CVD have a growing prevalence worldwide and are a leading cause of morbidity and mortality. These diseases can be present long before the onset of clinical symptoms(Reference Saxon, Reiter-Brennan and Blaha1). In addition, metabolic syndrome (MetS) is a universal epidemic defined by a cluster of cardiometabolic abnormalities, including abdominal obesity, elevated TAG, fasting blood glucose (FBG), blood pressure and decreased HDL-cholesterol levels(Reference Kassi, Pervanidou and Kaltsas2). Cardiometabolic risk factors and components of the MetS represent a comprehensive list of existing and emerging biomarkers of diabetes mellitus, CVD and obesity-related traits(Reference Despres, Cartier and Cote3). Researchers have shown that increased risk for cardiometabolic disorders and MetS occur due to lifestyle factors, genetic susceptibility and the interaction between them(Reference Perez-Martinez, M Phillips and Delgado-Lista4,Reference Vimaleswaran5) .
Diet is a major lifestyle factor with a critical role in preventing non-communicable diseases and preserving health(Reference Cena and Calder6). Since nutrients, food and food groups are consumed in combination with each other and also synergistic and interactive effects of food components, studying dietary patterns provides the opportunity to evaluate diet–disease associations(Reference Jacobs and Steffen7). Several studies have been undertaken regarding the association between cardiometabolic risk factors and dietary patterns(Reference Rocha, Milagres and Longo8). Moreover, many genes related to cardiometabolic risk and MetS are increasingly recognised, e.g., APOE, cyclin-dependent kinase inhibitor 2A and cluster of differentiation 36 (CD36)(Reference Whitfield9–Reference Love-Gregory, Sherva and Sun11). CD36 is a gene located on chromosome 7 (q11·2) and comprises fifteen alternatively spliced exons. This gene is expressed in various cell types including taste bud cells, adipocytes, skeletal, vascular endothelial cells and intestinal enterocytes(Reference Zhao, Varghese and Moorhead12). CD36 plays a decisive role in lipid metabolism, such as fat taste perception and dietary lipid intake, fatty acids utilisation by muscle and adipose tissues, lipoprotein production and transport, storage and lipolysis, and also is involved in inflammation, foam cell formation, atherosclerosis, cardiac function and insulin resistance(Reference Zhao, Varghese and Moorhead12,Reference Pepino, Kuda and Samovski13) . Some researchers suggested that post-translational modifications of CD36 (glycosylation, acetylation, phosphorylation, ubiquitination, palmitoylation and O-GlcNAcylation) play a role in altered fatty acid uptake rates in the heart and muscle(Reference Shu, Peng and Hang14).
The rs1761667 is a common polymorphism in the CD36 gene. This polymorphism is located in the intron of 5′ flanking exon 1A and is characterised by G (frequent allele) to A (minor allele) nucleotide substitution(Reference Ma, Bacci and Mlynarski15). There is growing evidence showing that CD36 rs1761667 is linked to decreased CD36 expression, sensitivity to fat taste and high fatty food intake as a compensatory reaction(Reference Melis, Carta and Pintus16). The evidence has shown the association of the A allele with a higher risk of stroke and type 2 diabetes mellitus(Reference Lee, Won and Lee17–Reference Zhang, Zang and Wang19). Moreover, a meta-analysis study demonstrated that the A allele was related to elevated total cholesterol and LDL-cholesterol and decreased HDL-cholesterol levels in Asians(Reference Yazdanpanah, Mozaffari-Khosravi and Mirzaei20). It was also found that the A allele was associated with higher BMI, WC, hip circumference, hypertension and coronary artery disease compared with the subjects with the GG genotype(Reference Momeni-Moghaddam, Asadikaram and Akbari21,Reference Muthuswamy, Shanmugamprema and Ponnusamy22) . Indeed, the A allele seems to be a risk factor for metabolic disturbances. On the other hand, some previous studies have shown an interaction between dietary factors and CD36 rs1761667 on cardiometabolic risk factors, for instance Lopez-Ramos et al. (Reference Lopez-Ramos, Panduro and Martinez-Lopez23) indicated that the high-fat diet and high serum cholesterol levels were related to the AA genotype. Furthermore, it is demonstrated that individuals carrying the A allele have a higher fat consumption but lower BMI(Reference Salim, Kartawidjajaputra and Suwanto24).
Although several studies have been conducted on different populations and health status, none have specifically assessed interactions between dietary patterns and rs1761667 polymorphism on cardiometabolic risk factors and the risk of MetS and its components. Therefore, the current study was undertaken to evaluate dietary patterns and rs1761667 interactions on cardiometabolic risk factors (anthropometric indices, lipid profile, blood pressure, FBG) and risk of MetS and its components in apparently healthy individuals aged 20–70 years in Yazd, Iran. We hypothesised that rs1761667 genotypes might change the association of diet with cardiometabolic risk factors and MetS and its components.
Methods
Study design and participants
For this cross-sectional study, 387 apparently healthy individuals (50·1 % females and 49·9 % males) were recruited using simple random sampling from the participants of the enrolment phase of the Yazd Health Study (YaHS) conducted from 2014 to 2015. In brief, YaHS is a comprehensive prospective study on the health and diseases in Yazd Greater Area, including 10 000 residents aged 20–70 years. Dietary intake was collected from the Yazd Nutrition Survey (a YaHS sub-study) which is locally known as the TaMYZ, which is the abbreviation of ‘Taghzieh-e-Mardome Yazd’ in Persian. A detailed description of the YaHS cohort study has been previously published in the literature(Reference Mirzaei, Salehi-Abargouei and Mirzaei25). This study was conducted according to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants. All procedures involving human subjects were approved by the ethics committee of Shahid Sadoughi University of Medical Sciences (Ethnic number: 17/1/73941 and IR.SSU.SPH.REC.1398·136). After merging data from YaHS and TaMYZ studies, participants who self-reported chronic diseases (e.g. diabetes, renal or CVD, stroke, cancer, liver disease, thyroid and hypertension) or missing data were removed. Furthermore, those with malignancies, drug abuse (e.g., alcohol consumption and smoking), neurologic disorder, pregnant or lactating women, implausible energy intake (<3347·2 or > 25104 kJ/day)(Reference Dimakopoulos, Magriplis and Mitsopoulou26,Reference Kwon, Lee and Lee27) , or under medications affecting body composition like corticosteroids and contraceptives were removed from the study.
Dietary assessment method
The participants’ dietary intakes in TaMYZ were evaluated via a 178-item semi-quantitative multiple-choice FFQ, which demonstrated acceptable validity and reliability(Reference Salehi-Abargouei, Zimorovat and Moghtaderi28). Participants were asked to report their intake frequency (number of times per month, week or day) and the amount of food taken each time in the last 12 months. Based on a food photograph book, the portion size of foods was estimated as a unit and then converted into weight (grams per day). Food items were categorised into twenty-nine food groups for extracting dietary patterns.
Demographic and physical activity
The demographic variables of age, sex, marital status, education, job status, smoking status and past medical history of chronic disease were obtained through a validated questionnaire by trained interviewers. Information on physical activity was evaluated using a short version of the International Physical Activity Questionnaire and converted to the metabolic equivalent in minutes per week (MET-min/wk)(Reference Moghaddam, Aghdam and Jafarabadi29). Finally, it was classified as sedentary, moderate and active according to the median of the MET-h/wk levels.
Anthropometric and blood pressure measures
The participant’s weight, body fat percentage and body muscle percentage were measured using Bioimpedance Analyzer (Omron-BF511, Japan). Height was measured using a tape measure on a straight wall. Trained interviewers performed the waist circumference (WC) and hip circumference measurements. BMI was calculated as weight (kg) divided by height in metres squared. Also, the waist-to-hip ratio was obtained by dividing WC by hip; ≥ 0·85 and 0·9 were considered abdominal obesity for women and men, respectively(30). Systolic and diastolic blood pressure were measured after 40 min rest in a sitting position (after completed two-thirds of the interview questions) and repeated three times with a 5-min interval between each measurement. The mean of the second and third measurements was considered the participant’s blood pressure. These measurements were performed using Reichter electronic sphygmomanometers (Model N-Champion, Reister GMBH)(Reference Mirzaei, Salehi-Abargouei and Mirzaei25).
Laboratory assessments
The levels of TAG, total cholesterol, LDL-cholesterol, HDL-cholesterol and FBG were measured using a commercial kit (Pars Azmoon) and calibrated Ciba Corning (Ciba Corp) auto-analyzers.
Index calculations
The visceral adiposity index was calculated as males: WC/(39·68 + (1·88 × BMI)) × TG/1·03 × 1·31/HDL-cholesterol or females: WC/ (39·58 + (1·89 × BMI)) × TG/0·81 × 1·52/HDL- cholesterol(Reference Amato, Giordano and Galia31). The logarithmic ratio of (TG to HDL-cholesterol) was used to calculate the atherogenic index of plasma (AIP)(Reference Dobiášová, Frohlich and Šedová32). TAG-glucose index (TyG index) was calculated as ln fasting TG (mg/dl) × FBG (mg/dl)/2(Reference Navarro-González, Sánchez-Íñigo and Pastrana-Delgado33).
Diagnosis of metabolic syndrome
MetS was diagnosed according to the International Diabetes Federation. Participants who had central adiposity with two of the following four were considered as subjects with MetS: WC ≥ 94 cm for men and ≥ 80 cm for women; HDL-cholesterol < 40 mg dl for men and HDL-cholesterol < 50 mg/dl for women; serum TAG ≥ 150 mg/dl; FBG ≥ 100 mg/dl and blood pressure ≥ 130/85 mmHg(Reference Alberti, Eckel and Grundy34).
DNA extraction and genotyping
Genomic DNA was extracted from 300 µl of whole blood using the FavorPrepTM DNA extraction mini kit (Favorgen Biotech Corp) based on silica technology. The CD36 rs1761667 (G > A) was genotyped by PCR-restriction fragment length polymorphism technique using the following primers: forward 5′-CAAAATCACAATCTATTCAAGACCA-3′; reverse 5′-TTTTGGGAGAAATTCTGAAGAG-3′. The volume of PCR reactions was 25 μl, containing 3 µl extracted DNA, 1 µl of each primer (with concentration 10 pmol/μl), 12·5 µl Taq DNA polymerase 2× master mix red (Ampliqon, Denmark) containing 1·5 mM MgCl2 and 7·5 µl distilled water. The PCR amplification was performed with denaturation at 95°C for 10 min, followed by 38 cycles at 95°C, 54°C and 72°C (each step for 30 s), and ended with a final extension at 72°C for 5 min. The PCR products (10 µl) were digested with 0·5 µl of HhaI restriction enzyme (Thermo Fisher Scientific) at 37°C for 4 h. All the mentioned methods were obtained from the study of Banerjee et al. (Reference Banerjee, Gautam and Saxena18), with minor modifications. The digested DNA fragments (10 µl) were visualised upon electrophoresis in 3·5 % agarose gel (SinaClon). Eventually, three possible genotypes of GG (52 and 138 bp), AG (52, 138 and 190 bp) and AA (190 bp) were identified. The accuracy of the PCR-restriction fragment length polymorphism results was confirmed using the sequencing process (ABI3130XL genetic analyzer, Applied Biosystems) for randomly selected samples.
Statistical analysis
The sample size was calculated by the Quanto software version 1.2.4 (Department of Preventive Medicine, University of Southern California)(Reference Gauderman35), assuming a minor allele frequency (MAF) of 0·36(Reference Momeni-Moghaddam, Asadikaram and Akbari21), power 0·80 (α = 0·05 and β = 0·20) and an OR of 2·75 for the CD36 rs1761667 polymorphism based on the study of Lopez-Ramos et al. (Reference Lopez-Ramos, Panduro and Martinez-Lopez23). A sample size of n 301 in total was calculated based on the mentioned formula. However, considering the probability of the high rate of attrition, 387 participants who had eligibility criteria were entered into the current study. The normal distribution of variables was assessed by the Kolmogorov–Smirnov test. The genotype frequencies of this SNP were tested for Hardy–Weinberg equilibrium by Pearson’s χ2 test and non-quantitative data were analysed by χ2 test. Principal component analysis was applied to identify dietary patterns from twenty-nine food groups. The independent Student’s t tests and one-way ANOVA with Bonferroni post hoc test were employed for comparing continuous variables. The interaction between dietary patterns and CD36 rs1761667 polymorphism on the quantitative and qualitative variables was evaluated using the general linear model and logistic regression, respectively, before and after adjustment for confounding variables including age, sex, energy intake, occupational, educational and marital status and physical activity. In this study, the individuals were divided into two groups, AA/AG and GG genotypes, based on previous studies(Reference Muthuswamy, Shanmugamprema and Ponnusamy22,Reference Pioltine, de Melo and Santos36) . Statistical analysis was performed using IBM SPSS version 22.0 (IBM Corp). P-values ≤ 0·05 were regarded as statistically significant, and less than 0·1 was considered marginally significant for interactions(Reference Yarizadeh, Mirzababaei and Ghodoosi37).
Results
The main characteristics of the participants are summarised in Table 1. This cross-sectional study was conducted on 387 apparently healthy individuals (194 (50·1 %) women and 193 (49·9 %) men) aged 20–70 years. The means and sd weight and BMI among individuals were 71·72 ± 14·26 kg and 26·51 ± 4·92 kg/m2. The frequencies of G and A alleles of rs1761667 were 52·97 % and 47·03 %, respectively, and genotype frequencies were consistent with the Hardy–Weinberg equilibrium (P = 0·52). The overall prevalence of rs1761667 genotypes was 17·30 % (n 67), 71·30 % (n 276), and 11·40 % (n 44) for GG, AG, and AA, respectively. The study results revealed that there was no significant difference in the general characteristics, anthropometric and clinical parameters between the two genotype groups (P > 0·05) (Table 1).
Table 1. Characteristics of the study participant across rs1761667genotypes*

WC: waist circumference; WHR: waist-to-hip ratio; VAI: visceral adiposity index; FBG: fasting blood glucose; TC: total cholesterol; SBP: systolic blood pressure; DBP: diastolic blood pressure; AIP: Atherogenic index of plasma; TyG index: TAG-glucose index.
* Values are provided as mean ± standard deviation (sd), otherwise explained. Independent Student’s t tests and chi-square analysis used for continuous and categorical variables, respectively.
Association between body composition, biochemical parameters and derived dietary patterns
The principal component analysis identified three main dietary patterns: (1) western dietary pattern (WDP; high in red and processed meats, condiments, snacks, soft drinks, sweets, mayonnaise, pizza and low in grain), (2) healthy dietary pattern (HDP; rich in seafood, vegetables, fruits and dairy products) and (3) traditional dietary pattern (TDP; high in eggs, tomatoes, salt, tea, grain and low in pizza), which could explain 24·82 % of the total variance in dietary intake (Table 2). As illustrated in Table 3, no significant relationship was observed between WDP and evaluated parameters. There was a significant association across tertiles of HDP for body muscle percentage (P = 0·04) and HDL-cholesterol (P = 0·02) in the crude model. However, they changed to non-significant (P ≥ 0·05) after adjusting in terms of age, sex, energy intake, physical activity, occupational, educational and marital status. Before adjustment for mentioned confounders, higher tertiles of TDP were associated with higher levels of total cholesterol/HDL-cholesterol ratio, TyG index and AIP (all of P = 0·03). These significant differences remained after adjustment, except in the variable of total cholesterol/HDL-cholesterol ratio (P = 0·06). Significant difference between tertiles of TDP and TG was observed after adjustment (P = 0·04) but was not appeared before adjusting (P = 0·06).
Table 2. Factor loadings of the food groups in the three extracted dietary patterns derived from factor analysis

WDP: western dietary pattern; HDP: healthy dietary pattern; TDP: traditional dietary pattern.
Factor loadings with an absolute value < ±0·30 were omitted.
Table 3. Associations of body composition and biochemical markers with the tertiles of the major dietary patterns*

WDP: western dietary pattern; HDP: healthy dietary pattern; TDP: traditional dietary pattern; WC: waist circumference; WHR: waist-to-hip ratio; VAI: visceral adiposity index; FBG: fasting blood glucose; TC: total cholesterol; SBP: systolic blood pressure; DBP: diastolic blood pressure; AIP: Atherogenic index of plasma; TyG index: TAG-glucose index.
Significant items with a P-value ≤ 0·05 are bolded.
* Data are presented as mean ± standard deviation (sd).
† Crude model (unadjusted).
‡ Adjusted for age, sex, energy intake, physical activity, occupational, educational and marital status.
The interaction between identified dietary patterns and CD36 rs1761667 variants on cardiometabolic risk factors
The significant and marginally significant interactions between rs1761667 genotypes and dietary patterns on cardiometabolic risk factors are shown in Fig. 1. The results show significant interactions between HDP and CD36 rs1761667 in terms of weight (P crude-interaction = 0·032, P adjusted-interaction = 0·006), BMI (P crude-interaction = 0·002, P adjusted-interaction = 0·009), WC (P crude-interaction = 0·007, P adjusted-interaction = 0·005) and body muscle percentage (P crude-interaction = 0·03, P adjusted-interaction = 0·02). Also, a marginally significant interaction was observed on the hip circumference (P crude–interaction = 0·03, P adjusted–interaction = 0·06), body fat percentage (P crude–interaction = 0·02, P adjusted–interaction = 0·09) and TyG index (P crude–interaction = 0·17, P adjustedc–interaction = 0·057). In addition, a marginally significant interaction was found between HDP and rs1761667 genotypes on the AIP in the adjusted model (P–interaction = 0·07); however, this result was not significant in the unadjusted model (P–interaction = 0·31). A significant difference was observed in weight and BMI between the three groups of HDP (P ≤ 0·05) in participants with A-allele. HDP was significantly associated with a lower body muscle percentage (P = 0·01) and marginally significantly with a higher body fat percentage (P = 0·06) in the second tertile of participants with the GG-allele. No statistically significant difference was observed in the mean WC, hip circumference, TyG index and AIP of participants with GG and A-allele genotypes in different categories of HDP. Unlike participants with the A allele, the mean of the mentioned variables in the second tertile of HDP was numerically higher in patients with the GG genotype. This difference was not statistically significant (Fig. 1). However, no relevant gene–diet (WDP, HDP and TDP) interaction was found regarding other parameters.
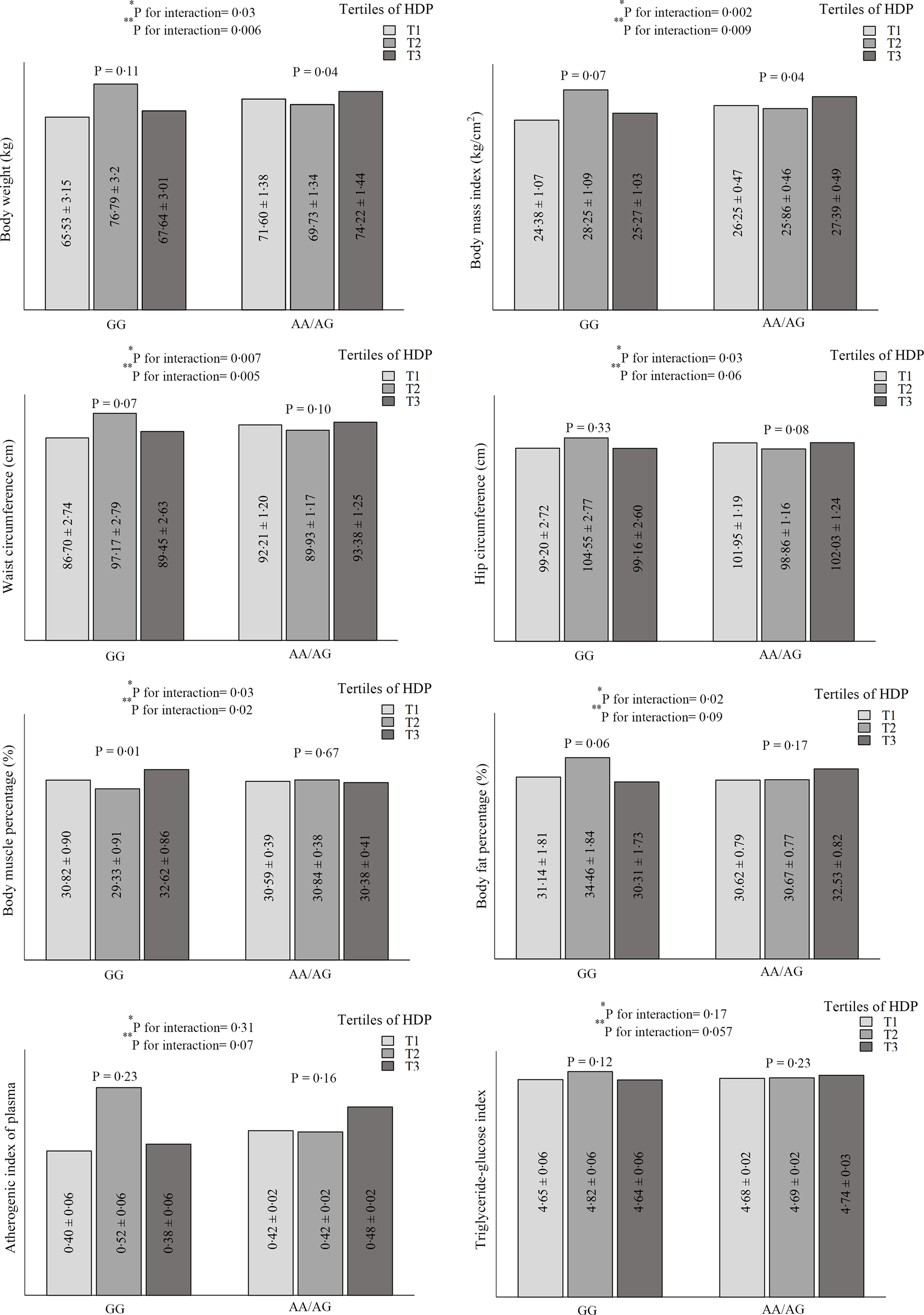
Fig. 1. Interaction between healthy dietary pattern (HDP) and CD36 rs1761667 polymorphism on cardiometabolic risk factors.
Variables are presented as mean ± standard error (SE).
*P for unadjusted interaction obtained from ANCOVA.
**P for adjusted interaction from ANCOVA. Adjusted for age, sex, energy intake, physical activity, occupational, educational, and material status.
The interaction between identified dietary patterns and CD36 rs1761667 genotypes on MetS and its components
The interaction between CD36 polymorphism (rs1761667) and dietary patterns (tertiles) on MetS and its components was investigated by considering the A-allele and the first tertile of the dietary pattern as reference groups.
A significant interaction was found between CD36 gene SNP rs1761667 and HDP on the probability of MetS in the crude (P-interaction = 0·04) and adjusted (P–interaction = 0·02) models. Also, there was a gene–diet interaction between HDP and rs1761667 genotypes in association with the risk of abdominal obesity (P-interaction = 0·05) and elevated blood pressure (P-interaction = 0·099) in the crude model. This interaction remained significant in the probability of abdominal obesity (P-interaction = 0·02) and the odds of elevated blood pressure (P–interaction = 0·07). Besides, CD36 rs1761667 polymorphism and TDP interaction were not significant in terms of TG in the crude (P–interaction = 0·11) but became significant after adjustment (P-interaction = 0·02). Although the interaction between the allele of rs1761667 and HDP was not significant regarding HDL-cholesterol levels (P-interaction = 0·60), higher adherence to the HDP (compared to the first quartile) was associated with higher odds of HDL-cholesterol (β (95 %CI) = 2·00 (1·04; 3·86), P = 0·03) in individuals with the A-allele. Individuals with the GG genotype had a higher probability of abdominal obesity in the second tertile of HDP (β (95 %CI) = 10·79 (1·90; 61·07), P = 0·007) compared with the first tertile of A-allele after adjusting potential confounders. Furthermore, lower adherence to the WDP was associated with higher odds of elevated BP among the individuals with the GG genotype as compared with A-allele (β (95 %CI) = 4·16 (1·17; 14·83), P = 0·02). No significant relation was observed between CD36 rs1761667 polymorphism and dietary patterns on other risks of MetS and its components in either crude or adjusted models (online Supplementary Table S1).
Discussion
The key findings of this cross-sectional study were the significant interaction between HDP and CD36 rs1761667 SNP on weight, BMI, WC and body muscle percentage and marginal interaction on the hip circumference, body fat percentage, AIP and TyG index. Also, when stratified by rs1761667 genotypes, the high HDP was associated with a significantly lower weight and BMI in A-allele. Among participants who carry GG genotype, the body muscle percentage was significantly lower in the second tertile of HDP. Moreover, a statistically significant interaction was observed between the CD36 gene rs1761667 and HDP on the odds of MetS and some of its components (hypertension and abdominal obesity). The interaction was also observed between TDP and the mentioned polymorphism on the odds of hypertriglyceridaemia. Higher intake of the HDP among the A-allele of rs1761667 was associated with higher odds of HDL-cholesterol. The probability of abdominal obesity in individuals with the GG genotype was higher in the second tertile of HDP than in the first tertile of A-allele. Furthermore, lower adherence to the WDP was associated with higher odds of elevated BP among individuals with the GG genotype. Based on these results, it appears that the presence of the A allele with high HDP is a protective factor against cardiometabolic risk factors. Thus, individuals with GG genotype may be more vulnerable to CVD despite moderate adherence to HDP which is rich in fish, vegetables and fruit.
The precise mechanism by which rs1761667 SNP interacts with dietary patterns is largely unknown. A noteworthy point about the HDP in this study is its high fibre content, and high-quality protein such as fish (rich in n-3 polyunsaturated fatty acids). According to previous studies(Reference Jovanovski, Mazhar and Komishon38–Reference Hidayat, Zhu and Peng40), these compounds reduce weight and BMI and increase HDL-cholesterol. Furthermore, the consumption of dairy products (low- and high-fat) was observed in this pattern. This item appears to be associated with a lower risk of MetS components, such as low HDL-cholesterol(Reference Lee, Lee and Kim41). Although some reviews have reported the benefit of fruits, vegetables, fish and dairy products on anthropometric and body composition, HDP interaction with rs1761667 genotypes may lead to increased and decreased WC and body muscle percentage, respectively, in the GG genotype. In addition, our results suggested that the GG genotype had a greater risk of hypertension in WDP. The WDP was characterised by high intakes of salty snacks, soft drinks, pizza, sweets and desserts, which reported that are associated with hypertension(Reference Neves, de Souza and Gorgulho42). The present study showed that a diet with high fibre, fish and dairy products could be more effective in the A carrier than the GG genotype. The finding of gene–diet interaction may account for some of the unexplained heritability of cardiometabolic and MetS traits. On the other hand, previous studies have focussed on the interplay of rs1761667 genotypes, dietary fat taste perception or fat preference, and the association between this polymorphism and dysmetabolic conditions such as CVD, dyslipidaemia, hypertension, diabetes, MetS and obesity(Reference Banerjee, Gautam and Saxena18,Reference Bayoumy, El-Shabrawi and Hassan43–Reference Karmous, Plesník and Khan46) . Scarce research has been conducted on the interaction between this polymorphism with total energy intake, macronutrients and other dietary factors(Reference Fujii, Hishida and Suzuki47–Reference Madden, Carrero and Brunner49), while the interplay between dietary patterns and this SNP has not been considered. Consistent with our results about cardiometabolic risk factors, a previous study demonstrated that individuals carrying the A allele have a lower BMI(Reference Salim, Kartawidjajaputra and Suwanto24). Furthermore, Fujii et al. (Reference Fujii, Hishida and Suzuki47) reported that participants with the AA genotype of rs1761667 had a higher intake of total fat and MUFA and a lower risk of hypertension than those with the GG genotype in Japan. In contrast with the findings, some studies have not reported any association between CD36 rs1761667 polymorphism and cardiometabolic risk factors and MetS(Reference Zhang, Ling and Deng45,Reference Solakivi, Kunnas and Nikkari50,Reference Noel, Lai and Mattei51) . These discrepancies in findings might be due to the variations in the genotyping methods, ethnicity, health status, gene-environment or gene–gene interactions and interactions of rs1761667 polymorphism with other variants in the CD36 gene. Another possible reason for these inconsistencies could be differences in the design of studies(Reference Yazdanpanah, Mozaffari-Khosravi and Mirzaei20).
Both deficiency and abnormally up-regulated CD36 lead to dyslipidaemia, metabolic disorders, inflammation and thrombosis(Reference Shu, Peng and Hang14). A genome-wide scan has provided evidence that SNP in CD36 including rs1761667 are strongly related to CD36 expression(Reference Ghosh, Murugesan and Chen52). Melis et al. (Reference Melis, Carta and Pintus16) have reported that the GG genotype of rs1761667 is characterised by the increased expression of the CD36 receptor, which plays an important role in regulating fatty acid entry into the cell. However, it has been observed that excessive fatty acid uptake can impair CD36 activity, induce ectopic fat deposition and reduce the activity of mitochondrial. Accordingly, it may decline lean mass and increase fat mass, especially in visceral adipose tissue(Reference Pepino, Kuda and Samovski13,Reference Samovski, Sun and Pietka53) . Hence, these conditions can correspond to a trend for increased WC in GG subjects. Moreover, some documents have suggested that the A allele of this SNP is related to a decreased expression of CD36 (Reference Ghosh, Murugesan and Chen52,Reference Love-Gregory, Sherva and Schappe54) . In this regard, an inverse relationship has been reported between CD36 expression and HDL-cholesterol level(Reference Love-Gregory, Sherva and Schappe54). One animal study indicated that hypertension is associated with increased CD36 gene expression. Also, the overexpression of CD36 may cause an intensified fatty acid uptake in cardiomyocytes(Reference Dumitrescu, Constantin and Nemecz55). Generally, it seems that CD36 polymorphisms and multiple factors could change the expression of this gene, and environmental factors including diet(Reference Koonen, Jacobs and Febbraio56,Reference Sung, Koonen and Soltys57) , probably could interact with them in altering this gene expression. Due to the limited financial resources, we could not carry out quantitative PCR or western blot to realise whether rs1761667 polymorphism alters the CD36 expression.
This cross-sectional research revealed that higher TDP adherence was associated with higher TG, AIP and TyG index. Grain (including whole- and refined-grain) and egg are among the contributors to the TDP in this study. Some studies have suggested that high refined-grains consumption is significantly related to elevated TG and FBG levels(Reference Parks58–Reference Sawicki, Jacques and Lichtenstein60). A systematic review and meta-analysis have reported that egg consumption had no significant effect on serum TG. Nevertheless, they observed an increasing influence on TG levels when egg interventions were compared with other foods(Reference Khalighi Sikaroudi, Soltani and Kolahdouz-Mohammadi61).
To the best of our knowledge, the present study is the first to investigate the interaction between dietary patterns and CD36 rs1761667 polymorphism on cardiometabolic risk factors and the risk of MetS and its components. Several potential confounders, such as age, sex, energy intake, physical activity, occupational, educational and marital status, were measured in this research. Moreover, another strength of the study is the application of a reliable and validated FFQ to collect dietary information by trained interviewers. However, some limitations need to be considered in interpreting this study. Since the design of this study was cross-sectional, causal inferences could not be drawn. The FFQ was applied for evaluating the dietary intake, which may potentially face recall bias. Dietary habits were not assessed as a confounder. Another limitation of the present study was that the health status and disease information were collected via self-reporting, which may lead to selection bias. This study was performed in a city in Iran with a unique culture and dietary intake. Therefore, the findings may not be generalisable to all Iranians or other countries and must be replicated in other populations.
Conclusions
In summary, the present study demonstrated an interaction between CD36 rs1761667 polymorphism and HDP on weight, BMI, WC, body muscle percentage, the risk of MetS and some of its components (hypertension and abdominal obesity). Also, it showed a marginal interaction on the hip circumference, body fat percentage, AIP and TyG index. Furthermore, a significant interaction was found between this polymorphism and TDP on hypertriglyceridaemia. Based on these results, the presence of the A allele with higher adherence to the HDP is a protective factor against cardiometabolic risk factors. However, cohort studies in different populations with varied health statuses are needed to elucidate these interactions further.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523000570
Acknowledgement
The authors thank all participants who participated in YaHS-TaMYZ studies and also the investigators for sharing the data.
This study was supported by grants (ID: 7173) from Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conceptualisation: Z. Y., A. S. A., M. H. S., M. M. and H. M-K; methodology: Z. Y., A. S. A. and H. M. K.; formal analysis: Z. Y., A. S. A. and H. M. K.; investigation: Z. Y. and M. M.; project administration: Z. Y., A. S. A. and H. M. K.; supervision: A. S. A. and H. M. K.; writing—original draft: Z. Y.; writing—review and editing: A. S. A, M. H. S, M. M. and H. M. K.
The authors declare that there is no conflict of interest.
Ethics of human subject participation
Written informed consent was obtained from all participants. All procedures involving human subjects were approved by the ethics committee of Shahid Sadoughi University of Medical Sciences (Ethnic number: 17/ 1/73941 and IR.SSU.SPH.REC.1398·136).







