The glycaemic index (GI) represents the incremental AUC of postprandial glucose excursions after ingestion of a certain food, compared with the postprandial excursion of an identical amount of carbohydrates from a reference food (either glucose or white bread)( Reference Jenkins, Wolever and Taylor 1 , Reference Atkinson, Foster-Powell and Brand-Miller 2 ). Recent systematic reviews have shown that low-GI diets are associated with a reduced risk of type 2 diabetes( Reference Barclay, Petocz and McMillan-Price 3 ), and that low-GI diets reduce fasting glucose and glycated Hb (HbA1c) levels in individuals with impaired glycaemic control( Reference Livesey, Taylor and Hulshof 4 ). Furthermore, a Cochrane review has indicated that low-GI diets reduce HbA1c levels in patients with type 1 and type 2 diabetes( Reference Thomas and Elliott 5 ), and a recent meta-analysis has shown a small significant improvement in HbA1c and HDL-cholesterol levels by the intake of low-GI diets specifically in patients with type 2 diabetes( Reference Ajala, English and Pinkney 6 ).
Specifically targeting the glycaemic response after breakfast seems to be a sound approach in the treatment of type 2 diabetes, since postprandial glucose excursions are higher in the morning than later in the day( Reference Peter, Dunseath and Luzio 7 ), even with a smaller carbohydrate content in breakfast compared with later meals( Reference Monnier, Colette and Rabasa-Lhoret 8 , Reference Polonsky, Given and Hirsch 9 ). This is probably due to a day–night rhythm of insulin sensitivity with more pronounced insulin insensitivity in the morning than in the evening in patients with type 2 diabetes( Reference Boden, Chen and Urbain 10 , Reference Stenvers, Jonkers and Fliers 11 ). However, studies investigating low-GI breakfasts in individuals with type 2 diabetes have yielded conflicting results. In one study conducted in nine patients with type 2 diabetes, a significant reduction in fasting plasma glucose and HbA1c levels has been shown after 3 months of a low-GI breakfast intervention( Reference Stilling, Mehlsen and Hamberg 12 ). In contrast, a study investigating a 4-week low-GI breakfast intervention in thirteen men with type 2 diabetes has shown reduced total cholesterol levels, but no effect on fasting glucose or HbA1c levels( Reference Kabir, Oppert and Vidal 13 ). A larger 6-month study performed in seventy-two patients with type 2 diabetes has shown no difference between the effects of morning consumption of low-GI or high-GI cereals on glycaemic control or cholesterol levels( Reference Tsihlias, Gibbs and McBurney 14 ). However, in the latter study, ambulant postprandial glucose excursions were not measured.
One possible advantage of using a low-GI liquid meal replacement over a regular, whole-food low-GI diet is the reduction in intensive dietary instructions and supervision, a necessity in most clinical trials on low-GI diets( Reference Ma, Olendzki and Merriam 15 – Reference Wolever, Gibbs and Mehling 17 ).
Here, we aimed to reduce postprandial glucose excursions by isoenergetic breakfast replacement with Glucerna SR (Abbott Nutrition). This liquid formula has a low glycaemic response (GR) due to a relatively high amount of MUFA, fructose and fibre( Reference Atkinson, Foster-Powell and Brand-Miller 2 , Reference Hofman, De Van and Kuipers 18 ). Its exact GI compared with that of white bread or glucose has not been determined yet. In the present study, we hypothesised that isoenergetic breakfast replacement with a low-GR liquid formula would reduce postprandial glucose excursions compared with an isoenergetic regular Dutch breakfast in both clinical and ambulant settings. Furthermore, we determined the effects of low-GR breakfast replacement on long-term glycaemia, glucose tolerance and cardiometabolic risk factors.
Subjects and methods
Subjects and setting
Eligibility criteria were a diagnosis of type 2 diabetes according to the 2010 American Diabetes Association criteria( 19 ), age 30–75 years and a BMI between 25 and 40 kg/m2. Exclusion criteria were use of any glucose-lowering agent other than metformin, any acute or chronic disorder interfering with digestion, absorption or metabolism, more frequent breakfast skipping than twice weekly and inability to give written informed consent. We carried out the present study between February 2011 and December 2012 at the Department of Endocrinology and Metabolism of the Academic Medical Center of the University of Amsterdam in The Netherlands. We recruited subjects from our outpatient clinic, via general physicians and by announcements in local newspapers and patient magazines.
The present study was approved by the Institutional Review Board of the Academic Medical Center, and conducted according to the Declaration of Helsinki of October 2004. The study was registered at The Netherlands Trial Registry (http://www.trialregister.nl) as NTR2773.
Study design and dietary intervention
We performed a randomised, controlled, cross-over trial to investigate the effect of isoenergetic breakfast replacement by a low-GR liquid formula. At baseline, the subjects recorded their food intake for 3 d for the determination of average breakfast energy content. Subsequently, they were assigned to a low-GR or control diet by block randomisation. Concealed envelopes were divided into blocks: one block of twenty and three blocks of ten. Subsequently, one concealed envelope was randomly selected by the department secretary. After completion of one intervention arm and a 1-month washout period, the subjects crossed over to the other arm (see Fig. 1).

Fig. 1 Flow chart depicting the progression of the study participants. Lab, laboratory; CGM, continuous subcutaneous glucose measurement; OGTT, oral glucose tolerance test; GR, low glycaemic response; GP, general practitioner.
In the low-GR arm, the subjects consumed a breakfast replacement consisting of an isoenergetic amount of Glucerna SR (Abbott Nutrition) for 3 months. The prescribed amount of low-GR breakfast intake was isoenergetic to the average baseline breakfast intake. The subjects were instructed to take the low-GR liquid breakfast as the first meal of the day, and were otherwise left free to choose their food intake. They were provided with sufficient amounts of the low-GR breakfast in the preferred taste (vanilla and/or chocolate) during their hospital visits. In the control arm, the subjects were allowed to consume a free-choice control breakfast. No additional dietetic support was provided.
End-points
Primary end-points were postprandial plasma glucose and insulin excursions in the clinical setting, postprandial glucose excursions measured using continuous subcutaneous glucose measurement (CGM) at home, and fasting plasma levels of glucose and insulin. Secondary end-points were fasting plasma levels of HbA1c and lipids, body weight and glucose tolerance. In addition, we measured plasma C-reactive protein concentration, waist circumference, body fat percentage, blood pressure and intakes of carbohydrates, lipids and protein.
Measurements
At randomisation, resting energy expenditure was assessed with a ViaSys Vmax Encore 29 (CareFusion).
Every 6 weeks, the subjects visited the Academic Medical Center for providing a fasting blood sample and for a physical exam. During this visit, a FreeStyle Navigator CGM sensor (Abbott Diabetes Care) was inserted. Subsequently, glucose was monitored at home for 4 d (or 3 d at the end of each 3-month intervention arm). In parallel, the subjects kept a 5 d food record (4 d at the end of an intervention arm) including exact mealtimes.
At the end of each 3-month intervention arm, the subjects were admitted to the clinical research unit for two consecutive days. On the 1st day, they entered the clinical research unit at 08.00 hours after an overnight fast. At 08.30 hours, the subjects in the low-GR arm consumed the low-GR liquid meal, while those in the control arm consumed an isoenergetic Dutch whole-food breakfast. At 12.30 hours, all subjects consumed a standard lunch of 2688 kJ (642 kcal; see Table 1). During the first postprandial hour, blood samples from a cannula inserted into a peripheral arm vein were obtained at an interval of 15 min, followed by an interval of 30 min during the subsequent hours.
Table 1 Composition of the meals in the clinical setting (Mean values and standard deviations)
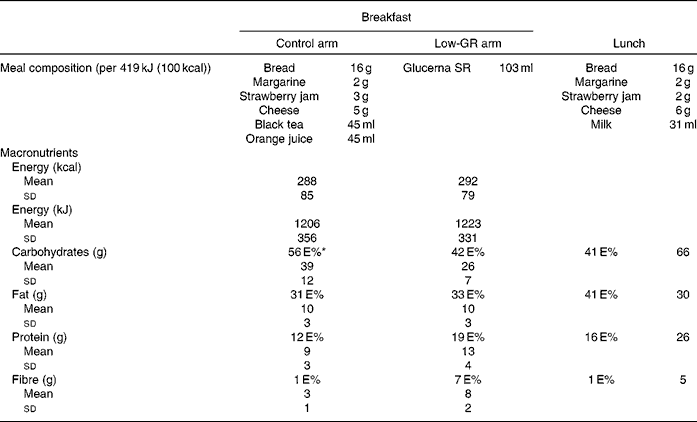
GR, glycaemic response; E%, percentage of energy.
* Percentages may not add up exactly to 100 due to rounding.
On the 2nd day, an oral glucose tolerance test (OGTT) was performed. The subjects entered the department after an overnight fast and consumed 75 g of dissolved glucose (Added Pharma), and then blood samples were obtained at an interval of 30 min.
Meal composition was determined with food analysis software based on the Dutch Food Composition Database (http://www.eetmeter.nl, Netherlands Nutrition Centre Foundation). Body fat percentage was measured by bioelectrical impedance analysis with a Maltron BF-906 body fat analyser (Maltron International).
At every visit during the low-GR treatment, the subjects were asked whether they experienced any side effects. At the end of the low-GR arm, the subjects were asked the question ‘If it would be proven that the low-GR liquid meal had a beneficial effect on your diabetes regulation, would you continue taking it for breakfast?’
Laboratory measurements
All laboratory measurements were performed in accredited diagnostic laboratories in the Academic Medical Center. Plasma glucose, cholesterol, TAG and C-reactive protein levels were assessed with a Cobas 8000 modular analyser (Roche Diagnostics). HbA1c levels were measured with a TOSOH G8 analyser (Sysmex). Insulin levels were determined with a chemiluminescent immunometric assay on an Immulite 2000 system (Siemens).
Power calculation and interim analysis
The present study was powered to detect a 1·0 mmol/l difference in fasting glucose levels, a 50 pmol/l difference in fasting insulin levels, a 100 mmol × min/l difference in postprandial glucose AUC levels, a 7500 pmol × min/l difference in postprandial insulin AUC levels and a 5 mmol/mol difference in plasma HbA1c concentrations, with a power of 90 % and a significance level of 0·05. An interim analysis was performed after 25 % of the initial target of fifty participants had completed the study. Based on this power calculation, the sample size was adjusted to twenty participants. Power calculation was performed using NQuery Advisor 7.0 (Statistical Solutions Limited).
Statistical analyses
Normally distributed variables are expressed as means and standard deviations or standard errors of the mean and non-normally distributed variables are expressed as medians and interquartile ranges (25th–75th percentiles).
Incremental AUC of postprandial plasma glucose and insulin levels were calculated using the trapezoid rule with GraphPad Prism for Windows (version 5.01; GraphPad Software, Inc.). For calculating the incremental AUC, the postprandial period was defined to extend until 180 min after meal onset. For clinical research unit data, baseline was defined as the pre-meal plasma value. For CGM data, baseline was defined as the average glucose concentration over the 60 min period before breakfast.
Further statistical analyses were performed with IBM SPSS Statistics (version 19; SPSS, Inc.). Data from the measurements at the end of the intervention arm (meal response and OGTT in the clinical setting) were analysed using the Wilcoxon signed-rank test for non-normally distributed variables and the paired-samples t test for normally distributed variables.
Data that were repeatedly measured within an intervention arm were log-transformed to achieve a normal distribution, if necessary, and subsequently analysed using a linear mixed-effects model for repeated measurements (the MIXED statement), with ‘time’, ‘intervention’ and ‘time × intervention’ as fixed effects. The repeated covariance type with an optimal model fit based on the Akaike information criterion was selected for each outcome measure.
Results
Participants
The progression of the participants through the study is shown in Fig. 1. Of the twenty-nine randomised patients, twenty patients (69 %) completed the study, and the baseline characteristics of these patients are shown in Table 2.
Table 2 Baseline characteristics of the patients (n 20) who completed the study (Medians and 25th–75th percentiles; mean values and standard deviations; number of patients and percentages)

REE, resting energy expenditure; BIA, bioelectrical impedance analysis; HbA1c, glycated Hb.
Glucose and insulin levels
During admission to the clinical research unit, postprandial glucose and insulin excursions were significantly reduced after ingestion of the low-GR breakfast compared with the isoenergetic control breakfast. After lunch, no differences were detected in glucose or insulin excursions between the dietary groups (see Fig. 2 and Table 3).

Fig. 2 Postprandial (a) glucose and (b) insulin excursions in the clinical setting. In the present cross-over study, participants were provided with either a low-glycaemic response breakfast (●) or an isoenergetic control breakfast (○) at 08.30 hours (time 0 min). At 12.30 hours (time 240 min), participants were provided with a standard lunch of 2688 kJ (642 kcal). Values are means, with their standard errors represented by vertical bars.
Table 3 Incremental AUC of postprandial plasma glucose and insulin excursions during admission to the clinical research unit (Medians and 25th–75th percentiles)
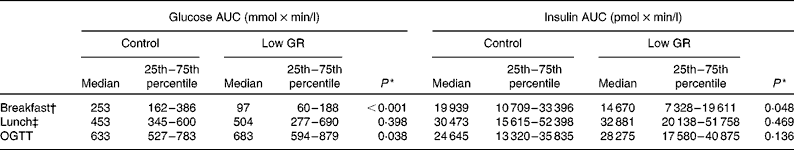
GR, glycaemic response; OGTT, oral glucose tolerance test.
* Between-group differences were assessed using the related-samples Wilcoxon signed-rank test.
† Participants were provided with either a low-GR breakfast or an isoenergetic control breakfast at 08.30 hours.
‡ At 12.30 hours, participants were provided with a standard lunch of 2688 kJ (642 kcal).
Using the CGM data, 312 (71 %) breakfast responses were obtained out of 440 attempts, with a mean of 16 measurements per subject (range 5–21). Missing data were due to sensor failures, calibration errors and/or logistic issues. Consistent with the clinical setting, ambulant recordings of postprandial glucose excursions after ingestion of breakfast showed a lower AUC in the low-GR arm compared with the control arm. The inverse log of the estimated fixed effect size of the low-GR breakfast was 0·63, indicating that the low-GR breakfast reduced the AUC of postprandial plasma glucose excursions at home by 37 (95 % CI 20, 50) % (see Table 4).
Table 4 Ambulant measurements of continuous subcutaneous glucose measurement (CGM) and food intake (Mean values and 95 % confidence intervals)
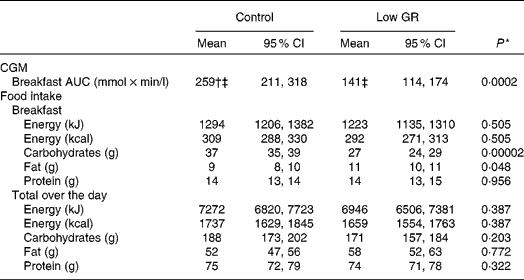
GR, glycaemic response.
* The effect of low-GR liquid meal replacement was evaluated with a repeated linear mixed model with ‘time’, ‘treatment’ and ‘time × treatment’ as fixed effects. P values represent the fixed effect of ‘treatment’. Covariance structure was selected based on the Akaike information criterion: for the AUC, ‘heterogeneous compound symmetry’; for food intake, ‘first-order ante-dependence’.
† Estimated marginal means of the linear mixed model (95 % CI) are depicted to correct for missing observations (all values in this table).
‡ Data were log-transformed to achieve normality for the linear mixed model analysis. The depicted estimated marginal means are the inverse log of the model output.
The 3-month breakfast replacement with a low-GR liquid meal did not affect fasting plasma glucose or insulin levels (see Table 5).
Table 5 Effects of 3-month breakfast replacement with a low-glycaemic response (GR) liquid meal on physical parameters and laboratory values (Medians and 25th–75th percentiles)

HbA1c, glycated Hb; NA, not available; CRP, C-reactive protein; BIA, bioelectrical impedance analysis.
* P value for the treatment × time interaction term, indicating the effect of low-GR liquid meal replacement.
† Data were log-transformed to achieve normality for the linear mixed model analysis.
Glycated Hb and lipid levels, and glucose tolerance
The 3-month low-GR liquid meal replacement did not affect fasting HbA1c or lipid levels (see Table 5). At the end of each 3-month dietary period, glucose tolerance was assessed by an OGTT in nineteen subjects (see Fig. 3). In one subject, no OGTT was performed due to family issues. Glucose values 2 h after ingestion of glucose were not found to be different between the dietary groups (low GR: mean 13·4 (sd 4·1) mmol/l; control: mean 12·6 (sd 4·0) mmol/l; P= 0·190). However, the AUC of glucose excursions after ingestion of glucose was slightly lower in the control group than in the low-GR breakfast group (see Table 3).
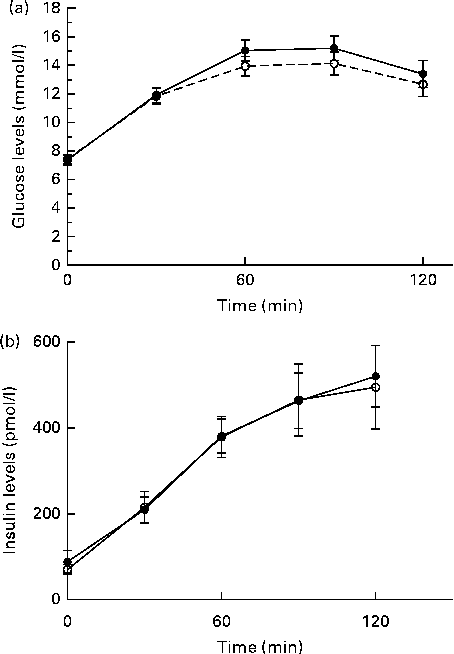
Fig. 3 Oral glucose tolerance test in the clinical setting. After a 3-month dietary period of either a low-glycaemic response breakfast (●) or a free-choice control breakfast (○), patients ingested 75 g of liquid glucose at 0 min. Blood samples for determination of plasma (a) glucose and (b) insulin levels were obtained at an interval of 30 min. Values are means, with their standard errors represented by vertical bars.
Physical parameters
The low-GR liquid meal replacement did not affect waist circumference, body weight, body composition or blood pressure (see Table 5).
Food intakes
A total number of 511 (91 %) completed food records were obtained out of 560 attempts, with a mean of 26 food records per patient (range 21–28). Missing data were due to planning issues or loss of the 5 d food record by the patient. Consumption of breakfast carbohydrate was significantly reduced in the low-GR arm, and intake of breakfast fat was slightly increased in the low-GR arm compared with the control arm. Otherwise, there were no differences between the intervention arms in terms of macronutrient intakes at breakfast or total over the day (see Table 4).
Evaluation of low-glycaemic response breakfast
During the present study, there were no serious adverse events with a likely relationship to breakfast replacement. However, two participants discontinued the study because they did not tolerate the low-GR liquid meal: one patient reported increased nausea; another patient reported an increase in pre-existing pretibial oedema. Among the patients who completed the study, self-reported side effects during the low-GR liquid meal replacement were none in ten participants (50 %); however, there were self-reports of an altered defecation pattern and/or flatulence in eight participants (40 %), nausea in one participant (5 %) and a mild attack of gout in one participant (5 %). When asked whether participants would like to continue the low-GR breakfast, sixteen (80 %) answered ‘yes’, while four (20 %) answered ‘no’.
Discussion
Low-GI diets are known to reduce HbA1c levels in patients with type 2 diabetes( Reference Ajala, English and Pinkney 6 ). In the present study, we showed that isoenergetic breakfast replacement with a low-GR liquid formula reduced postprandial glycaemia compared with an isoenergetic regular Dutch breakfast under controlled circumstances in a clinical setting.
One major advantage of using a liquid meal replacement to reduce postprandial glycaemia is the simplicity of the intervention. Patients were instructed to consume a certain amount of the liquid meal replacement, and no further dietary counselling was provided. Here, we also showed that despite the limited counselling, breakfast replacement reduced postprandial glycaemia in an ambulant setting at home compared with a free-choice control breakfast.
We verified the isoenergetic nature of the low-GR breakfast by measuring food intake and body weight. Since the low-GR arm and the control arm did not differ in the amount of energy consumed or body-weight change, the low-GR breakfast replacement was indeed isoenergetic to the control breakfast.
The low-GR liquid breakfast was well tolerated, side effects were mild and the majority of participants were willing to continue using the low-GR breakfast. This fits with earlier studies that have shown that Glucerna SR (Abbott Nutrition) is safe and well tolerated( Reference Leon-Sanz, Garcia-Luna and Sanz-Paris 20 , Reference McCargar, Innis and Bowron 21 ). The most frequently reported side effect was an altered defecation pattern and/or flatulence, which is probably the result of the high fibre content.
In the present study, we observed a clear effect on postprandial glucose and insulin levels; however, there was no effect on fasting plasma HbA1c, glucose or lipid levels. Unexpectedly, we even observed a small but significant increase in the AUC of the OGTT, implying decreased glucose tolerance after the 3-month low-GR liquid meal replacement. However, glucose values 2 h after glucose ingestion in the OGTT (an alternative and frequently used end-point) were not different between the intervention arms.
The observed absence of an effect on fasting HbA1c and glucose levels has two likely explanations. First, breakfast consumption in the population of the present study comprises approximately 20 % of total daily energy intake. Replacing breakfast only may be insufficient to affect overall glycaemic control, and it may be necessary to replace multiple meals per d in order to affect fasting HbA1c or glucose levels. Second, due to the low baseline HbA1c values in the patients of the present study, there was little room for improvement of HbA1c levels by the intervention. Accordingly, participants in the low-GI breakfast study( Reference Stilling, Mehlsen and Hamberg 12 ) that has found an effect on HbA1c levels had higher baseline HbA1c levels than those in the two low-GI breakfast studies( Reference Kabir, Oppert and Vidal 13 , Reference Tsihlias, Gibbs and McBurney 14 ) that observed no effect on HbA1c levels. The low baseline HbA1c values in the present study are probably due to the exclusion of patients using glucose-lowering medication other than metformin. This restriction represents a major limitation to the interpretation of the present study. Possibly, in poorly controlled patients with type 2 diabetes, the low-GR liquid meal replacement will actually reduce HbA1c levels.
In conclusion, meal replacement with a low-GR liquid meal replacement is a potential dietary approach to reduce ambulant postprandial glycaemia in patients with type 2 diabetes. However, in the present study population of well-controlled type 2 diabetic patients, we observed no beneficial effect of the low-GR liquid meal replacement on long-term glycaemia. Thus, before an isoenergetic low-GR liquid meal replacement can be adopted as a dietary approach to the treatment of diabetes mellitus, clinical trials are required to investigate the effects of a low-GR liquid replacement of multiple meals on long-term glycaemia and lipid profiles in patients with poor baseline glycaemic control.
Acknowledgements
The authors are grateful to Nan van Geloven for valuable statistical advice.
The present study is an investigator-initiated study, supported by Abbott Nutrition with a financial grant and in-kind contribution of Glucerna SR and FreeStyle Navigators.
The authors' contributions are as follows: D. J. S., E. F. and P. H. B. designed the research; D. J. S., L. J. S. and J. J. conducted the research; D. J. S. analysed the data; D. J. S., E. E., A. K., E. F. and P. H. B. wrote the paper; P. H. B. had primary responsibility for the final content. All authors read and approved the final manuscript.
There are no conflicts of interest.










