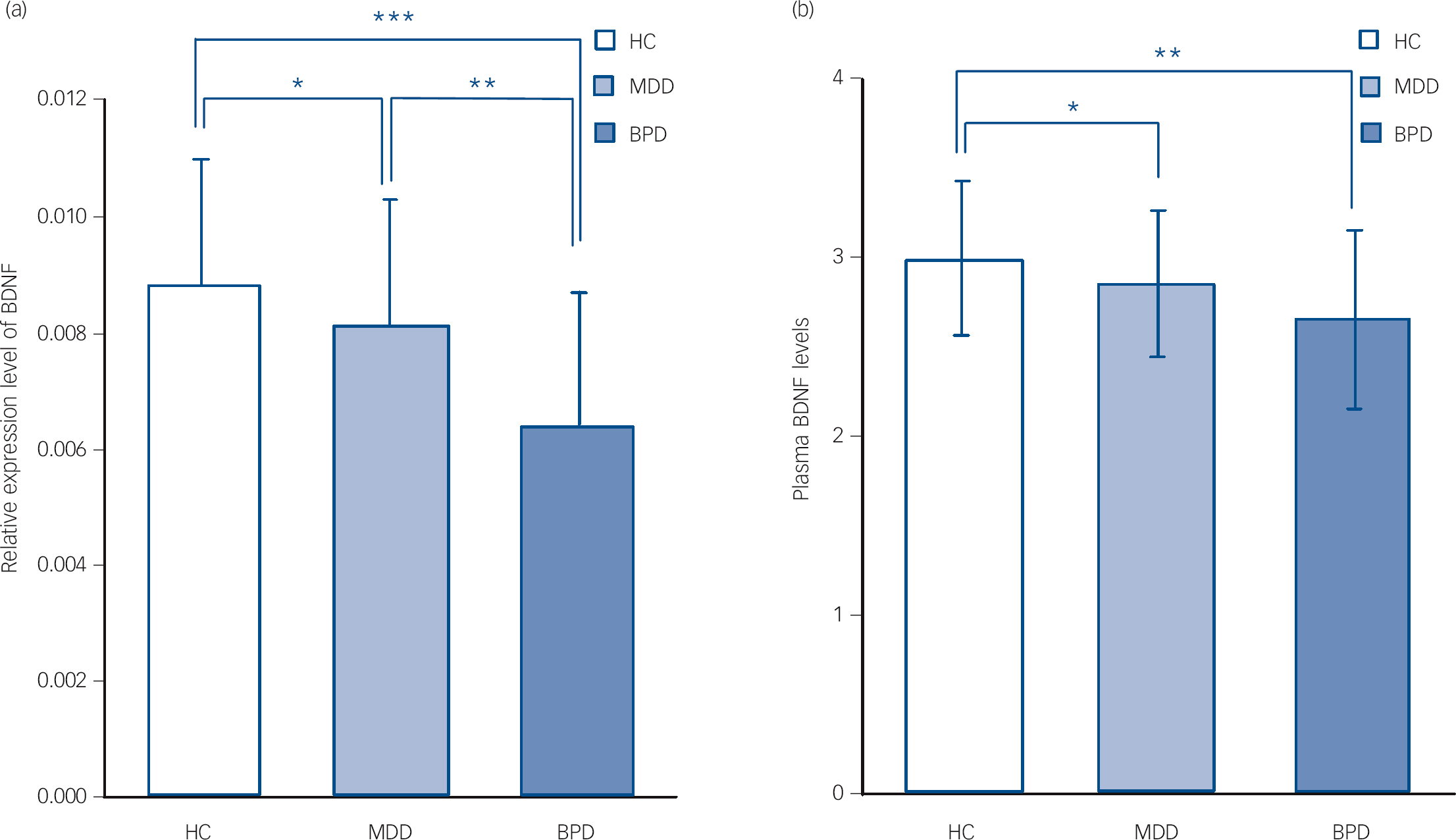
Fig. 2 Brain-derived neurotrophic factor (BDNF) levels in the three groups on admission (data presented as means (s.e.m.).
On admission, BDNF expression levels decreased in both the bipolar disorder (BPD) and major depressive disorder (MDD) groups compared with healthy controls (HCs); and BDNF expression levels in the BPD group were lower than those in the MDD group (ANOVA followed by Bonfrerroni multiple comparison test) (a). On admission, plasma BDNF levels decreased in the BPD and MDD groups compared with the HC group; but no difference of plasma BDNF levels was found between the BPD group and MDD group (ANOVA followed by Bonfrerroni multiple comparison test) (b). * P<0.05; ** P<0.005; *** P<0.001.
Plasma BDNF levels in the BPD group, MDD group and healthy controls on admission
There was a significant difference of plasma BDNF levels among the BPD group (2.66, s.d. = 0.50), the MDD group (2.86, s.d. = 0.41) and healthy controls (3.00, s.d. = 0.43) (F = 8.35, d.f. = 2, P<0.001). ANOVA (one-way) followed by Bonferroni multiple comparison test showed that plasma BDNF levels were decreased in both the BPD and MDD groups compared with the healthy controls (P = 0.002 and P = 0.01 respectively), although no difference in plasma BDNF levels was found between the BPD and MDD groups (P = 0.16) (Fig. 2(b)). There was no observed difference in BDNF plasma levels between patients with bipolar I and bipolar II disorder (t = 0.91, d.f. = 19, P = 0.37).
Association between BDNF expression/plasma levels and severity of disease
To test the potential association between BDNF expression/plasma levels and the severity of disease on admission, linear regression models were used to clarify the potential effects of confounding factors. These models were composed of both independent (e.g. relative expression levels of BDNF or log-transformed plasma BDNF levels, age, gender, BMI and duration of depressive episode and dependent variables (HRSD-17 score). The results showed that neither the relative levels of BDNF or plasma BDNF levels were correlated with HRSD-17 in either the MDD or BPD group (P>0.05) (Table 2).
Table 2 Linear regression models for association studyFootnote a
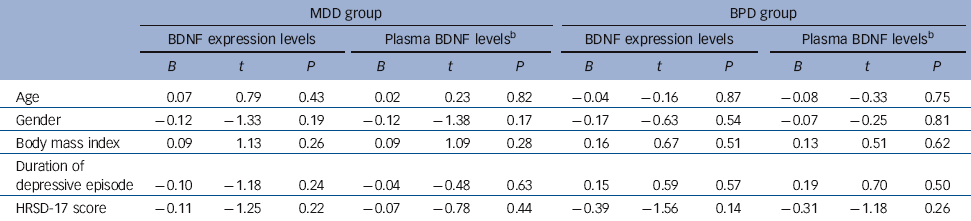
| MDD group | BPD group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDNF expression levels | Plasma BDNF levelsFootnote b | BDNF expression levels | Plasma BDNF levelsFootnote b | |||||||||
| B | t | P | B | t | P | B | t | P | B | t | P | |
| Age | 0.07 | 0.79 | 0.43 | 0.02 | 0.23 | 0.82 | −0.04 | −0.16 | 0.87 | −0.08 | −0.33 | 0.75 |
| Gender | −0.12 | −1.33 | 0.19 | −0.12 | −1.38 | 0.17 | −0.17 | −0.63 | 0.54 | −0.07 | −0.25 | 0.81 |
| Body mass index | 0.09 | 1.13 | 0.26 | 0.09 | 1.09 | 0.28 | 0.16 | 0.67 | 0.51 | 0.13 | 0.51 | 0.62 |
| Duration of depressive episode | −0.10 | −1.18 | 0.24 | −0.04 | −0.48 | 0.63 | 0.15 | 0.59 | 0.57 | 0.19 | 0.70 | 0.50 |
| HRSD-17 score | −0.11 | −1.25 | 0.22 | −0.07 | −0.78 | 0.44 | −0.39 | −1.56 | 0.14 | −0.31 | −1.18 | 0.26 |
MDD, major depressive disorder; BPD, bipolar disorder; BDNF, brain-derived neurotrophic factor; HRSD-17, 17-item Hamilton Rating Scale for Depression.
a. Age, gender, duration of disease and body mass index were also tested in linear regression models for adjustment.
b. Log-transformed plasma BDNF levels.
Best model to predict bipolar disorder in the first depressive episode
Although there was no observed correlation between BDNF gene expression levels and plasma levels in the BPD group (r = 0.20, P = 0.38), there was a marginal correlation between BDNF gene expression level and plasma level in the MDD group (r = 0.21, P = 0.02). To determine the best model for predicting bipolar disorder in the first depressive episode, discriminatory capacity was analysed by calculating the area under the ROC curve using two different statistical methods: logistic regression and a decision tree. With logistic regression, the areas under the ROC curves of gene expression level of BDNF, plasma BDNF level and the combination of both were 0.69, 0.61 and 0.80 respectively (Fig. 3). Using a decision tree, the area under the ROC curve of the combination of BDNF expression levels with plasma BDNF levels was 0.84 (the process of a decision tree is shown in online Fig. DS1). Both these methods showed that BDNF expression levels combined with plasma BDNF levels served as the most accurate model for predicting the occurrence of bipolar disorder in patients with a first major depressive episode.
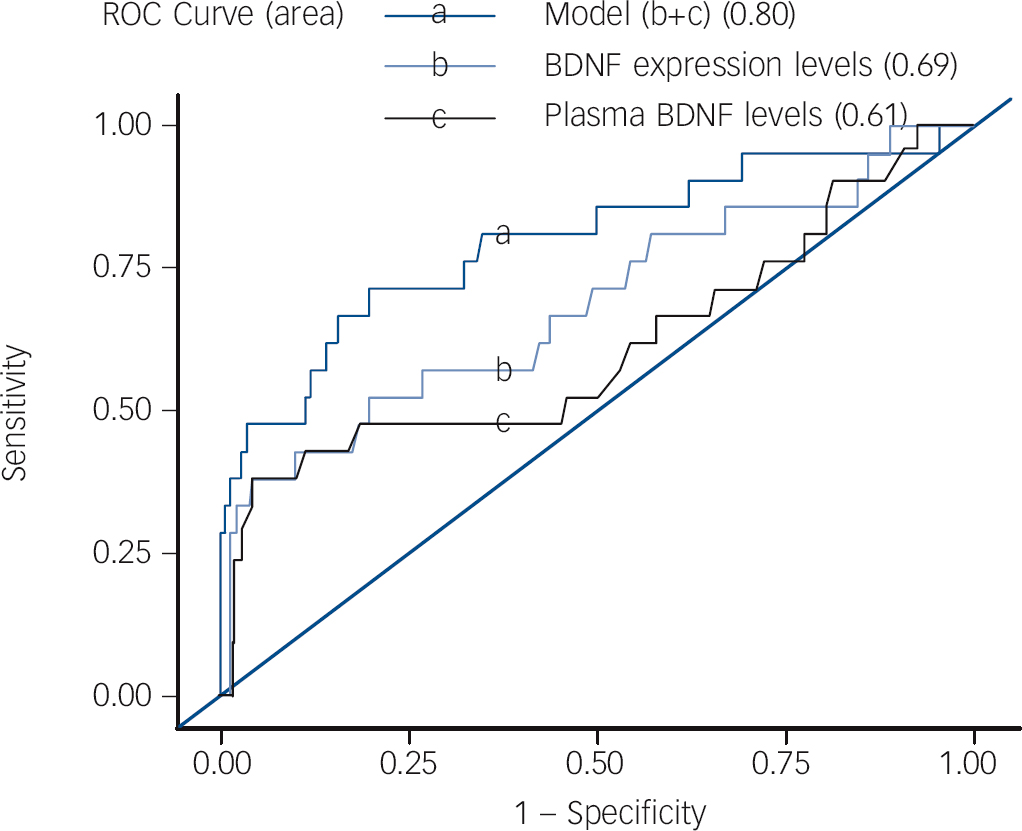
Fig. 3 Best model to predict bipolar disorder in first depressive episode.
Brain-derived neurotrophic factor (BDNF) expression levels combined with plasma BDNF levels was the best model to predict the occurrence of bipolar disorder in patients with major depressive episode using logistic regression (area under curve = 0.80) and decision tree (online Fig. DS1). ROC, receiver operating characteristic.
We also conducted a sensitivity analysis, including the patients who dropped out. If we categorise these patients as having MDD, with logistic regression the areas under the ROC curves of gene expression level of BDNF, plasma BDNF level and the combination of both were 0.67, 0.61 and 0.73 respectively. Using a decision tree, the area under the ROC curve of the combination of BDNF expression levels with plasma BDNF levels was 0.84. In summary, missing values - whether deleted or identified as MDD - did not change the diagnosis value of the variables.
Discussion
Despite numerous advances in understanding the aetiology and underlying mechanisms of bipolar disorder, the outcome for many patients remains poor. Since improvements could be made with earlier intervention and treatment, there is a strong need to develop adequate early detection methods (e.g. BDNF biomarkers) 23 However, core clinical features overlap between bipolar disorder depression and MDD, and a depressive episode is often the first mood syndrome at the onset of bipolar disorder in particular. Finding a reliable and robust biomarker identifying bipolar disorder early during a patient’s first presenting episode of depression is critical.
BDNF plays a critical role in neuronal processes including neurogenesis, neuronal survival, growth, plasticity and synaptic efficacy. Previous data have also indicated that BDNF-related neuronal function may be a crucial mediator of the effects of brain volume, psychosocial stress and psychopathology in mood disorders. Reference Grande, Fries, Kunz and Kapczinski24 To the best of our knowledge, this is the first prospective longitudinal study to investigate whether BDNF expression levels and/or plasma levels are capable of predicting bipolar disorder during a first depressive episode. We performed a large-scale study in a cohort of patients with a first major depressive episode who were drug-naive, and followed them for 3 years to identify those with bipolar disorder. The low proportion of males in our sample was consistent with a recent systematic review about the epidemiology of major depressive disorder in mainland China. Reference Gu, Xie, Long, Chen, Chen and Pan25 Our main results showed that gene expression levels of BDNF and plasma BDNF levels were both decreased in the BPD and MDD groups prior to treatment compared with the levels in healthy controls. Similarly, our analyses showed that a combination of gene expression levels of BDNF and plasma BDNF levels were the best models to predict bipolar disorder in patients with a first major depressive episode.
Despite promising results in our study, earlier reports show that BDNF levels among individuals with mood disorders is far from consistent. Some studies found decreased BDNF levels in the depressive phase of bipolar disorder, whereas others did not. De Oliveira’s group, for example, found that serum BDNF levels among patients in the depressed phase of bipolar disorder were decreased compared with controls. Reference de Oliveira, Cereser, Fernandes, Kauer-Sant'anna, Fries and Stertz26 Fernandes et al Reference Fernandes, Gama, Kauer-Sant'Anna, Lobato, Belmonte-de-Abreu and Kapczinski27 also found that patients in the depressed phase of bipolar disorder showed decreased BDNF levels compared with healthy controls. Machado-Vieira et al Reference Machado-Vieira, Dietrich, Leke, Cereser, Zanatto and Kapczinski28 demonstrated that BDNF levels were significantly decreased in unmedicated patients with bipolar disorder during manic episodes compared with healthy controls and the severity of the manic episode was negatively correlated with plasma BDNF levels. However, Mackin et al Reference Mackin, Gallagher, Watson, Young and Ferrier29 found that BDNF levels in patients during the depressed phase of bipolar disorder were similar to those in healthy controls. One meta-analysis of 107 patients and 118 healthy participants demonstrated that BDNF levels decreased during a bipolar disorder depressive episode compared with healthy controls. Reference Fernandes, Gama, Ceresér, Yatham, Fries and Colpo8 Such inconsistent results are likely due to the comparatively small sample sizes used as well as the unspecified (or undetermined) psychopharmacological states of the patients (i.e. drug-naive v. medicated patients, or distinct medications being given).
To the best of our knowledge, no study to date has examined BDNF levels in patients with bipolar disorder in their first depressive episode. Similarly, our larger sample size (composed of patients who were drug-naive and in a first major depressive episode) provides provocative evidence that, compared with healthy controls, both BDNF expression levels and plasma levels have already decreased in patients with bipolar disorder even during their first depressive episode. Our results also showed that BDNF levels (both expression levels of BDNF and plasma levels) were lower in the MDD group than in healthy controls, consistent with most previous studies. Reference Sen, Duman and Sanacora30 Further evidence from several magnetic resonance imaging (MRI) studies has shown a decreased expression of BDNF in volumetric brain reductions in psychiatric disorders. Reference Blugeot, Rivat, Bouvier, Molet, Mouchard and Zeau31,Reference Taylor32 Arnone et al Reference Arnone, McKie, Elliott, Juhasz, Thomas and Downey33 previously reported that experiencing depression is associated with a decrease in hippocampal volume and an increase in grey matter following clinical improvement. Our findings, as well as those previously reported, strengthen the hypothesis that down-regulated BDNF levels, although playing a vital role in a number of developmental processes, synaptic-plasticity and reconstruction, Reference Kelleher, Govindarajan and Tonegawa13 may also be involved in the pathophysiology of both MDD and bipolar disorder.
Previous reports suggested that BDNF is a physiopathological biomarker in psychiatry. Reference Sen, Duman and Sanacora30,Reference Cubeddu, Bucci, Giannini, Russo, Daino and Russo34,Reference Nakazato, Tchanturia, Schmidt, Campbell, Treasure and Collier35 However, even though BDNF can be considered a biomarker, whether BDNF levels can predict bipolar disorder in a first depressive episode is not clear. Fernandes et al Reference Fernandes, Gama, Kauer-Sant'Anna, Lobato, Belmonte-de-Abreu and Kapczinski27 first reported that serum BDNF levels can differentiate MDD and bipolar disorder and that serum BDNF levels were lower in patients with bipolar disorder than those in either patients with MDD or healthy controls. Their observations, however, were based on a study of 10 patients with MDD, 40 patients with bipolar disorder and 30 healthy controls. In their cross-sectional study, patients with MDD and bipolar disorder were diagnosed at baseline, thus serum BDNF levels could not be used to predict bipolar disorder in patients in their first depressive episode. Furthermore, in the absence of a follow-up observation, it is not possible to rule out hypomanic or manic episodes in the future. More importantly, their samples were medicated patients, which could confound the results, a problem shared in most studies, Reference Li, Qi, Chen, Zhang, Yi and Yuan36 because both mood stabilisers and antidepressants may potentially affect BDNF levels. Reference Jacobsen and Mork37,Reference Omata, Murata, Takamatsu, Maruoka, Mitsuya and Yonekura38 They also performed a meta-analysis, which still found peripheral BDNF as a state-marker of mood episodes in bipolar disorder. Reference Fernandes, Gama, Ceresér, Yatham, Fries and Colpo8
In the present study, we found that gene expression levels of BDNF were lower in patients with bipolar disorder initially presenting a depressive episode compared with patients with MDD. The most effective model for predicting bipolar disorder in a first depressive episode was a combination of BDNF gene expression and plasma BDNF levels. These results suggest that BDNF level may be a potential biomarker for bipolar disorder that can be detected in the first depressive episode.
Interestingly, our results showed that the expression level of BDNF was lower in the BPD group than in the MDD group. Previously, Arnone et al Reference Arnone, McIntosh, Ebmeier, Munafò and Anderson39,Reference Arnone, Cavanagh, Gerber, Lawrie, Ebmeier and McIntosh40 and Kempton et al Reference Kempton, Geddes, Ettinger, Williams and Grasby41 conducted meta-analysis studies which indicated that volumetric brain reductions in depression appeared localised in specific brain regions; conversely, there may be a more widespread volumetric loss in bipolar disorder that results in decreased intracranial brain volume. If true, this may, at least to some degree, explain the lower BDNF levels in bipolar disorder and reflect a different magnitude of genetic expression. This is only speculation, but it is an intriguing possibility that warrants further study.
Limitations
Despite the suggestive results, there are some limitations to consider. First, although we followed patients with a first major depressive episode for 3 years in an attempt to identify those patients with bipolar disorder, some patients may have experienced manic or hypomanic episodes in the following years. A longer longitudinal follow-up is required in the future to further confirm or clarify our results. Second, we performed a naturalistic observation, and patients were interviewed by our research team every 6 months, so YMRS scores were not obtained when manic/hypomanic symptoms actually occurred. Finally, most patients were at remission stage when they were interviewed and some patients were interviewed at their house every 6 months, so most of them did not want to have their blood drawn and therefore their BDNF levels were not obtained.
Implications
This study represents the first prospective longitudinal attempt to explore a predictive biomarker for bipolar disorder among patients in their first depressive episode. Our findings demonstrate that a combination of gene expression levels of BDNF and plasma BDNF levels may potentially serve to predict bipolar disorder in those experiencing their first depressive episode on admission; and it could potentially be significant in understanding the biological discrimination of affective disorders. Ultimately, down-regulated BDNF may contribute to the pathophysiology of bipolar disorder and MDD, although further evidence is needed to make any definitive determination.
Funding
This study was supported by the National Natural Science Foundation of China (91232719, 30971047, 81000581, 81171272), National High-tech R&D Program (863 Program, 2006AA02Z430, Ministry of Science and Technology of China), the “12th Five-year Plan” of National Key Technologies R&D Program (2012BAI01B04, Ministry of Science and Technology of China), National Key Clinical Disciplines at Shanghai Mental Health Center (OMA-MH, 2011-873), Shanghai Natural Science Foundation (13ZR1460500, 10ZR1426600), Shanghai Health Bureau Project, Shanghai Changhai Hospital Foundation, and Postdoctoral Grant of Secondary Military Medical University.
Acknowledgements
The authors are very grateful to all participants. We especially express our gratitude to Mrs Mary Beth Serrano from Case Western Reserve University, USA, for grammatical assistance.








eLetters
No eLetters have been published for this article.