Anaemia and Fe-deficiency anaemia (IDA) have a negative impact(Reference Gardner, Edgerton and Senewiratne1) on physical and cognitive development of children, on immune status and resistance to infection and on work capacity. Fe deficiency (ID) affects nearly 3·5 billion people throughout the world, mainly women of reproductive age, infants and young children(2). It is estimated that in developing countries, more than 40 % of preschool children and women of reproductive age are affected by anaemia(3). Vietnam has experienced considerable economic growth in the last 10 years(4) and the living standard of the population has gradually increased, mainly in urban areas(5). Malnutrition including micronutrient deficiencies remains a serious problem among vulnerable groups, in particular those living in rural or mountainous zones. In 2000, 34 % of the children aged < 5 years were underweight, and this has fallen to < 20 % over the last decade(6). Stunting prevalence remains high, with more than 30 % of children < 5 years old being stunted(6). Anaemia affects about 36 % of < 5-year-olds, 26 % of women of reproductive age and 33 % of pregnant women(7). Dietary data indicate that Fe consumed has a low availability because the diet consists mainly of cereals (75 % of energy intake) and contains many inhibitors for Fe absorption, such as phytates and polyphenols(Reference Ninh, Khan and Vinh8). Studies have suggested that in Vietnam, ID is probably the main cause of anaemia(9, Reference Le Hung, de Vries and Giao10). Lastly, parasitic infections, such as intestinal parasites and malaria, also significantly contribute to anaemia(Reference Ninh, Khan and Vinh8, 9). Recent studies in Vietnam have focused particularly on children < 5 years old and women of reproductive age(Reference Van Thuy, Berger and Nakanishi11, Reference Cavalli-Sforza, Berger and Smitasiri12). However, fewer interventions have involved school children, even though recent studies from developing countries(3, Reference Nga, Winichagoon and Dijkhuizen13) have suggested that anaemia is a serious health problem in school children, affecting about 48 % of 5–14-year-olds. This could have a negative impact on development by limiting the intellectual and physical development of children through impaired learning capacity and resistance to disease(14).
To control anaemia and ID, the WHO and UNICEF(14) recommend using an integrated and long-term multidisciplinary approach such as increasing Fe intake from diets and controlling infections. Supplementation and food fortification are regarded as the most cost-effective strategies for the control of ID and anaemia(Reference Baltussen, Knai and Sharan15). In Vietnam, the efficacy of these interventions has been proven in infants and young children(Reference Berger, Ninh and Khan16–Reference Phu, Hoan and Salvignol18), school children(Reference Nga, Winichagoon and Dijkhuizen13, 19, Reference Le, Brouwer and de Wolf20) and reproductive-age women(Reference Van Thuy, Berger and Nakanishi11, Reference Thuy, Berger and Davidsson21).
However, some studies have shown that the provision of Fe alone might not be enough to treat anaemia, as deficiencies of other micronutrients needed for erythropoiesis are likely to be present(Reference Zimmermann, Biebinger and Rohner22). Multiple micronutrient interventions also target other causes of nutritional anaemia besides Fe, and might therefore have a greater impact on reducing anaemia prevalence. Biscuits proved to be a good vehicle for micronutrient fortification and improved micronutrient status of school children in South Africa(Reference van Stuijvenberg, Kvalsvig and Faber23, Reference van Stuijvenberg, Dhansay and Smuts24). Biscuits given to children at the school were perceived by parents as a snack, and daily total food intake at home was not affected. In Vietnam, biscuits produced by local factories are well distributed throughout the country, including rural settings. Therefore, the first objective of the present study was to evaluate the impact of the consumption of biscuits fortified with multiple micronutrients on Fe status and anaemia in Vietnamese school children.
However, previously, we showed that a weekly Fe supplement was effective in improving Fe status and anaemia in Bolivian school children, with similar effects as Fe-fortified foods consumed daily(Reference Berger, Aguayo and Tellez25). As weekly Fe supplements are potentially cheaper than fortified biscuits and are also produced in Vietnam, the second objective of the present study was to evaluate the effect of a weekly Fe supplement as a potential alternative and sustainable intervention.
Participants and methods
A randomised, double-masked, placebo-controlled intervention trial was carried out in November 2005 to May 2006. School children aged 6–9 years in grade one to three of five primary schools were recruited. The schools were located in three communes of two districts (Bac Tra My and Tien Phuoc) of Quang Nam province, 900 km south of Hanoi, Vietnam where micronutrient deficiencies were known to exist. The schools were selected based on their proximity to the general hospital of Tam Ki so that blood samples could be processed within 4 h of collection. The study was carried out within the framework of the Fasevie programme(Reference Bruyeron, Khan and Berger26), a cooperative programme between Vietnam and France, carried out jointly by the Groupe de Recherche et d'Echanges Technologiques, the National Institute of Nutrition, Hanoi, Vietnam (NIN) and the Institute of Research for Development, France (IRD) and aimed at improving the nutritional status of vulnerable groups in Vietnam.
From an alphabetical name list of the children attending the five selected schools, children were randomly assigned into three treatment groups using a computer-generated random list and all children from all schools were allocated to the three groups. The treatment groups were as follows: daily fortified biscuit group and placebo tablet once per week (FB); daily non-fortified biscuits and placebo tablet once per week (control group, C); Fe tablet once per week and daily non-fortified biscuits (weekly Fe pharmaceutical supplementation group, SUP). All the children were dewormed by the health services of the Quang Nam province with Mebendazole (500 mg) a few days after the start of the study.
Sample size was estimated based on an expected difference in Hb concentration at the end of the study of 5·5 g/l between the FB and SUP groups and the C group, with a standard deviation of 12 g/l, a significance of 0·05 and a power of 90 %. The sample size was estimated to be 100 per group and with the assumption of a 20 % dropout rate; a total of at least 360 children were needed for the study.
Exclusion criteria were children who were severely underweight (weight-for-age < − 3 Z-score), with a congenital or mental anomaly, or severe anaemia (Hb < 70 g/l). At the end of the study, all children who were still anaemic were referred to the health centres of the communes to receive a weekly Fe tablet (50 mg) for 3 months. Additionally, all children with vitamin A deficiency received a dose of 60 mg vitamin A.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Scientific and Ethics Committees of the National Institute of Nutrition – Ministry of Health (Hanoi, Vietnam). All parents with children in the selected classes were informed orally and in writing about the aims and procedures of the study, and written informed consent was obtained from all parents.
The two types of biscuits were produced by the HAIHA Company, Hanoi, Vietnam. The non-fortified biscuits were produced according to a standard recipe from the HAIHA, and contained wheat flour, sugar, palm oil, skimmed milk, salt and food additives including lecithin (soya), (NH4)2CO3, NaHCO3, aroma and instant yeast. The FB were produced by adding a mixture of micronutrients (premix) to the paste of biscuits before forming and cooking. This premix was produced by the Fortitech Company, Malaysia. Its formulation was calculated so that a daily ration of five biscuits (approximately 30 g) covered 50 % of the Recommended Nutrient Intake (RNI)(27) of a 9-year-old child for vitamin A (all-trans retinol), Fe (iron fumarate), Zn (zinc sulphate)(Reference Rosado28) and iodine, 40 % of the requirements of Cu(Reference Rosado28), vitamin C, thiamin, riboflavin, vitamins B6, B12, E and niacin, 35 % of the requirements of Mg(Reference Abrams and Atkinson29, Reference Seelig30), 20 % of the requirements of Ca, vitamin D and folate and 7 % for the requirements of Mn, Se, K, chloride, Na, fluoride, pantothenic acid, vitamin K and biotin. The single serving of five biscuits corresponded to about 627 kJ. Both types of biscuits were packaged in aluminium foil bags weighing 150 g and identified with distinguishable coloured labels. All field staff and researchers as well as teachers were blinded for the group allocation that was kept in a sealed envelope at the NIN until the end of data analysis. For practical reasons and to ensure the quality of biscuits, the production was divided into two batches (the first batch was produced at the beginning of the study and the second 3 months later).
The Pharmacy University, Hanoi, produced the Fe tablets (ferrous fumarate) and placebo. The Fe tablets were made in two forms. The first contained 30 mg ferrous fumarate and the second 40 mg, allowing easy distribution to each child of a dose of 1–2 mg Fe/kg body weight. Children with a body weight < 20 kg received a 30 mg tablet/week and children weighing ≥ 20 kg received a 40 mg tablet/week. Fe and placebo tablets looked similar but were stored in two containers with a different colour code, and teachers were instructed to give the tablets to each child according to the colour.
The composition and stability over a 3-month storage of the different micronutrients in the biscuits (Fe, Zn, vitamin A, vitamin C, Ca and Mg) were analysed by the IRD laboratory in Montpellier, France, and by DSM Nutritional Products, Switzerland. The hygienic quality of biscuits was analysed at the laboratory of the NIN, Hanoi, Vietnam. The micronutrient content and the safety of the biscuits were adequate (see Table S1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). Fe content was 8·8 mg for 30 g FB (one serving) and 0·3 mg for 30 g of non-fortified biscuits.
Biscuits were distributed for 6 months during the break time (09.00–09.30 hours), 5 d/week excluding school holidays, weekends or public holidays. Over the 6-month intervention period, biscuits were distributed between 112 and 116 d and Fe tablets between 21 and 23 d, depending on schools. To avoid the exchange of biscuits and tablets between the children, three different rooms were reserved in each school for the distribution of biscuits and tablets according to their coloured labels. The children of each group were required to go to the room assigned to their group with strict supervision by a teacher who was carefully trained. Teachers recorded the daily consumption of each child and reasons for any absence or consumption of less than one ration of biscuits. If all five biscuits were not consumed by a child (which happened only a few times), supervisors were instructed to keep leftovers in the room and store them for counting. Internal monitoring was done by the headmaster of each school. External follow-up was ensured by recruited and trained supervisors (one person per school) who visited the school every day to double-check the distribution to the pupils in their respective rooms, as well as the teachers' follow-up records.
Anthropometric and biochemical data were collected twice: before (t 0) and at the end (t 6) of the intervention in non-fasted children. Children were weighed by a balance (precision 0·1 kg; SECA, Unicef) without shoes or sandals and wearing light clothes. Height was measured by using a height-measuring apparatus (precision 0·1 cm; STANLEY). Dietary intakes of energy and nutrients in the three study groups were estimated from single 24 h recalls by surveying 375 mothers at baseline and 310 at the end of the study by using the Nutritive Composition Table of Vietnamese Foods(31).
For biochemical data, 4 ml of venous blood were taken between 07.00 and 09.30 hours by technicians of the Hematology Service of Quang Nam province, Vietnam, and stored in a tube containing an anticoagulant (EDTA). A thick smear was done by technicians of the Center of Malaria of Quang Nam and the blood tube was stored immediately in an isothermal box (4–8°C) and transported the same morning to the Hematology Service. After having measured Hb concentration, plasma was obtained by centrifugation of the whole blood sample at 2000 g for 10 min at 4oC. Plasma was divided into aliquots into six 0·25 ml Eppendorf tubes and frozen at − 70°C before being transported to the NIN laboratory on dry ice where the samples were stored at − 70°C until analysis. All plasma samples were analysed at the end of the study in May 2006 at the laboratory of the Micronutrients Department of the NIN.
Hb concentration was measured by an automatic blood counter (Celdyn1700; Abbott Diagnostics). Standardisation was carried out by using standards and controls provided by an independent laboratory (Biorad). Plasma concentration of ferritin (PF) that measures the size of Fe stores and transferrin receptor (TfR) that increases with increased cellular Fe needs were analysed by the ELISA method (Ramco Laboratories, Inc.). Standardisation was carried out using the standards and controls provided by the same producer. Plasma retinol concentrations (PR) were analysed using standard HPLC methods (reverse-phase HPLC, LC-10 ADVP), using an internal standard, and plasma Zn concentration was analysed using a flame atomic absorption spectrophotometer (GBC, Avanta) using controls to check for Zn contamination. Anaemia was defined as Hb < 115 g/l(3), ID as PF < 15 μg/l and/or TfR>7·6 mg/l(3, Reference Cook32–Reference Zimmermann, Muthayya and Moretti35). IDA was defined as the simultaneous presence of ID and anaemia. Body Fe was calculated according to the formula of Cook et al. (Reference Cook, Flowers and Skikne33): body Fe (mg/kg) = − (log (TfR/PF ratio) − 2·8229)/0·1207. The diagnosis of malaria was carried out by professional technicians of the Center of Malaria of Quang Nam through the direct microscopic visualisation of the parasite on thick blood smears.
Compliance was calculated as the (number of tablets eaten)/(number of tablets intended to receive), and (number of biscuits eaten)/(number of biscuits given). If a child was absent on the day of Fe tablet distribution (Monday), s/he received the Fe (SUP) or placebo (FB and C) tablets on the day s/he returned to school and the corresponding serving of biscuits every day of presence in the week.
Statistical analyses were carried out with SPSS software version 13.0 (SPSS, Inc.), and Epi-Info software version 7.0. For continuous variables, ANOVA general linear modelling was used to determine differences among the groups, controlling for baseline values when required. If the overall F test was significant, differences between the groups were tested with Bonferroni post hoc test. P < 0·05 and P < 0·1 were considered to be statistically significant for main effects and interaction terms, respectively. Variables not normally distributed (PF and TfR) were transformed to logarithms to obtain normality. These variables are expressed as medians and inter-quartile ranges. Estimated marginal means were used to assess the size of change in biochemical indicators. Differences between baseline and endpoint biochemical indicators were tested with the paired t test. Differences in prevalence were tested with Pearson's χ2 and binary logistic regression analyses.
Results
A total of 403 children (see Fig. S1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn) were eligible and all recruited for the study and randomised into the three groups. However, only 384 children participated in the study as nineteen children were absent from schools on the day of baseline evaluation. From these, 381 (99 %) followed the study for 6 months: three children left the study because of changing school (one in the FB group and two in the SUP group). However, at the final phase of the evaluation (t 6), eighty-four parents (21·7 %) refused to bring their children to school because of their apprehension about the blood sampling (twenty-nine, thirty and twenty-five for the FB, C and SUP groups, respectively). Hence, only 297 children took part in the final evaluation and 290 blood samples were obtained, as blood sampling was insufficient in seven children (three, one and three in the FB, C and SUP groups, respectively).
At the beginning of the study, the mean age of the children was 7·7 (sd 0·9) years. There were no statistical differences for any of the parameters (age, anthropometry, Hb, PF, TfR, retinol and Zn) measured between children from whom a blood sample was collected and children absent on the last day (data not shown). At recruitment, 43·8 % of the children had anaemia, of which 12·4 % had IDA; 6·3 % had ID without anaemia; 12·8 % had low PF values, 13·1 % high TfR values and 6·3 % had both low PF and high TfR values; 7·4 % had negative values for body Fe; 38·8 % were underweight; 34·0 % were stunted and 9·9 % were wasted. There were no significant differences among the groups for biochemical and anthropometric measures (Table 1).
Table 1 Baseline characteristics of Vietnamese school children receiving either micronutrient-fortified biscuits (FB), non-fortified biscuits (C) or a weekly iron supplement (SUP) (Mean values, standard deviations and 95% confidence intervals)
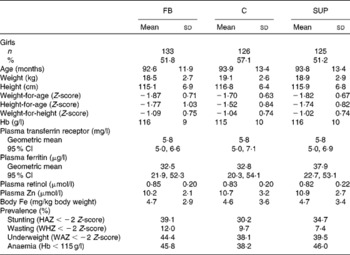
HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; WAZ, weight-for-age Z-score.
No significant differences (P>0·05) were found among the three groups for any of the variables.
After 3 months of storage, the micronutrient content of the serving size of the five FB was still close to the targeted values for Fe (47 % of the RNI), Zn (55 % of the RNI), Mg (32 %) and Ca (21 %), and somewhat higher for vitamin A (66 % of the RNI) and vitamin C (58 % of the RNI). In the non-fortified biscuits, only vitamin C (7·3 mg) and Mg (8·7 mg) made substantial contributions to daily intake (21 and 9 % of the RNI, respectively). Fe (0·3 mg, 2 % of the RNI), Zn (0·2 mg, 1 % of the RNI), Ca (7·1 mg, 1 % of the RNI) and vitamin A (0·4 μg, 0 % of the RNI) intake from the non-fortified biscuits was minimal.
After 6 months of intervention, Hb concentration was significantly higher in the FB group than in the C group (P = 0·014, ANOVA controlling for the baseline value; Table 2). In the SUP group, Hb concentration was intermediate and not significantly different from either the C or FB group. Hb concentration in all three groups was significantly higher after 6 months of intervention (P < 0·01; paired t test).
Table 2 Impact on biochemical indicators in Vietnamese school children of 6-month intervention including either micronutrient-fortified biscuits (FB), non-fortified biscuits (C) or a weekly iron supplement (SUP) (Mean values, standard deviations and 95% confidence intervals)

a,b Mean values within a row having the same superscript letters did not differ significantly (P>0·05).
* The values represent the overall P value for the group comparison (either ANOVA or Pearson's χ2) controlling for sex and baseline value.
After 6 months of intervention, the concentration of TfR was significantly lower in the FB group compared with the C group (P = 0·035), and TfR tended to be lower in the SUP group compared with the C group (P = 0·07). There was no significant difference between the FB and SUP groups in TfR concentrations. At the end of the intervention, PF concentrations of both the FB and SUP groups were significantly higher (P < 0·001) than those in the C group. As a result of these changes in TfR and PF, body Fe was significantly higher in the FB and SUP groups compared with the C group. PF concentrations and body Fe increased significantly during the intervention period in both the FB and SUP groups, whereas they decreased in the C group.
After 6 months of intervention, the prevalence of anaemia was significantly reduced in all groups (Table 2), but was significantly lower in the FB group than in the C group (P < 0·05), whereas the prevalence was intermediate in the SUP group, and not significantly different from the other two groups (Table 2). At the end of the intervention, the prevalence of ID (with or without anaemia) decreased in both the FB and SUP groups, whereas it increased in the C group (Fig. 1). At the end of the intervention, the prevalence of ID was significantly lower (P = 0·001) in the FB and SUP groups compared with the C group, with no significant difference between the FB and SUP groups (Table 2). The use of negative values for body Fe to define ID(Reference Cook32) gave similar results between the groups, although the prevalence of ID was slightly lower when using this indicator. At the end of the study, the prevalence of ID defined by a negative body Fe value was 1, 0 and 12·5 % in the FB, SUP and C groups, respectively (P < 0·001). The proportion of children with low PF values was significantly lower in the FB and SUP groups compared with the C group (5·0, 10·7 and 21·4 %, respectively, P < 0·001). The proportion of high TfR was also significantly lower in the FB and SUP groups compared with the C group (2·0, 11·1 and 14·3 %, respectively, P = 0·007). No children in the FB group, and only 2·0 % in the SUP group, had both a low PF and a high TfR value. However, 9·2 % of children in the C group had both a low PF and a high TfR value (P < 0·001). PR and plasma Zn concentrations were not significantly different between the groups, either at baseline or at the end of the intervention, and did not change significantly from baseline to the end of the intervention in the whole sample. After 6 months of intervention, there were no statistical differences in any of the anthropometric parameters among the three groups.

Fig. 1 Prevalence of anaemia, iron deficiency (ID) and iron-deficiency anaemia (IDA) in Vietnamese children at baseline and at the end of the 6-month intervention period including either micronutrient-fortified biscuits (FB), non-fortified biscuits (C) or a weekly iron supplement (SUP). Values are mean percentages. At baseline, endpoint comparisons were made between the groups for each category, respectively, anaemia without ID (□), IDA (■) and ID without anaemia (![]() ). No significant differences were found between the groups at baseline. a,b Mean values with the same letters did not differ significantly (P>0·05).
). No significant differences were found between the groups at baseline. a,b Mean values with the same letters did not differ significantly (P>0·05).
As vitamin A deficiency at baseline was a significant effect modifier of the intervention on the final PR and Hb concentrations (P < 0·01), we analysed the effect of the intervention in children who were vitamin A deficient at baseline (n 65 with twenty, twenty-two and twenty-three, respectively, in the FB, C and SUP groups): change in PR concentration was significantly higher in the FB group (0·16 (sd 0·13) μmol/l, P = 0·039) than in the C group (0·03 (sd 0·12) μmol/l), whereas the change was intermediate in the SUP group (0·09 (sd 0·22) μmol/l, not different from the C or FB group). Moreover, in these vitamin A-deficient children, Hb concentrations after 6 months of intervention were estimated to be 8·0 and 7·1 g/l higher in the FB group than in children receiving the control biscuits or SUP, respectively (P < 0·005 for both), with no difference in Hb concentration between the C and SUP groups. In contrast, in children who were vitamin A replete (PR>0·70 μmol/l), the effect of FB on endpoint Hb concentrations was estimated to be only 2·0 g/l higher compared with the C group, which was not statistically significant and was similar to the SUP group ( − 0·1 g/l). The prevalence of anaemia at endpoint evaluation in children who were vitamin A deficient at baseline was 0 % in the FB group, compared with 22·7 % in the C group and 13·6 % in the SUP group (P = 0·08, Pearson's χ2).
At the end of the intervention, there was a significant difference in weight-for-height Z-score (WHZ-score) between the three groups (P = 0·03). Children in the FB group had significantly higher WHZ-scores than children in the SUP group (P = 0·009, estimated difference 0·19 Z-score, correcting for baseline values and sex). Children in the C group had intermediate WHZ-scores and were not significantly different from the SUP and FB groups. There were no significant differences for the height-for-age or weight-for-age Z-scores between the three groups.
Compliance with the treatments averaged at 95 % and was not significantly different among the three groups (91·9 % in the FB group, 93·5 % in the C group and 99·2 % in the SUP group), and did not diminish over the study period. The main reason for not consuming biscuits or tablets was children's absence from school. The compliance in the SUP group was slightly higher than in the other groups because there was the possibility to give a missed Fe tablet later during the week in the SUP group.
Dietary intake did not differ by intervention group or by sex. Excluding biscuits and tablets, the average daily energy intake was 5597 (sd 1810) kJ at baseline and 4836 (sd 1672) kJ at the end point, i.e. about 70 % of the Vietnamese Recommended Daily Intake. Daily Fe intake averaged 7·4 (sd 3·3) mg/d at baseline and 6·2 (sd 2·9) mg/d at the end of the study, representing only 41 % of the RNI, assuming a low (5 %) bioavailability diet. About 1·0 mg Fe was provided from animal sources. Vitamin A and vitamin C daily intakes averaged 150 (sd 178) and 126 (sd 194) μg/d and 37·3 (sd 45·2) and 22·6 (sd 27·8) mg/d, respectively, at baseline and endpoint, representing on average < 30 % of the RNI for vitamin A, and between 60 and 100 % of the RNI for vitamin C. In the FB group, the consumption of Fe-fortified biscuits provided an average of 44·3 (sd 7·7) mg Fe/kg body weight during the intervention period, whereas the weekly Fe tablet plus the non-fortified biscuits provided 41·8 (sd 5·52) mg Fe/kg body weight in the SUP group and the non-fortified biscuits 1·7 (sd 0·3) mg Fe/kg body weight in the C group.
Discussion
The consumption of a serving of 30 g FB 5 d/week for 6 months significantly improved the Fe status of Vietnamese school children: Hb concentrations increased and the prevalence of ID and anaemia decreased. Children who received the Fe supplements once per week also showed a significant improvement in their Fe status, i.e. a significant increase in Fe stores and body Fe in comparison with the C group. These results indicated that the intake of a weekly Fe supplement is as effective as the regular consumption of FB in the prevention and treatment of ID. In the present study, the two interventions provided almost the same amount of Fe over the 6-month intervention period, while the non-fortified biscuits provided a much lower quantity of Fe.
However, whereas endpoint Hb concentration was higher in children receiving the FB, endpoint Hb concentration was not significantly different from the control in children receiving the weekly Fe supplements. This suggests that ID was not the only cause of anaemia and that other nutrients present in the biscuits had an effect on the Hb concentration.
The low prevalence of IDA at baseline (12·4 %) that represented less than one-third of the anaemia observed supports this hypothesis. However, this low prevalence could be due to the presence of inflammation, as ferritin concentration is increased by subclinical inflammation leading to its underestimation. In the present study, acute-phase proteins were not measured. Nevertheless, only 15 % of children had elevated leucocyte counts, suggesting a low prevalence of infections overall. Moreover, using negative values of body Fe, an indicator of exhausted Fe stores which is less affected by inflammation(Reference Cook, Flowers and Skikne33), the prevalence of ID was not higher. In the present study, 20·0 % of the children had a PF concentration < 20 μg/l and 42·2 % < 30 μg/l, indicating that many children had low Fe stores and were at risk of ID.
Other micronutrient deficiencies such as vitamin A or vitamin B12 deficiencies could be additional causes of anaemia(Reference Hieu36). The effect of the interventions in the subgroup of children with vitamin A deficiency at baseline strengthens this assumption. Indeed, Fe pharmaceutical supplementation had no effect on Hb concentration in these children, whereas provision of multiple micronutrients through FB significantly increased Hb concentrations. Among the children who were vitamin A-deficient at baseline, the final Hb concentration was significantly higher in those who consumed the FB compared with the C or SUP groups. Moreover, in the vitamin A-deficient children, prevalence of anaemia at the end of the intervention was 13·6 % in the SUP group compared with 0 % in the FB group, despite a higher body Fe status in the former, confirming the positive impact of vitamin A on Hb synthesis(Reference Zimmermann, Biebinger and Rohner22). The precise mechanism by which vitamin A affects Hb concentrations remains unclear at the moment, although increased erythropoiesis and mobilisation of Fe from stores have been proposed(Reference Zimmermann, Biebinger and Rohner22, Reference Roodenburg, West and Hovenier37).
A similar impact on micronutrient status in school-age children consuming daily FB (with Fe, iodine and β-carotene) has been found in a study in South Africa(Reference van Stuijvenberg, Kvalsvig and Faber23, Reference van Stuijvenberg, Dhansay and Smuts24), where the prevalence of anaemia decreased from 29·6 to 15·6 % over a 12-month period. We observed a decline in anaemia prevalence from 45 to 1 % over the 6-month intervention. Whereas children were dewormed at baseline in both the studies, the quantity of Fe provided in the present study was higher (8·8 mg/d) than in the study in South Africa (5 mg/d), and baseline Hb concentration was much lower in the present study (about 116 g/l v. 125 g/l in South Africa), which might explain the bigger effect found in Vietnam. In another study carried out in Vietnamese school children, mean initial Hb concentration was about 108 g/l, and 6-month consumption of noodles fortified with 10·7 mg Fe in the form of NaFeEDTA increased the Hb concentration by 18 g/l and reduced the prevalence of anaemia from 89 to 10 %(Reference Le Huong, Brouwer and Nguyen38).
In the present study, Hb concentration increased significantly in all groups over the intervention period, suggesting other causes of anaemia. Although the study was carried out in an area at risk for malaria, we did not identify any malaria case. However, all the children were dewormed by a government programme some days after baseline, which would probably have contributed to the observed increase in Hb concentrations in all groups. Another study in Vietnamese school children showed a modest impact (0·5 g/l) of deworming on Hb concentrations over a 4-month intervention(Reference Nga, Winichagoon and Dijkhuizen13). Interestingly, in the study in Vietnam with fortified noodles(Reference Le Huong, Brouwer and Nguyen38), the authors concluded that deworming had no effect on Hb levels and Fe status. Hence, the impact of deworming on Hb concentrations remains unequivocal. In addition, we cannot exclude that the information provided to all parents and teachers about the causes of micronutrient deficiencies and the ways to prevent them provided at the time of the preparation of the study, and the constant follow-up during the study by members of the research team, had a positive effect on reducing anaemia prevalence in children in the C group.
The present study also showed some effects on growth, with children receiving FB having higher WHZ-scores at the end of the study than children receiving a weekly Fe supplement. Earlier studies have reported negative effects of daily Fe pharmaceutical supplementation on weight and height gain(Reference Idjradinata, Watkins and Pollitt39, Reference Dewey, Domellof and Cohen40). From the present study, we cannot conclude whether the difference between the two interventions was due to a negative effect of the supplemented Fe on ponderal growth, improved ponderal growth in the FB group, or a combination of both, as the C group is intermediate and change in the WHZ-score between the FB and SUP groups was not significantly different. However, the present study supports the case for providing multiple micronutrients instead of single-nutrient interventions. Providing micronutrients to subjects with an energy-deficit diet, such as in the present study, is not optimal as a complete array of nutrients is likely to be the most beneficial. Indeed, effects of micronutrient pharmaceutical supplementation on growth are likely to be absent or less pronounced in subjects with concurrent deficiency of macronutrients or type-II nutrients(Reference Golden41), even though in the present study, the biscuits provided about 600 kJ/d, an addition of >10 % to the normal daily energy intake of these children.
In conclusion, the distribution of FB was easy to implement within the school system framework during the morning break and was effective in improving the Fe and anaemia status of school children. Compliance was high at more than 90 % and the same for fortified and non-fortified biscuits. Organoleptic tests (data not shown) carried out after production by the HAIHA Company did not show any difference between the two types of biscuits. However, the present study was implemented in controlled conditions, and the effectiveness of the consumption of FB must now be evaluated in real conditions where the FB are available and with an affordable cost to the population. Following the present efficacy study, a pilot intervention including the sale of FB throughout the school system was implemented with success in seven communes with a cost of 10·17 €/year per child(Reference Khoury and Bruyeron42). The weekly Fe supplement was as effective as the FB to improve the Fe status of children and represents a potential alternative when the distribution of fortified food is difficult or not available. However, in such settings, where anaemia has several nutritional causes, weekly supplementation with multi-micronutrients should be considered and evaluated.
Acknowledgements
This study was funded by the Decentralized French cooperation, Sight and Life and IRD. We thank Professor Ha Huy Khoi, director of the NIN at the beginning of the Fasevie programme, and Dr Pham Van Phu for their support and help to the Fasevie programme. We would like to thank Dr Michelle Holdsworth for proofreading the manuscript. J. B., N. T. H. and A. L. with contributions from the NCK, designed the study. N. T. H., F. S., A. S., N. P. T., A. L., O. B. and J. B. conducted the study. J. B. and F. T. W. performed the statistical analysis. J. B., F. T. W. and N. T. H. wrote the paper. J. B., N. T. H. and F. T.W. had primary responsibility for the final content. All authors read and approved the final manuscript. N. T. H., F. S., A. S., A. L., N. P. T., N. C. K., O. B., F. T. W. and J. B. have no conflicts of interest. A. S. is currently employed with the European Food Safety Authority (EFSA) in its Nutrition Unit that provides scientific and administrative support to the Panel on Dietetic Products, Nutrition, and Allergies. The present article is published under the sole responsibility of the author and may not be considered as an EFSA scientific output. The positions and opinions presented in this article are those of the author alone and are not intended to represent the views or scientific works of the EFSA. To know about the views or scientific outputs of the EFSA, please consult its website under http://www.efsa.europa.eu.





